Abstract
The synthetic amino acid copolymer copolymer 1 (Cop 1) suppresses experimental autoimmune encephalomyelitis (EAE) and is beneficial in multiple sclerosis. To further understand Cop 1 suppressive activity, we studied the cytokine secretion profile of various Cop 1-induced T cell lines and clones. Unlike T cell lines induced by myelin basic protein (MBP), which secreted either T cell helper type 1 (Th1) or both Th1 and Th2 cytokines, the T cell lines/clones induced by Cop 1 showed a progressively polarized development toward the Th2 pathway, until they completely lost the ability to secrete Th1 cytokines. Our findings indicate that the polarization of the Cop 1-induced lines did not result from the immunization vehicle or the in vitro growing conditions, but rather from the tendency of Cop 1 to preferentially induce a Th2 response. The response of all of the Cop 1 specific lines/clones, which were originated in the (SJL/J×BALB/c)F1 hybrids, was restricted to the BALB/c parental haplotype. Even though the Cop 1-induced T cells had not been exposed to the autoantigen MBP, they crossreacted with MBP by secretion of interleukin (IL)-4, IL-6, and IL-10. Administration of these T cells in vivo resulted in suppression of EAE induced by whole mouse spinal cord homogenate, in which several autoantigens may be involved. Secretion of anti-inflammatory cytokines by Cop 1-induced suppressor cells, in response to either Cop 1 or MBP, may explain the therapeutic effect of Cop 1 in EAE and in multiple sclerosis.
Keywords: immunoregulation, multiple sclerosis, cytokines
The existence of T lymphocyte subsets that produce distinct sets of cytokines and, as a result, perform distinct effector functions, has been well established in both mice and humans (1–3). Naive CD4+ lymphocytes (Th0) triggered by antigen differentiate either into T helper type 1 (Th1) or into T helper type 2 (Th2) cells that can crossregulate one another; their respective cytokines act antagonistically. Th1 cells produce interleukin (IL)-2, interferon (IFN)-γ, and tumor necrosis factor, activate cell-mediated immunity, and induce delayed-type hypersensitivity. Th2 cells produce IL-4, IL-5, IL-6, and IL-10 and down-regulate cell-mediated immunity. During the last few years many studies have implicated the preferential development and activation of Th1/Th2 subpopulations in a variety of autoimmune diseases (3–5). Experimental autoimmune encephalomyelitis (EAE) is an inflammatory autoimmune disease of the central nervous system (CNS) that serves as an animal model for multiple sclerosis (MS) (6). EAE can be induced in several animal species by immunization with myelin basic protein (MBP) (6), proteolipid protein (7), or myelin oligodendrocyte glycoprotein (8). During the progression of EAE, Th1, and not Th2, cytokines are present in the inflammatory lesions in the CNS (4, 9). The autoreactive T cells that induce the disease generally display a Th1 phenotype, and the adoptive transfer of these Th1 cells is sufficient to induce EAE (4–6, 10, 11). On the other hand, recovery from EAE is associated with an elevation of Th2 cells and cytokines in the CNS (4, 12). Regulatory T cells that suppress the development of EAE produce cytokines that correspond to the Th2 profile and mediate their activity by secretion of these suppressive cytokines (4, 13, 14). These findings, along with the observation that Th2 cytokines can inhibit the action of Th1 cytokines (3, 4, 11), suggest that the induction and activation of Th2 cells may potentially prevent EAE and other autoimmune diseases that are mediated by Th1 cells.
Copolymer 1 (Cop 1) is a synthetic random copolymer of amino acids composed of l-Ala, l-Glu, l-Lys, and l-Tyr (15, 16). Cop 1 exerts a marked suppressive and protective effect on EAE in various animal species, including primates, and on chronic relapsing EAE. Cop 1 also was shown to slow the progression of disability and to reduce the relapse rate in MS patients (17), and it recently was approved as a drug for MS under the trade name of Copaxone. The mechanism of Cop 1 activity in EAE and MS involves high-affinity promiscuous binding to various class II major histocompatibility complex (MHC) molecules (18). This efficient MHC binding results in both competition with myelin antigens for T cell activation and induction of specific regulatory T cells. We previously have demonstrated that the unresponsiveness to EAE induced by Cop 1 is regulated by T suppressor (Ts) cells, because it can be adoptively transferred to normal recipients and abrogated by pretreatment with cyclophosphamide (19). Cop 1-specific Ts cell lines and Ts hybridomas were established from spleens of mice that had been rendered unresponsive to EAE by Cop 1 (20). These Cop 1-induced suppressor cells, or their supernatants, inhibited the in vitro response of an encephalitogenic line to MBP when cocultured. Furthermore, they prevented the development of EAE induced by whole mouse spinal cord homogenate (MSCH) in vivo. To further understand the mechanism of Cop 1 activity, we studied the cytokine secretion profile of various Cop 1-induced T cell lines and clones in response to Cop 1, as well as to the autoantigen MBP. In the following we demonstrate that Cop 1 induces CD4+ T cell lines/clones of the Th2 subtype, which secrete anti-inflammatory Th2 cytokines in response to either Cop 1 or MBP, and suppress EAE induced by whole spinal cord homogenate.
MATERIALS AND METHODS
Mice.
SJL/J, BALB/c and (SJL/J×BALB/c)F1 mice were purchased from The Jackson Laboratory. Female mice, 7–12 weeks old, were used for all experiments.
Antigens and Antibodies.
Cop 1 is a synthetic random basic polymer, prepared by polymerization of the N-carboxyanhydrides of l-alanine, γ-benzyl-l-glutamate, ɛ,N-trifluoroacetyl l-lysine, and l-tyrosine (15) followed by removal of blocking groups. Two Cop 1 batches (batches 02095 and 55495) obtained from Teva Pharmaceutical Industries (Petach Tikva, Israel) were used through the study, with average molecular mass of 6,000 Da and 5,800 Da, respectively. MBP was isolated from spinal cords of mice or rats, as previously described (21). MSCH was prepared as previously described (19). Lysozyme from egg white was obtained from Sigma. Cell lines producing the mAbs, mouse anti-mouse I-Ad (MK-D6), were obtained from American Type Culture Collection. Monoclonal anti-mouse I-Ek antibodies crossreactive with I-Ed were purchased from Serotec.
T Cell Lines and Clones.
T cell lines were established either from spleens of mice that had been rendered unresponsive to EAE by subcutaneous injection of Cop 1 or MBP (5–10 mg/mouse), emulsified in incomplete Freund’s adjuvant (ICFA, Difco) 15 to 35 days earlier, or from lymph nodes (LN) of mice that had been immunized with Cop 1 or MBP (200 μg/mouse) emulsified in complete Freund’s adjuvant (CFA, Difco) supplemented with 4 mg/ml of Mycobacterium tuberculosis H37Ra (Difco), 10 days earlier. Cells were cultured and selected in vitro using the immunizing antigen (1–0.5 mg/plate), as described (20). Every 14–21 days, cells were stimulated by 3-day exposure to Cop 1 or MBP presented on syngeneic-irradiated (3,000 rad) spleen cells (50 × 106/plate), followed by propagation in 10% supernatant of Con A-activated normal mouse spleen cells as T cell growth factor. Control lines specific to lysozyme were similarly obtained by subcutaneous immunization with lysozyme (5 mg/mouse) emulsified in ICFA and stimulated by exposures to lysozyme. Cloning of T cell lines was performed by limiting dilution at 0.3 cells/well.
Proliferation Assay.
T-Cells (1.5 × 104) were cultured with irradiated spleen cells (5 × 105) and with the indicated antigens. At the end of 48-hr incubation, cultures were pulsed with 1μCi[3H]thymidine and harvested 6–12 hr later. Results are expressed as mean cpm thymidine incorporation for triplicate cultures. SDs were under 20% of the mean cpm.
Cytokine Assays.
Spleen cells (5 × 106/ml) or T cell from lines and clones (1 × 106/ml), were incubated with the indicated antigens, presented on irradiated spleen cells (5 × 106/ml), in a final volume of 1 ml. Supernatants were collected 24 hr later and assayed for cytokine levels using either indicator cells or mAbs in ELISA.
Cytokine assay by indicator cells.
The presence of IL-2 or IL-4 in culture supernatants was evaluated by their ability to support the proliferation of the IL-2-dependent CTLD line and the IL-4-dependent CT4-S line, respectively. The tested supernatants were incubated with the indicator cells (1 × 104/well) at a 1:1 dilution for 48 hr and then labeled with 1 μCi-thymidine. Results are expressed as mean cpm thymidine incorporation for triplicate cultures, and the SDs were less then 20%.
Cytokine assay by ELISA.
IL-2, IL-4, IL-6, IL-10, IFN-γ, and tumor necrosis factor-α were measured using a quantative sandwich ELISA using pairs of mAbs obtained from PharMingen, according to the manufacturer’s instructions. The threshold detection for all cytokines was 10–50 pg/ml. Results are expressed in ng as mean concentration of duplicate culture supernatants (SDs under 20%), measured in duplicate wells by ELISA (SDs under 10%).
Induction of EAE.
(SJL/J×BALB/c)F1 2–3-month-old female mice were injected in all four footpads with 3.5 mg/mouse spinal cord homogenate emulsified in a 1:1 ratio in CFA supplemented with 4 mg/ml H37Ra. Pertussis toxin (0.25 ml, 250 ng, Sigma) was injected intravenously, immediately after, and 48 hr later. Mice were examined daily for signs of EAE and assessed for clinical severity using a 1–5 score as described (20).
RESULTS
Cop 1-specific T cell lines were established from spleens of (SJL/J×BALB/c)F1 mice that had been rendered unresponsive to EAE by injection of Cop 1 in ICFA 15–35 days earlier or from lymph nodes of mice that had been immunized with Cop 1 in enriched CFA 10 days earlier. The cells were cultured and selected in vitro by repeated exposures to Cop 1. Twelve different Cop 1-specific T cell lines and clones were generated and characterized. The lines were tested for their CD4/CD8 phenotype, and in all cases 96% of the cells were found to bear the CD4 phenotype.
IL-2 and IL-4 Secretion by Cop 1-Specific Lines.
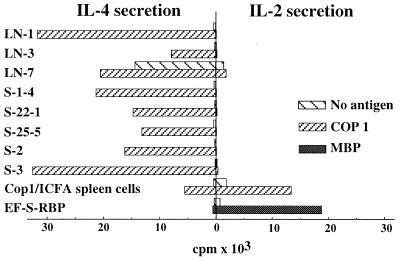
The IL-2 and IL-4 secretion of eight different Cop 1 Ts lines and clones first was measured using specific indicator cell lines. CTLD and CT4-S lines were used for measuring IL-2 and IL-4 secretion, respectively. T cell lines/clones were tested with various antigen concentrations, and the results obtained with the optimal antigen dose is presented. All of the Cop 1-specific lines and clones secreted IL-4, and not IL-2, when incubated with Cop 1 (Fig. 1). This restriction toward IL-4 secretion was observed in the Cop 1 lines and clones originating from spleens of mice that had been rendered unresponsive to EAE with Cop 1 in ICFA (S-1–4, S-22–1, S-22–5, S-2, and S-3), as well as in the Cop 1 lines originating from LN of mice that had been immunized with Cop 1 in CFA (LN-1, LN-3, and LN-7). The whole spleen cell population from the Cop 1/ICFA immunized mice (Cop1/ICFA spleen cells) secreted both IL-2 and IL-4 in response to Cop 1 (see Figs. 1 and 3A) However, after one or two cycles of stimulation with Cop 1, when Cop 1-specific lines have been established, only IL-4 could be detected. The clones originating from LN of mice immunized with Cop 1/CFA exhibited a mixed IL-2/IL-4 secretion for a longer period (IL-2 was measured for 10 stimulations), but eventually they lost their ability to secrete IL-2 as well. In contrast to the Cop 1 lines/clones, the EF-S-RBP effector line originating in mice that had been immunized with the encephalitogenic antigen rat MBP in CFA and selected using MBP, secreted IL-2 from the initial stimulation through the whole study period, when cocultured with the immunizing antigen. IL-4 could not be detected by the MBP-specific line in response to MBP (Fig. 1).
Figure 1.
IL-2 and IL-4 secretion by T cell lines/clones. Eight different Cop 1-specific T cell lines and clones, spleen cells of mice injected with Cop 1 in ICFA, and a rat MBP-specific line (EF-S-RBP) were cultured with Cop 1 (10 μg/culture) or rat MBP (20 μg/culture). The presence of IL-2 and IL-4 in the supernatants was determined by their ability to support IL-2-dependent CTLD and IL-4-dependent CT4S cell lines. Results are expressed as mean cpm of thymidine incorporation for triplicate cultures. SDs were under 20% of the mean cpm. Results are from one representative experiment of more than 10 performed.
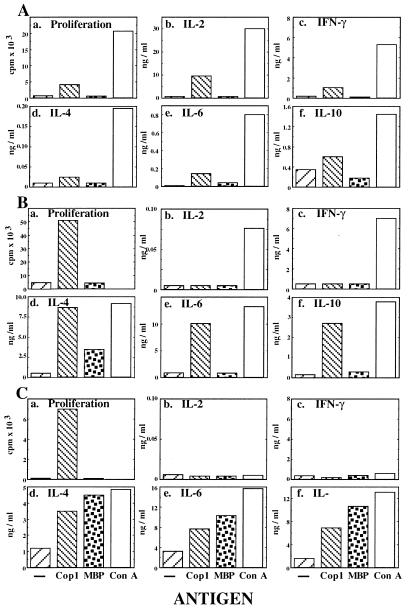
Figure 3.
Proliferation and cytokine secretion profile of S-2 line during its development. (A) Initial response of spleen cells. (B) After 6 weeks in culture. (C) After 6 months in culture. Cells were cultured with no antigen, Cop 1 (50 μg/ml), MBP (100 μg/ml), and ConA (5 μg/ml). Proliferation was measured by thymidine incorporation for triplicate cultures. Cytokine concentration was measured by quantitative ELISA in duplicate wells for each one of duplicate culture supernatants. SDs were under 20% of the mean. Results represent one of three independent experiments.
Comparison Between Cop 1- and MBP-Induced Suppressor Lines.
Two different lines originating in mice that had been rendered unresponsive to EAE by injection of either Cop 1 or mouse MBP emulsified in ICFA were selected in vitro using the homologous antigen. Each line exhibited a high proliferation response to its specific antigen. The cytokine profile of these two lines, after three in vitro stimulations, was tested by using mAbs in ELISA (Fig. 2). For each line the response to the optimal antigen concentration is presented. Whereas both lines responded to the nonspecific mitogen Con A with production of high amounts of all the cytokines tested, they differed in the secretion profile obtained when each line was exposed to its specific antigen. The MBP line reacted to its specific antigen MBP by secretion of Th1 cytokines (IL-2, IFN-γ and tumor necrosis factor-α), as well as Th2 cytokines (IL-4, IL-6, and IL-10). In contrast, the Cop 1 line secreted only Th2 cytokines, but not Th1 cytokines, in response to Cop 1, although it still had the potential of producing Th1 cytokines as indicated by the response to Con A. Thus, whereas the MBP line exhibited a mixed Th1/Th2 cytokine secretion, the Cop 1 line was confined to the Th2 pathway.
Figure 2.
Comparison between cytokine secretion by Cop 1- and MBP-induced Ts lines. Quantative ELISA of supernatants of two T cell lines established from spleens of mice, which had been rendered unresponsive to EAE by injection of Cop 1 or MBP in ICFA, stimulated by no antigen, Cop 1 (50 μg/ml), MBP (100 μg/ml), and ConA (5 μg/ml). Results are expressed as mean cytokine concentration of duplicate wells. SDs for both assays were under 20% of the mean.
Restriction of Cop 1 Specific F1-Ts Lines.
The F1-Ts-Cop 1 lines/clones were originated from (SJL/J×BALB/c)F1 mice. The restriction of S-2 line to the two parental haplotypes SJL/J (H-2s) and BALB/c (H-2d) is shown in Table 1. Whereas the response to the mitogen Con A was not restricted to one of the parental haplotypes, a restricted pattern was observed in response to Cop 1. Maximal proliferation as well as secretion of IL-4, IL-6, and IL-10 were obtained when Cop 1 was presented on BALB/c or F1 antigen-presenting cells (APC). On the other hand, the proliferation and Th2 secretion in response to Cop 1 on SJL/J APC were much lower, and IL-4 was not secreted when Cop 1 was presented by SJL/J. Neither IL-2 nor IFN-γ were measured in response to Cop 1 with all strains of APC. Similar results were obtained with the other Cop 1-specific lines/clones. Thus, the activity of F1-Ts-Cop1 lines/clones toward Cop 1, manifested both by proliferation and by Th2 cytokine secretion, was restricted to the BALB/c H-2d haplotype.
Table 1.
Restriction of Cop 1-specific S-2 line to the parental haplotypes
| Antigen | F1 | SJL/J | BALB/c | |
|---|---|---|---|---|
| Proliferation, ×10−3 | — | 0.21 | 0.26 | 0.16 |
| Cop 1 | 19.50 | 4.80 | 21.61 | |
| Con A | n.d. | n.d. | n.d. | |
| IL-2, ng/ml | — | <0.05 | <0.05 | <0.05 |
| Cop 1 | <0.05 | <0.05 | <0.05 | |
| Con A | 16.7 | 14.7 | 18.7 | |
| INF-γ, ng/ml | — | <0.05 | <0.05 | <0.05 |
| Cop 1 | <0.05 | <0.05 | <0.05 | |
| Con A | 9.8 | 9.2 | 9.1 | |
| IL-4, ng/ml | — | <0.3 | <0.3 | <0.3 |
| Cop 1 | 2.0 | <0.3 | 2.1 | |
| Con A | 10.8 | 10.3 | 11.7 | |
| IL-6, ng/ml | — | <0.1 | <0.1 | <0.1 |
| Cop 1 | 14.1 | 6.5 | 15.1 | |
| Con A | 36.6 | 36.8 | 35.5 | |
| IL-10, ng/ml | — | <0.2 | <0.2 | <0.2 |
| Cop 1 | 9.8 | 2.1 | 8.6 | |
| Con A | 12.5 | 10.9 | 11.4 |
The Cop 1-specific line S-2 was tested for its restriction to the parental haplotype, by comparing its proliferation and cytokine secretion in response to Cop 1 (50 μg/ml) or Con A (5 μg/ml), on irradiated spleen cells (5 × 106 cells/ml) from (SJL/J×BALB/c)F1, SJL/J, or BALB/c origin. SDs of all the indicated values were under 20%. Results are from one representative experiment of two performed. n.d., not determined.
A further analysis of the restriction of the Cop 1-specific lines/clones within the H-2d region was performed using anti-I-Ad and anti-I-Ed mAbs (Table 2). Whereas no inhibition was obtained by antibodies to the I-E subregion of the BALB/c haplotype, the response to Cop 1 manifested by both proliferation and IL-4 secretion was strongly inhibited in the presence of I-Ad antibodies. Similarly the other Cop 1-specific lines/clones were inhibited only by antibodies to the I-Ad. Thus a restriction of the response toward Cop 1 to the I-A subregion of the H-2d was demonstrated.
Table 2.
Restriction of Cop 1-specific S-2 line within the H-2d region
| Antigen | Inhibitor
|
|||
|---|---|---|---|---|
| — | Anti-I-Ad | Anti-I-Ed | ||
| Proliferation, cpm | — | 243 | 748 | 390 |
| Cop 1 | 7,405 | 796 | 8,573 | |
| IL-4, cpm | — | 28 | 43 | 64 |
| Cop 1 | 9,685 | 61 | 8,563 | |
The Cop 1-specific line S-2 was tested for its restriction within the H-2d region, by inhibition of the proliferation and IL-4 secretion in response to Cop 1 (10 μg/well) presented on BALB/c irradiated spleen cells (5 × 106/well), with mAbs specific either to the I-Ad or I-Ed region. SDs of all the indicated values were under 20%. Results are from one representative experiment of two performed.
Crossreactivity of Cop 1-Specific F1-Ts Lines/Clones with MBP.
The pattern of cytokine secretion by several F1-Ts-Cop 1 lines/clones in response to Cop 1 and to the autoantigen MBP was studied. A detailed proliferation and cytokine profile of S-2 line during its development is demonstrated in Fig. 3. Initially, when whole spleen cell population from mice that had been rendered unresponsive to EAE by Cop 1 was tested (Fig. 3A) a low response to Cop 1 was measured in comparison to the response obtained with the mitogen Con A. The spleen cells reacted to Cop 1 by proliferation and by secretion of both Th1 (IL-2 and IFN-γ) and Th2 (IL-4, IL-6, and IL-10) cytokines. On the other hand, neither proliferation nor cytokine secretion could be measured when the whole spleen cell population was incubated with MBP.
Six weeks later, after the cells had been exposed to Cop 1 for three cycles of stimulation, the response to Cop 1 was more confined (Fig. 3B). The S-2 line responded to Cop 1 by proliferation, but not by secretion of IL-2 and IFN-γ. These Th1 cytokines were secreted only in response to the mitogen Con A. On the other hand, higher amounts of IL-4, IL-6, and IL-10 were secreted by these cells in response to Cop 1, approaching the amounts induced by Con A. Moreover, S-2 cells secreted IL-4 not only in response to Cop 1 but also in response to MBP (7-fold more than the secretion with no antigen).
After 6 months of in vitro growth in which the S-2 line had been repeatedly exposed to Cop 1, IL-2 and INF-γ were not detected in response to Cop 1, nor after stimulation with Con A (Fig. 3C). Although the cells did not secrete Th1 cytokines, they secreted high amounts of Th2 cytokines in response to Cop 1 and to Con A. The crossreactive response to MBP, which had been observed after 2 months only on the level of IL-4 secretion, now was demonstrated on the level of all Th2 cytokines measured (IL-4, IL-6, and IL-10). Furthermore, this line secreted even higher amounts of Th2 cytokines in response to MBP than to Cop 1.
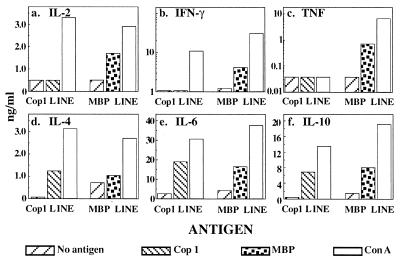
It should be noted that although all of the Cop 1-specific lines/clones secreted significant amounts of Th2 cytokines in response to Cop 1, they differed in the extent of crossreactivity with MBP. Other lines/clones obtained demonstrated a smaller degree of crossreactivity with MBP, usually on the level of IL-4 secretion. The crossreactivity with MBP was exhibited only by Cop 1-induced lines/clones. Thus control lysozyme specific lines, which secreted Th2 cytokines in response to lysozyme, did not respond at all to MBP or to Cop 1 (data not shown).
Suppressive Activity of Cop 1-Specific F1-Ts Lines in Vivo.
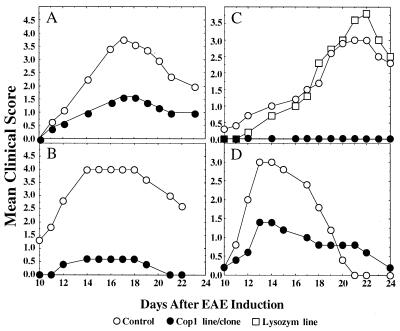
The ability of the Cop 1-specific lines/clones to prevent EAE induced by whole spinal cord homogenate in vivo was investigated. Activated F1-Ts-Cop1 cells were injected to (SJL/J×BALB/c)F1 mice followed by EAE induction. The S-2 line originating from spleens of mice that had been rendered unresponsive to EAE with Cop 1 in ICFA was tested both after 2 months (Fig. 4A) and after 6 months (Fig. 4B) of in vitro growth. This line was significantly effective in suppressing EAE, because the mean clinical score of mice injected with the line was always lower than that of the control group. However the S-2 cells grown in vitro for 6 months were more effective in disease prevention than those grown for 2 months. Thus, after 2 months 20 × 106 cells inhibited only 40% and 58% of disease incidence and severity respectively, P < 0.04, whereas 10 × 106 cells of the line grown for 6 months inhibited 80% incidence and 86% severity of the disease, P < 0.01.
Figure 4.
Inhibition of MSCH-induced EAE by T cell lines and clones. (A) Cop 1-specific line S-2 after 2 months in culture (20 × 106/mouse). (B) S-2 line after 6 months in culture (10 × 106/mouse). (C) Cop 1-specific clone S-22–1 and lysozyme-specific line Lys-1 after 6 months in culture (15 × 106/mouse). (D) Cop 1-specific clone LN-3 after 6 months in culture (20 × 106/mouse). Cells were injected intravenously 3 days after stimulation with Cop 1 to (SJL/J×BALB/c)F1 mice 5–10 in a group, followed by EAE induction by MSCH. Control mice were induced with EAE alone. Results are expressed as mean daily clinical score of 5–10 mice in a group. Statistical analysis by Student’s t test: (A) S-2 after 2 months in culture, P < 0.04. (B) S-2 after 6 months in culture, P < 0.01. (C) l-22–1, P < 0.002, Lys-1, P < 0.7. (D) LN-3 clone, P < 0.09.
The Cop 1-specific clone S-22–1, originating similarly to S-2, completely prevented the disease, and none of the animals showed any signs of EAE (P < 0.002). This is in contrast to the lysozyme-specific line, which did not show any inhibitory effect on the disease (P < 0.7), even though the same number of cells were injected (Fig. 4C).
The suppressive activity of another Cop 1-specific clone, LN-3, originating from LN of mice that had been immunized with Cop 1 in CFA and grown in vitro for 6 months, also was tested. As demonstrated (Fig. 4D), this clone also induced EAE suppression, but it was less effective than S-2 and S-22–1, because even after 6 months, 20 × 106 cells induced only 60% and 50% inhibition of the disease incidence and severity, respectively (P < 0.09).
DISCUSSION
The cumulative evidence on the protective role of Th2 cells in a variety of cellular immune responses, including autoimmune diseases, led to the design of immuno-intervention approaches aimed at inducing an antigen-specific shift from the Th1 pathogenic response to the protective Th2 response (4, 5, 13, 14, 22). In this study we demonstrated that Cop 1, which had been shown to suppress EAE as well as MS (16, 17), induces a predominant Th2 response. All 12 T cell lines and clones generated from Cop 1-immunized mice and selected for Cop 1 exhibited a Th2 cytokine profile and secreted high amounts of IL-4, IL-6, and IL-10 in response to Cop 1 (Figs. 1 and 3A, Tables 1 and 2). Secretion of tumor necrosis factor-α never was detected by Cop 1-induced cells (data not shown). IL-2 and IFN-γ were secreted when the whole spleen cell population was tested (Figs. 1 and 3). However, after the cells were repeatedly exposed to Cop 1 and T cell lines/clones were established, these Th1 cytokines could not be detected, whereas CD4+ Th2 cells prevailed.
The bias toward the Th2 phenotype was found in the Cop 1 lines/clones originating from spleens of mice that had been rendered unresponsive to EAE by injection of Cop 1 in ICFA 15–35 days earlier. This regimen has been previously shown to induce antigen-specific supressor cells (19, 20), and the Cop 1 lines/clones originating in this way showed strong disposition toward the Th2 pathway, because after 1–3 cycles of stimulation with Cop 1 they already were confined to the Th2 pathway (Figs. 2 and 3B). Furthermore, even clones originating from lymph nodes of mice that had been immunized with Cop 1 in enriched CFA 10 days earlier, a procedure usually used to obtain effector T cell lines (6), exhibited this tendency to Th2 diversion. In this case a mixed Th1/Th2 secretion was detected for a longer period but eventually, those clones, too, lost the ability to secrete IL-2, and a complete shift to IL-4 secretion was observed. T cell lines obtained from LN of mice that produced Th2 cytokines also were demonstrated in the case of mice that had been immunized with altered peptide ligand of proteolipid protein and CFA (23).
It should be noted that our T cell lines were not grown in the presence of either IL-4 or neutralizing antibodies to Th1 interleukins, which frequently are used to selectively promote Th2 polarized cells (3). Rather, T cell growth factor medium that contained the whole spectrum of cytokines secreted by normal spleen cells in response to Con A was used as a growth factor source. In this way the role of the specific antigen in determining the cytokine profile could be revealed. Indeed, the effector line EF-S-RBP, secreted IL-2, and not IL-4, when cocultured with its specific antigen MBP (Fig. 1). Furthermore, even when MBP was injected according to the suppression regime (in ICFA, 1 month earlier), the MBP-specific line obtained exhibited a mixed Th1/Th2 cytokine profile (Fig. 2). In contrast, the Cop 1-induced lines were confined to the Th2 pathway already after three stimulations. These findings indicate that the polarization of Cop 1-induced lines was not obtained due to the immunization vehicle or the in vitro culture conditions, but rather due to the uniqueness of Cop 1, which preferentially induces Th2 protective response. This trait could stem from the polymeric nature of Cop 1, which contains multiple major histocompatibility complex class II binding epitopes, and does not require processing for presentation (18). This results in high density of the antigen on the antigen-presenting cells, thus favoring Th2 development (3).
Of interest is the genetic restriction of the Cop 1-specific lines that were originated from (SJL/J×BALB/c)F1 mice to the BALB/c (H-2d) parental haplotype (Table 1), and within the H-2d region, to the I-A and not the I-E subregion (Table 2). This restriction was manifested by proliferation and by Th2 cytokine secretion. We demonstrated before that the unresponsiveness to EAE of the BALB/c strain results from the high level of regulatory cells because it could be abrogated by pretreatment with cyclophosphamide (19). The restriction of the Cop 1 Th2 lines to the BALB/c haplotype is also consistent with the dominant BALB/c genetic predisposition toward Th2 differentiation (24), which was reported in the literature for Leishmania major (4, 25) and autoimmune diabetes (4, 26).
The crossreactivity of Cop 1 with the natural autoantigen MBP, on the level of B as well as T cell response, previously was demonstrated (16, 27, 28). This crossreactivity was shown to correlate with Cop 1 suppressive activity in vivo (29). It was therefore interesting to find that the crossreactivity between Cop 1 and MBP also is expressed on the level of cytokine secretion (Fig. 3). This crossreactivity, manifested only on the level of the Th2 cytokine secretion, became more evident with more stimulations with Cop 1, probably due to selection of crossreactive clone from the cell line population. Thus, initially it was confined to the signature Th2 cytokine, IL-4 (Fig. 3B), but with additional stimulations with Cop 1, the crossreactivity with MBP became much more pronounced and expanded to the three Th2 cytokines tested (Fig. 3C). The S-2 line secreted even higher amounts of Th2 cytokines in response to MBP to which it had never been exposed before, than to Cop 1, which had been used for immunization and stimulation. Interestingly, whereas the response of encephalitogenic lines to MBP is Th1- and H-2s-restricted (4, 6), the crossreactive response to MBP of our Cop 1-specific lines is Th2- and H-2d-restricted. The way by which Cop 1 induces this crossreactive Th2-confined response has not been revealed in this study. Whether the Cop 1-specific cells recognize a common “suppressive” determinant shared by Cop 1 and MBP, but different from the encephalogenic regions of MBP that induce Th1 inflammatory processes, or bear a mutated T cell receptor that transduces different signals as a consequence of antigen binding, is not clear at present.
The most meaningful criterion for the biological relevance of the Cop 1-specific Th2 lines is their ability to suppress the disease in vivo. Indeed a significant suppression was demonstrated by these cells on the development of EAE, as reflected both in the incidence and in the clinical score of the disease (Fig. 4). The relevance of Cop 1 Th2-specific lines to the in vivo effect induced by Cop 1 is not obvious, because whole spleen population demonstrated a mixed Th1/Th2 response (Figs. 1 and 3A). However it should be noted that adoptive transfer of protection was demonstrated using whole spleen cell population from Cop 1-treated mice as well (19). Because activated T cells cross the blood brain barrier irrespective of their antigenic specificity (30), it is possible that Cop 1-specific cells induced or stimulated by the injection of Cop 1 in the periphery, penetrate through the blood brain barrier to the CNS. The Cop 1-induced cells, which crossreact with MBP on the level of Th2 cytokines, are further stimulated by exposure to MBP, which is abundantly present in the CNS, and can be presented by Ia-inducible glia cells (30). Thus the secretion of the anti-inflammatory cytokines is carried on or even amplified in the case of the crossreactive response to MBP (Fig. 3C). Interestingly, even those Cop 1 lines that showed only small or moderate crossreactivity with MBP induced an in vivo protective effect on the disease (Fig. 4 A andD). This effect can be attributed to the dominant Th2-inducing effect of IL-4. It was found that small amounts of IL-4 produced by T cells and accumulated at the site of the T cells can reach a threshold necessary to induce the development of polarized Th2 response (3).
This proposed mechanism supports our results demonstrating specificity, namely that lysozyme-specific Th2 lines did not induce any suppressive effect on EAE (ref. 21 and Fig. 4C). Hence, because they did not crossreact with MBP, they could not be stimulated to secret these Th2 cytokines in situ. In contrast, the ability of the Cop 1-specific cells to respond to one of the naturally occurring myelin antigens, MBP by Th2 secretion, enable them to suppress inflammatory processes induced by MBP and the other encephalitogenic antigens, which are included in whole MSCH that was used for EAE induction (7, 8). The effect of Cop 1-specific Th2 lines to elicit bystander suppression on EAE induced by individual antigens that are included in the whole MSCH, but different than MBP (e.g., proteolipid protein), is now under study.
Cop 1 (Copaxone) is one of the few drugs authorized to treat MS. The relevance of Cop 1-induced Th2 cells in MS patients treated with Cop 1 has yet to be established. Increasing knowledge indicates the importance of cytokines in the pathogenesis of MS (31). Furthermore, recent studies demonstrated that the pattern of cytokines secreted correlates with disease status (32). It therefore is likely that Cop 1-specific cells of the Th2 type similar to those demonstrated in the mouse system are involved in the induction of the therapeutic effect of Cop 1 in MS.
Acknowledgments
We thank Ada Veksler and Carmit Bar-Natan for their skillful technical assistance. We are grateful to Dr. Rivka Rivan Kreitman for helpful discussions and continued support. This work was supported by a grant from Teva Pharmaceutical Industries, Ltd, (Israel) to D.T., R.A., and M.S.
ABBREVIATIONS
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- MSCH
mouse spinal cord homogenate
- MBP
myelin basic protein
- Cop 1
copolymer 1
- Ts
T suppressor
- Th1 and Th2
T helper types 1 and 2
- IL
interleukin
- IFN-γ
interferon-γ
- LN
lymph nodes
- ICFA
incomplete Freund’s adjuvant
- CFA
complete Freund’s adjuvant
- CNS
central nervous system
References
- 1.Mosmann T R, Cherwinski H, Bond M W, Gieldin M A, Coffman R L. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Mosmann T R, Coffman R L. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.Abbas A K, Murphy K M, Sher A. Nature (London) 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 4.Liblau R S, Singer S M, McDevitt H O. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson L B, Kuchroo V K. Curr Opin Immunol. 1996;8:837–842. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 6.Zamvil S S, Steinman L. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 7.Lees M B, Kuchroo V K, Sobel R A. Int Pediatr. 1991;6:84–90. [Google Scholar]
- 8.Mendel I, Kerlero de Rosbo N, Ben-Nun A. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 9.Merrill J E, Kono D H, Clayton J, Ando D G, Hinton D R, Hofman F M. Proc Natl Acad Sci USA. 1992;89:574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller S D, Karpus W J. Immunol Today. 1994;15:356–361. doi: 10.1016/0167-5699(94)90173-2. [DOI] [PubMed] [Google Scholar]
- 11.Van der Veen R C, Stohlman S A. J Neuroimmunol. 1993;48:213–220. doi: 10.1016/0165-5728(93)90194-4. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy M K, Torrance D S, Picha K S, Mohler K M. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 13.Kuchroo V K, Prabha D M, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Kuchroo V K, Inobe J, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 15.Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Eur J Immunol. 1971;1:242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- 16.Arnon R, Sela M, Teitelbaum D. J Neurol. 1996;243:S8–S13. doi: 10.1007/BF00873696. [DOI] [PubMed] [Google Scholar]
- 17.Johnson K P, Brooks B R, Cohen J A, Ford C C, Goldstein J, Lisak R P, Myers L W, Panitch H S, Rose J W, Sciffer R B, Vollmer T, Weiner L P, Wolinski J S The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 18.Fridkis-Hareli M, Teitelbaum D, Gurevich E, Pecht I, Brautbar C, Kwon O J, Brenner T, Arnon R, Sela M. Proc Natl Acad Sci USA. 1994;91:4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lando Z, Teitelbaum D, Arnon R. J Immunol. 1979;132:2156–2160. [PubMed] [Google Scholar]
- 20.Aharoni R, Teitelbaum D, Arnon R. Eur J Immunol. 1993;23:17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- 21.Hirschfeld H, Teitelbaum D, Arnon R, Sela M. FEBS Lett. 1970;7:317–320. doi: 10.1016/0014-5793(70)80193-4. [DOI] [PubMed] [Google Scholar]
- 22.Brennan F M, Feldmann M. Curr Opin Immunol. 1996;8:872–877. doi: 10.1016/s0952-7915(96)80018-5. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson L B, Greer J M, Sobel R A, Lees M B, Kuchroo V K. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 24.Gorham J D, Guler M L, Steen R G, Mackey A J, Daly M J, Frederick K, Dietrich W F, Murphy K. Proc Natl Acad Sci USA. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sher A, Coffman R L. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 26.Scott B, Liblau R, Degermann S, Marconi L A, Ogata L, Caton A J, McDevitt H O, Lo D. Immunity. 1994;1:73–82. doi: 10.1016/1074-7613(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 27.Webb C, Teitelbaum D, Arnon R, Sela M. Eur J Immunol. 1973;3:279–286. doi: 10.1002/eji.1830030506. [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum D, Aharoni R, Sela M, Arnon R. Proc Natl Acad Sci USA. 1991;88:9528–9532. doi: 10.1073/pnas.88.21.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webb C, Teitelbaum D, Herz A, Arnon R, Sela M. Immunochemistry. 1976;13:333–337. doi: 10.1016/0019-2791(76)90344-x. [DOI] [PubMed] [Google Scholar]
- 30.Wekerle H, Linington C, Lassmann H, Meyermann R. Trends Neurosci. 1986;9:271–277. [Google Scholar]
- 31.Olsson T. Eur J Neurosci. 1994;1:7–12. [Google Scholar]
- 32.Correale J, Gilmore W, McMillan M, Li S, McCarthy K, Le T, Weiner L P. J Immunol. 1995;154:2959–2968. [PubMed] [Google Scholar]