Abstract
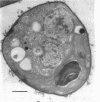
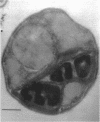
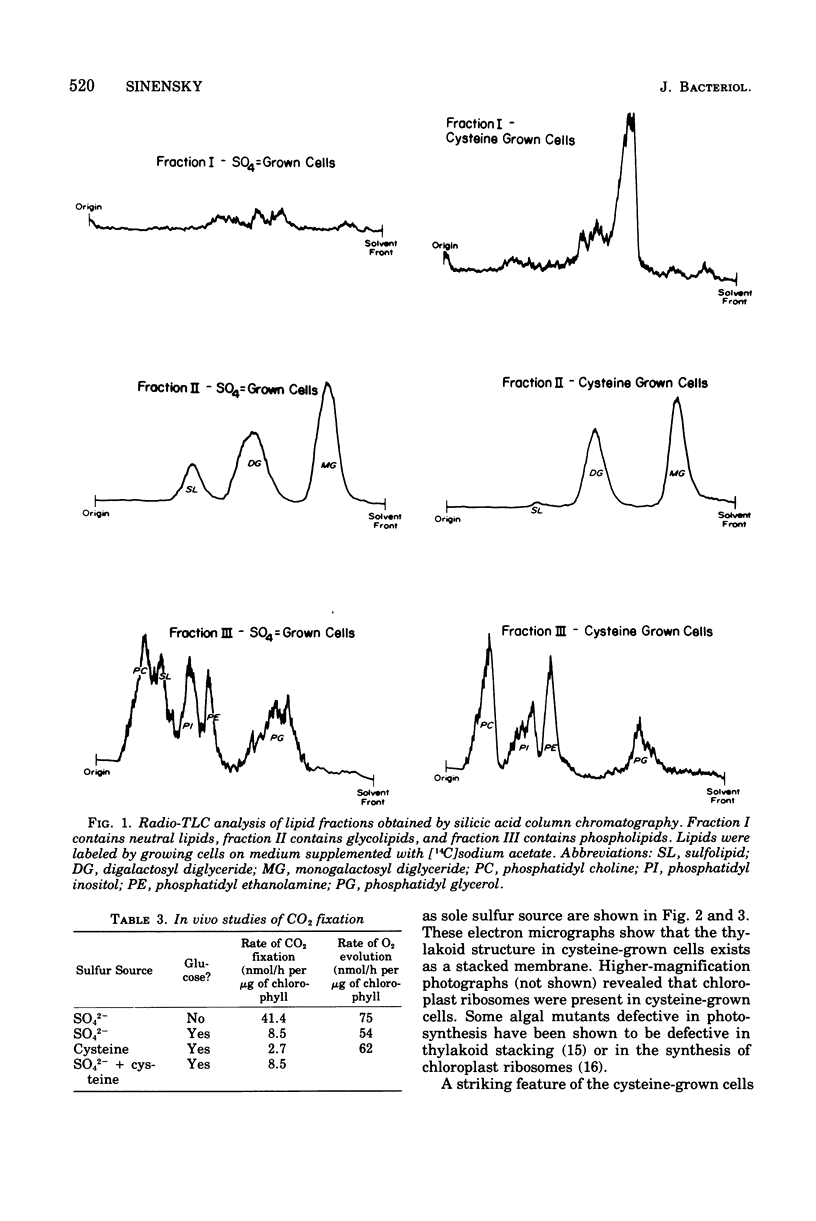
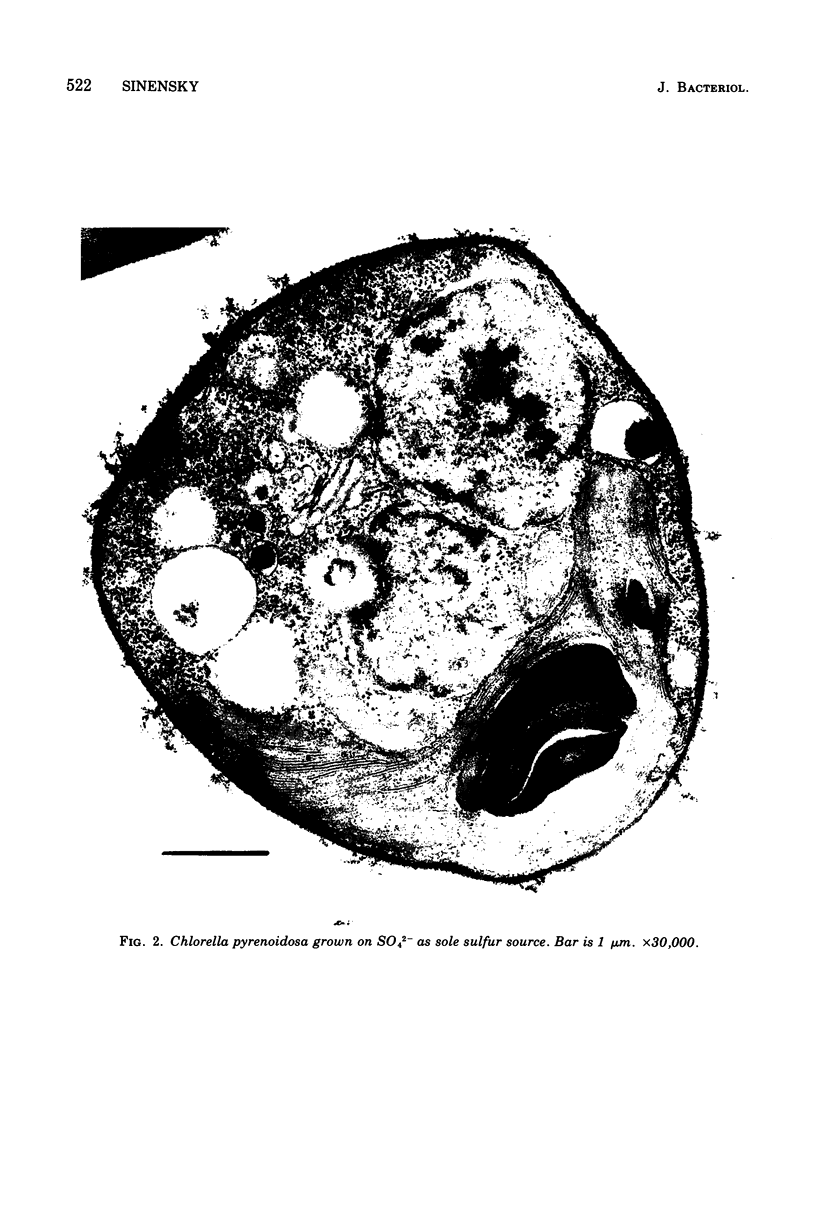
It was found that when Chlorella pyrenoidosa was grown on cysteine as the sole sulfur source, it lost the ability to grow photoautotrophically. When grown in the presence of glucose, cysteine-grown cells displayed a doubling time in the light or dark of 45 h, which is identical to that of cells grown on glucose and SO4 in the dark. This suggests that cells grown on cysteine as sole sulfur source can only grow heterotrophically. In support of this hypothesis, it was found that cysteine-grown cells were defective both in vivo and in vitro in CO2 fixation, although O2 evolution in such cells was normal. Assays of the enzymes of the Calvin cycle indicated that the deficit in CO2 fixation could be ascribed to a lowered phosphoribulokinase activity. A total lipid analysis of Chlorella grown on cysteine revealed that such cells showed a 100-fold deficiency in the purportedly chloroplast-associated 6-sulfoquinovsyl diglyceride. This agrees with earlier reports that cysteine could not serve as a precursor of sulfolipid in Chlorella. No other polar lipid was affected. Large amounts of triglyceride, however, were found in cysteine-grown cells. The biosynthesis of triglyceride provides a means of utilizing reduced nicotinamide adenine dinucleotide reducing equivalents not being used for CO2 fixation.
Full text
PDF




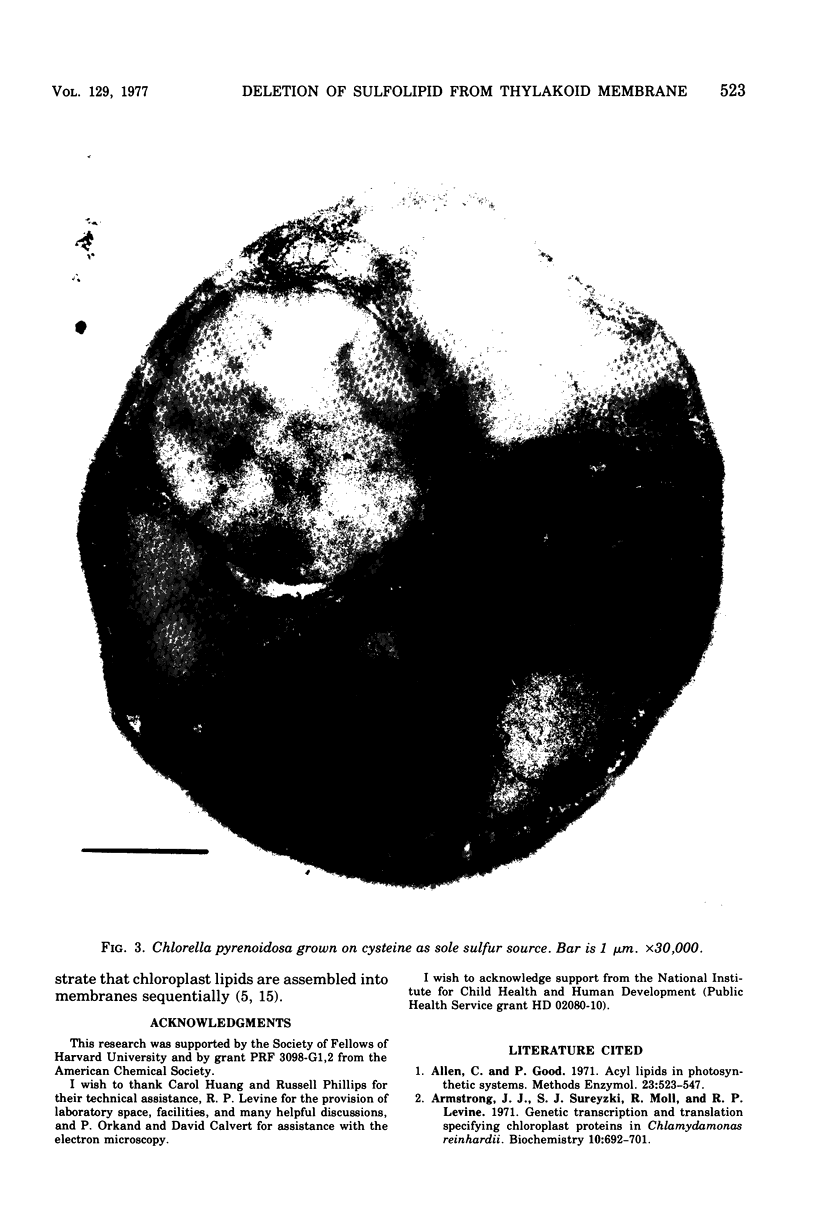

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. J., Moll B., Surzycki S. J., Levine R. P. Genetic transcription and translation specifying chloroplast components in Chlamydomonas reinhardi. Biochemistry. 1971 Feb 16;10(4):692–701. doi: 10.1021/bi00780a022. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beck D. P., Levine R. P. Synthesis of chloroplast membrane polypeptides during synchronous growth of Chlamydomonas reinhardtii. J Cell Biol. 1974 Dec;63(3):759–772. doi: 10.1083/jcb.63.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson A. A., Daniel H., Wiser R. A SULFOLIPID IN PLANTS. Proc Natl Acad Sci U S A. 1959 Nov;45(11):1582–1587. doi: 10.1073/pnas.45.11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr A new method for selection of Escherichia coli mutants defective in membrane lipid synthesis. Nat New Biol. 1972 Nov 1;240(96):21–22. doi: 10.1038/newbio240021a0. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcans B., Jain M. K. Role of phospholipids in transport and enzymic reactions. Adv Lipid Res. 1974;12(0):147–226. doi: 10.1016/b978-0-12-024912-1.50011-9. [DOI] [PubMed] [Google Scholar]
- Freeman C. P., West D. Complete separation of lipid classes on a single thin-layer plate. J Lipid Res. 1966 Mar;7(2):324–327. [PubMed] [Google Scholar]
- Goodenough U. W., Armstrong J. J., Levine R. P. Photosynthetic Properties of ac-31, a Mutant Strain of Chlamydomonas reinhardi Devoid of Chloroplast Membrane Stacking. Plant Physiol. 1969 Jul;44(7):1001–1012. doi: 10.1104/pp.44.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Levine R. P. Chloroplast structure and function in ac-20, a mutant strain of Chlamydomonas reinhardi. 3. Chloroplast ribosomes and membrane organization. J Cell Biol. 1970 Mar;44(3):547–562. doi: 10.1083/jcb.44.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U. W., Levine R. P. Methods for the study of chloroplast membranes of wild-type and mutant strains of Chlamydomonas reinhardi. Methods Enzymol. 1974;32:871–880. doi: 10.1016/0076-6879(74)32089-7. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XX. Identification of phosphatidylglycerol and cardiolipin as cofactors for isoprenoid alcohol phosphokinase. J Biol Chem. 1970 Jul 25;245(14):3691–3696. [PubMed] [Google Scholar]
- Hodson R. C., Schiff J. A., Mather J. P. Studies of sulfate utilization by algae: 10. Nutritional and enzymatic characterization of chlorella mutants impaired for sulfate utilization. Plant Physiol. 1971 Feb;47(2):306–311. doi: 10.1104/pp.47.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan-Zur V., Lips S. H. Studies on the intracellular location of enzymes of the photosynthetic carbon-reduction cycle. Eur J Biochem. 1975 Nov 1;59(1):17–23. doi: 10.1111/j.1432-1033.1975.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Latzko E., Gibbs M. Enzyme activities of the carbon reduction cycle in some photosynthetic organisms. Plant Physiol. 1969 Feb;44(2):295–300. doi: 10.1104/pp.44.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. P., Togasaki R. K. A mutant strain of Chlamydomonas reinhardi lacking ribulose diphosphate carboxylase activity. Proc Natl Acad Sci U S A. 1965 May;53(5):987–990. doi: 10.1073/pnas.53.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P. Inositol deficiency resulting in death: an explanation of its occurrence in Neurospora crassa. Science. 1966 Jan 7;151(3706):86–88. doi: 10.1126/science.151.3706.86. [DOI] [PubMed] [Google Scholar]
- Moll B., Levine R. P. Characterization of a Photosynthetic Mutant Strain of Chlamydomonas reinhardi Deficient in Phosphoribulokinase Activity. Plant Physiol. 1970 Oct;46(4):576–580. doi: 10.1104/pp.46.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISSEN P., BENSON A. A. ABSENCE OF SELENATE ESTERS AND "SELENOLIPID" IN PLANTS. Biochim Biophys Acta. 1964 Feb 10;82:400–402. doi: 10.1016/0304-4165(64)90313-7. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Benson A. A. Isolation and fatty acid composition of the plant sulfolipid and galactolipids. J Lipid Res. 1964 Jul;5(3):432–434. [PubMed] [Google Scholar]
- RACKER E. The reductive pentose phosphate cycle. I. Phosphoribulokinase and ribulose diphosphate carboxylase. Arch Biochem Biophys. 1957 Jul;69:300–310. doi: 10.1016/0003-9861(57)90496-4. [DOI] [PubMed] [Google Scholar]
- Renkonen O., Bloch K. Biosynthesis of monogalactosyl diglycerides in photoauxotrophic Euglena gracilis. J Biol Chem. 1969 Sep 25;244(18):4899–4903. [PubMed] [Google Scholar]
- Sandermann H., Jr The reactivation of C55-isoprenoid-alcohol-phosphokinase apoprotein by lipids. Evidence for lipid hydration in lipoprotein function. Eur J Biochem. 1974 Apr 1;43(2):415–422. doi: 10.1111/j.1432-1033.1974.tb03427.x. [DOI] [PubMed] [Google Scholar]
- Trosper T., Sauer K. Chlorophyll a interactions with chloroplast lipids in vitro. Biochim Biophys Acta. 1968 Jul 16;162(1):97–105. doi: 10.1016/0005-2728(68)90218-1. [DOI] [PubMed] [Google Scholar]
- Vanderkooi J., Erecińska M., Chance B. Cytochrome c interaction with membranes. II. Comparative study of the interaction of c cytochromes with the mitochondrial membrane. Arch Biochem Biophys. 1973 Aug;157(2):531–540. doi: 10.1016/0003-9861(73)90672-3. [DOI] [PubMed] [Google Scholar]