Abstract
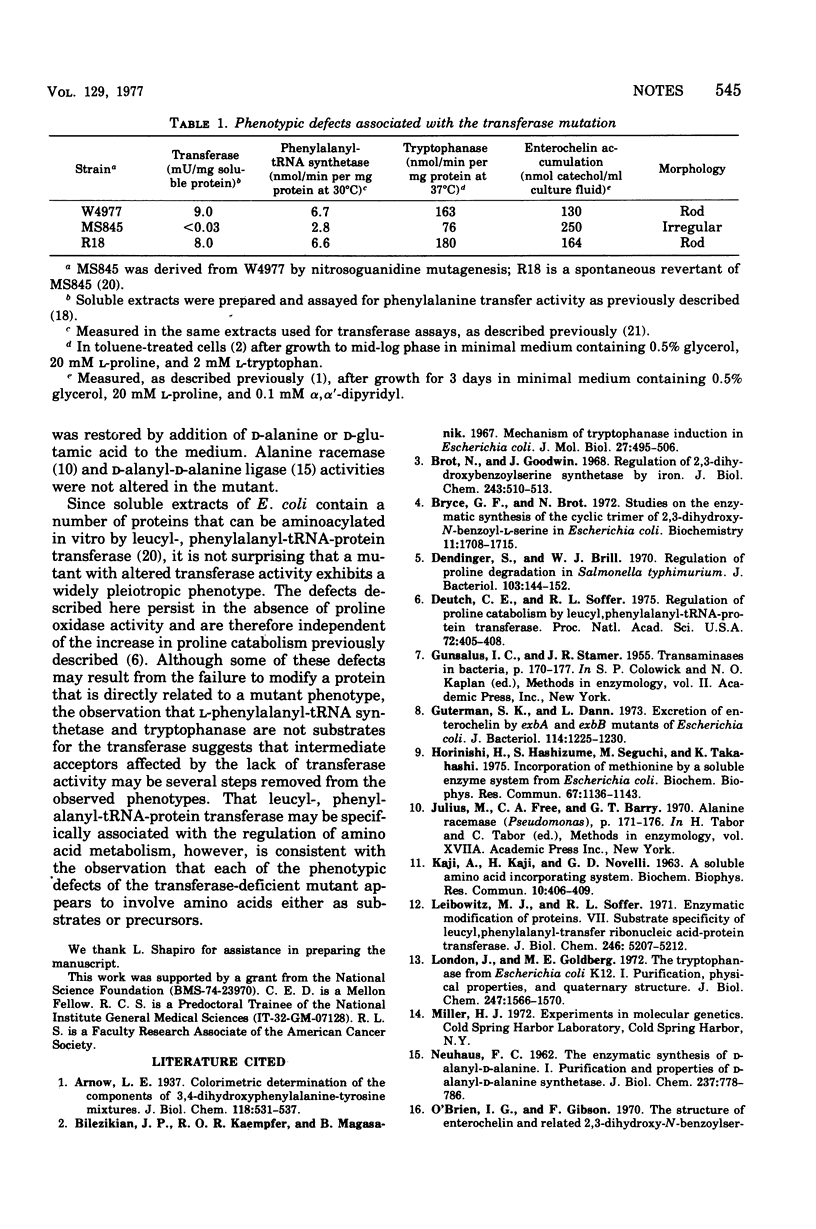
A mutant of Escherichia coli that lacks leucyl-, phenylalanyl-transfer ribonucleic acid-protein transferase had diminished activities of L-phenylalanyl-transfer ribonucleic acid synthetase and tryptophanase, grew faster than its parent with aspartic acid as the sole nitrogen source, accumulated higher levels of enterochelin in the medium during iron limitation, and exhibited an abnormal morphology.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brot N., Goodwin J. Regulation of 2,3-dihydroxybenzoylserine synthetase by iron. J Biol Chem. 1968 Feb 10;243(3):510–513. [PubMed] [Google Scholar]
- Bryce G. F., Brot N. Studies on the enzymatic synthesis of the cyclic trimer of 2,3-dihydroxy-N-benzoyl-L-serine in Escherichia coli. Biochemistry. 1972 Apr 25;11(9):1708–1715. doi: 10.1021/bi00759a028. [DOI] [PubMed] [Google Scholar]
- Dendinger S., Brill W. J. Regulation of proline degradation in Salmonella typhimurium. J Bacteriol. 1970 Jul;103(1):144–152. doi: 10.1128/jb.103.1.144-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch C. E., Soffer R. L. Regulation of proline catabolism by leucyl,phenylalanyl-tRNA-protein transferase. Proc Natl Acad Sci U S A. 1975 Jan;72(1):405–408. doi: 10.1073/pnas.72.1.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Dann L. Excretion of enterochelin by exbA and exbB mutants of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1225–1230. doi: 10.1128/jb.114.3.1225-1230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinishi H., Hashizume S., Seguchi M., Takahashi K. Incorporation of methionine by a soluble enzyme system from Escherichia coli. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1136–1143. doi: 10.1016/0006-291x(75)90792-5. [DOI] [PubMed] [Google Scholar]
- KAJI A., KAJI H., NOVELLI G. D. A soluble amino acid incorporating system. Biochem Biophys Res Commun. 1963 Mar 5;10:406–409. doi: 10.1016/0006-291x(63)90546-1. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. J., Soffer R. L. Enzymatic modification of proteins. VII. Substrate specificity of leucyl,phenylalanyl-transfer ribonucleic acid-protein transferase. J Biol Chem. 1971 Sep 10;246(17):5207–5212. [PubMed] [Google Scholar]
- London J., Goldberg M. E. The tryptophanase from Escherichia coli K-12. I. Purification, physical properties, and quaternary structure. J Biol Chem. 1972 Mar 10;247(5):1566–1570. [PubMed] [Google Scholar]
- NEUHAUS F. C. The enzymatic synthesis of D-alanyl-D-alanine. I. Purification and properties of D-alanyl-D-alanine synthetase. J Biol Chem. 1962 Mar;237:778–786. [PubMed] [Google Scholar]
- Scarpulla R. C., Deutch C. E., Soffer R. L. Transfer of methionyl residues by leucyl, phenylalanyl-tRNA-protein transferase. Biochem Biophys Res Commun. 1976 Jul 26;71(2):584–589. doi: 10.1016/0006-291x(76)90827-5. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Aminoacyl-tRNA transferases. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):91–139. doi: 10.1002/9780470122853.ch4. [DOI] [PubMed] [Google Scholar]
- Soffer R. L. Peptide acceptors in the leucine, phenylalanine transfer reaction. J Biol Chem. 1973 Dec 25;248(24):8424–8428. [PubMed] [Google Scholar]
- Soffer R. L., Savage M. A mutant of Escherichia coli defective in leucyl, phenylalanyl-tRNA-protein transferase. Proc Natl Acad Sci U S A. 1974 Mar;71(3):1004–1007. doi: 10.1073/pnas.71.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stulberg M. P. The isolation and properties of phenylalanyl ribonucleic acid synthetase from Escherichia coli B. J Biol Chem. 1967 Mar 10;242(5):1060–1064. [PubMed] [Google Scholar]


