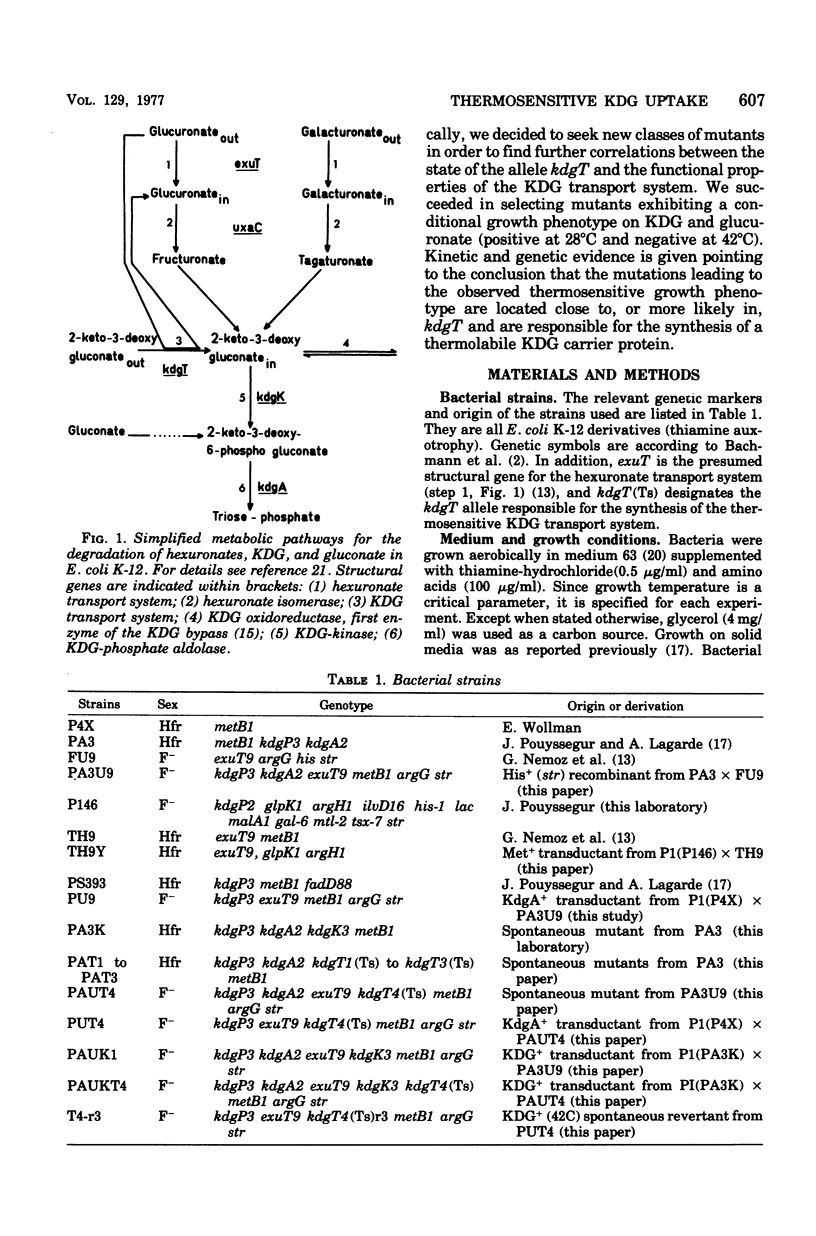
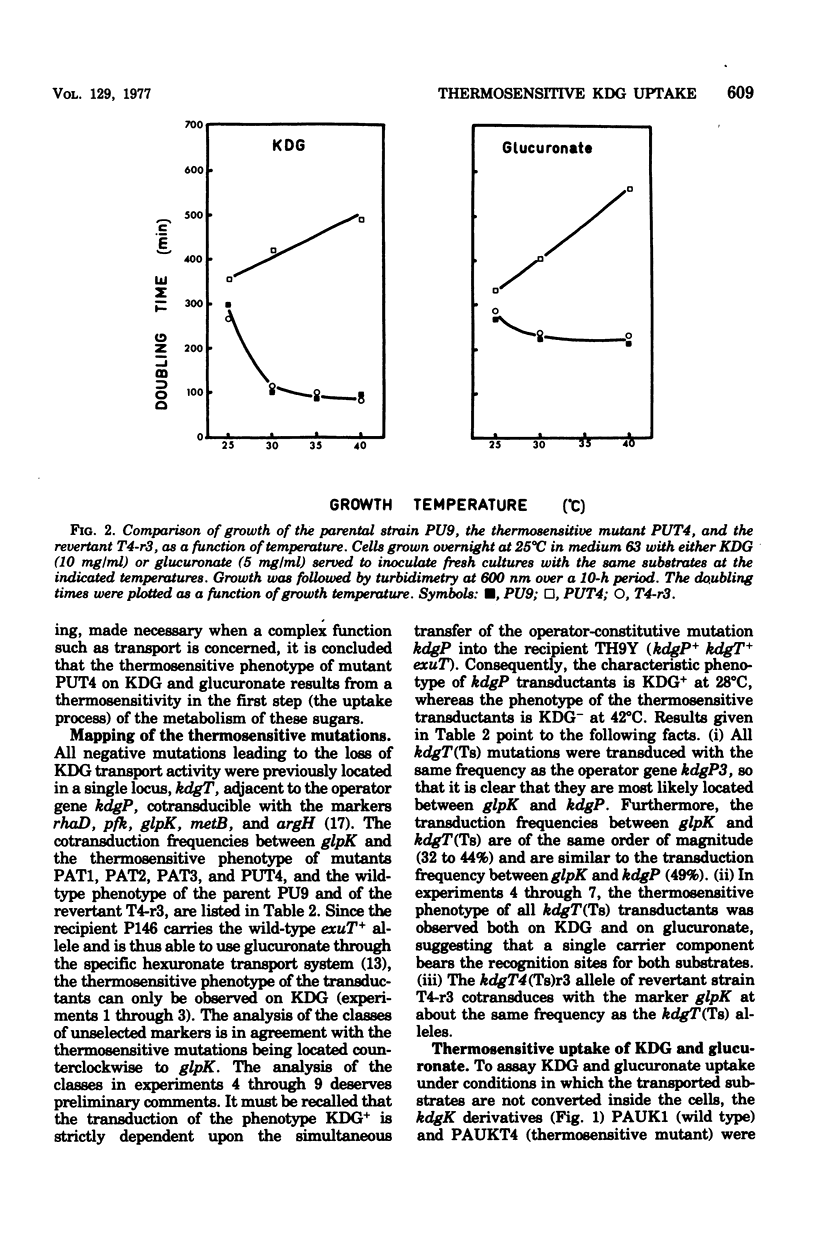
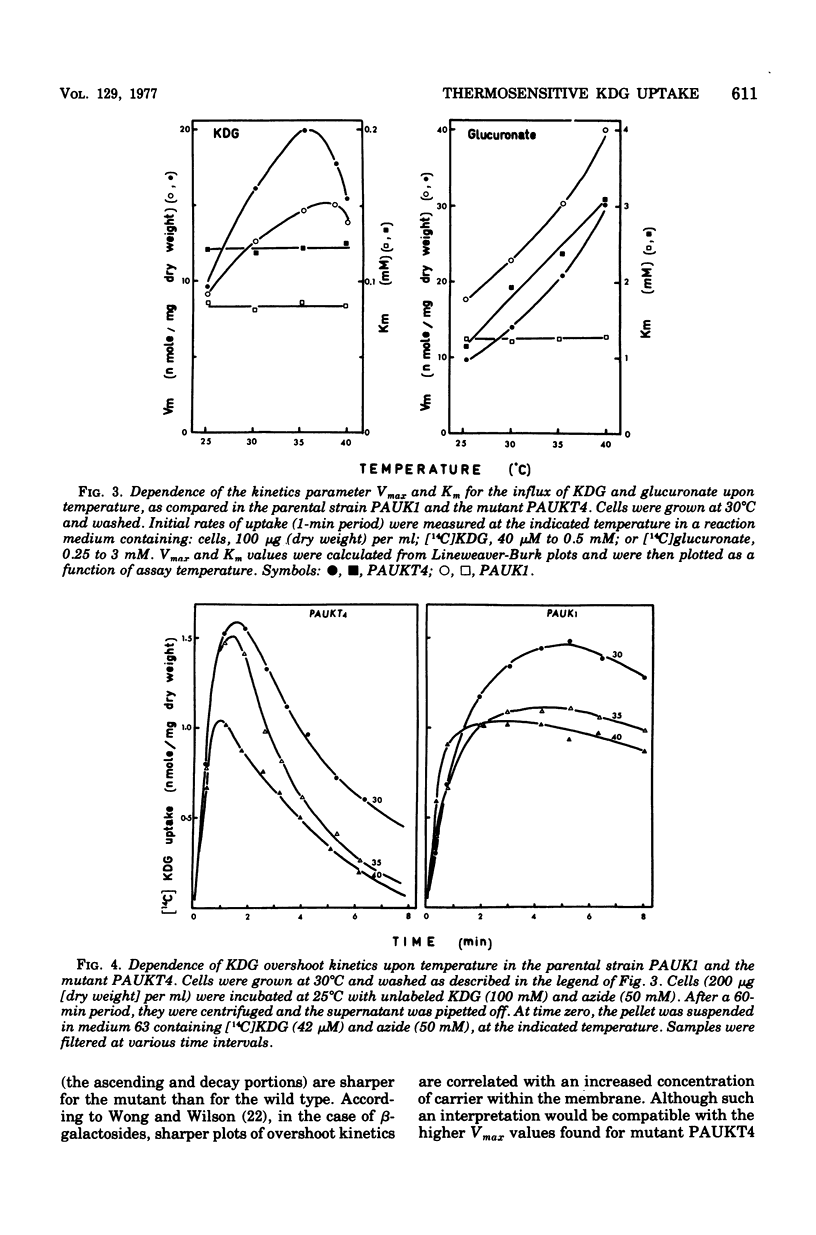
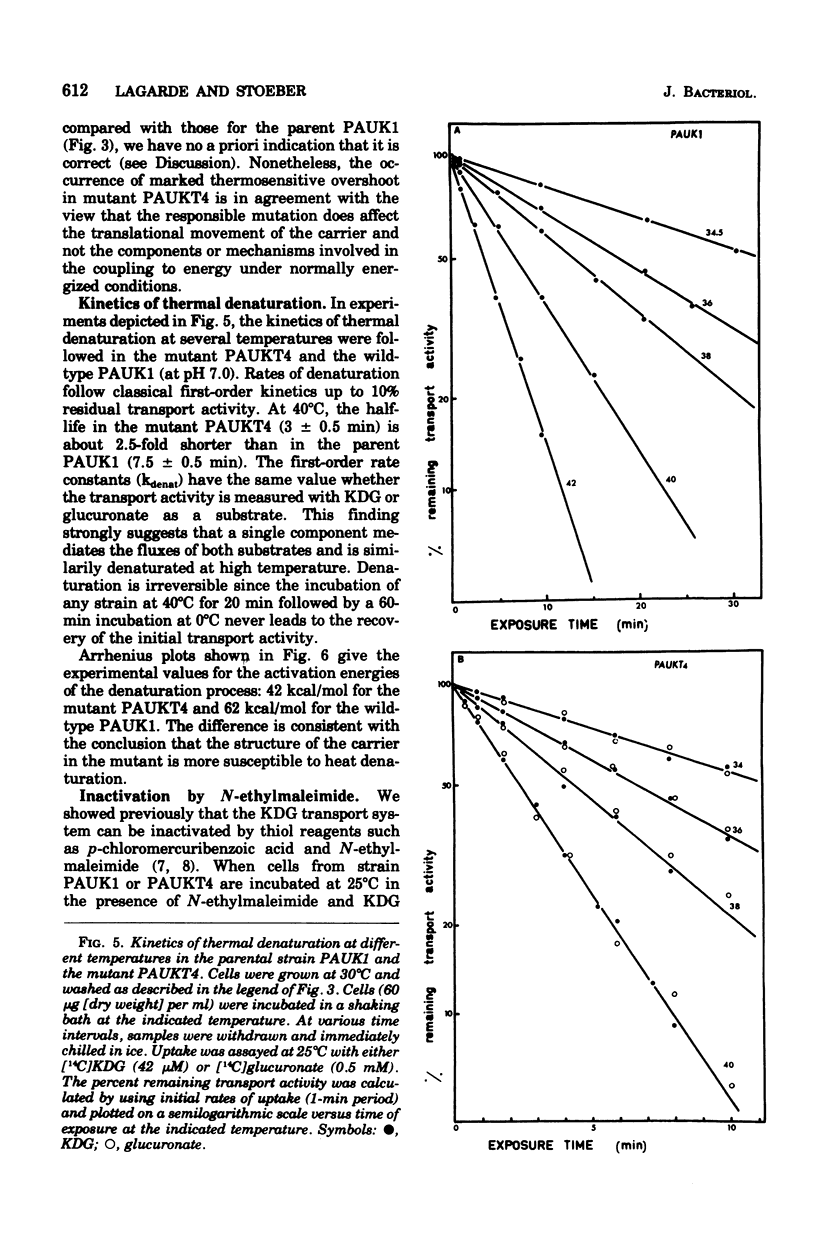
Abstract
A specific method is described for selecting thermosensitive mutants of Escherichia coli K-12 able to grow on 2-keto-3-deoxy-D-gluconate (KDG) and D-glucuronate at 2, but not at 42 degrees C. The extensive analysis of one such mutant is consistent with the conclusion that the carrier molecule responsible for KDG and glucuronate uptake becomes thermolabile. (i) Growth on a variety of carbon sources is perfectly normal at 28 and 42 degrees C, whereas in the same temperature range it gradually diminishes on KDG and glucuronate. (ii) The apparent Km value for KDG is about twofold in the range 25 to 40 degrees C. In the same temperature range, the Vmax values for KDG influx are higher for the mutant compared with those of the wild-type strain, but the optimum temperature is 34 degrees C instead of 38 degrees C. On the contrary, the Vmax values for glucuronate influx are lower for the mutant than for the parental strain, and the optimum temperature for both strains is shifted beyond 40 degrees C. (iii) The activation energies for KDG and glucuronate uptake are about twofold higher in the mutant than in the wild-type strain. (iv) Kinetics of counterflow under deenergized conditions (overshoot) at different temperatures indicate that the defect is located in the translocation step rather than in the processes involved in energy coupling. (v) The first-order rate constants for thermal denaturation are, respectively, 2.5- and 5-fold higher at 40 and 30 degrees C in the mutant than in the wild-type strain, and the activation energy for thermal denaturation is lower. (vi) The carrier molecule in the mutant is also much more sensitive to denaturation by N-ethylmaleimide. (vii) Four independent thermosensitive mutations and one revertatn were located by transduction in or near the kdgT locus, defined previously as the site of nonconditional KDG transport-negative mutations. These results support the conclusion that kdgT represents the structural gene coding for the KDG transport system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. E. The histidine-binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem. 1972 Jul 10;247(13):4309–4316. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall M., Koch A. L. Temperature-sensitive mutants of Escherichia coli affecting beta-galactoside transport. J Bacteriol. 1971 Feb;105(2):609–619. doi: 10.1128/jb.105.2.609-619.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Carter J. R., Kennedy E. P. GENETIC CONTROL OF THE MEMBRANE PROTEIN COMPONENT OF THE LACTOSE TRANSPORT SYSTEM OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):698–705. doi: 10.1073/pnas.57.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde A. E., Pouysségur J. M., Stoeber F. R. A transport system for 2-keto-3-deoxy-D-gluconate uptake in Escherichia coli K12. Biochemical and physiological studies in whole cells. Eur J Biochem. 1973 Jul 16;36(2):328–341. doi: 10.1111/j.1432-1033.1973.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. The energy-coupling controlled efflux of 2-keto-3-deoxy-D-gluconate in Escherichia coli K 12. Eur J Biochem. 1975 Jul 1;55(2):343–354. doi: 10.1111/j.1432-1033.1975.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. Transport of 2-keto-3-deoxy-D-gluconate in isolated membrane vesicles of Escherichia coli K12. Eur J Biochem. 1974 Mar 15;43(1):197–208. doi: 10.1111/j.1432-1033.1974.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Lieberman M. A., Hong J. S. A mutant of Escherichia coli defective in the coupling of metabolic energy to active transport. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4395–4399. doi: 10.1073/pnas.71.11.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden C. D., Fox C. F. A comparison of characteristic temperatures for transport in two unsaturated fatty acid auxotrophs of Escherichia coli. J Supramol Struct. 1973;1(6):535–544. doi: 10.1002/jss.400010608. [DOI] [PubMed] [Google Scholar]
- Nemoz G., Robert-Baudouy J., Stoeber F. Physiological and genetic regulation of the aldohexuronate transport system in Escherichia coli. J Bacteriol. 1976 Aug;127(2):706–718. doi: 10.1128/jb.127.2.706-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Hill F. F., Lamnek-Hirsch I. Biogenesis of E. coli membrane: evidence for randomization of lipid phase. Nat New Biol. 1971 Dec 29;234(52):264–267. doi: 10.1038/newbio234264a0. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J. M. Localisation génétique de mutations 2-céto-3-désoxy-6-P-gluconate aldolase négatives chez E. coli K 12. Mol Gen Genet. 1971;113(1):31–42. [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol. 1974 Feb;117(2):641–651. doi: 10.1128/jb.117.2.641-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Synthèse enzymatique du 2-céto-3-désoxy-D-gluconate. Bull Soc Chim Biol (Paris) 1970;52(12):1419–1428. [PubMed] [Google Scholar]
- Pouysségur J., Lagarde A. Système de transport du 2-céto-3-désoxy-gluconate chez E. coli K 12: localisation d'un gène de structure et de son opérateur. Mol Gen Genet. 1973 Mar 1;121(2):163–180. doi: 10.1007/BF00277530. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Stoeber F., Lagarde A., Nemoz G., Novel G., Novel M., Portalier R., Pouyssegur J., Robert-Baudouy J. Le métabolisme des hexuronides et des hexuronates chez Escherichia coli K 12: aspects physiologiques et et génétiques de sa régulation. Biochimie. 1974;56(2):199–213. doi: 10.1016/s0300-9084(74)80379-2. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Wilson T. H. Counterflow of galactosides in Escherichia coli. Biochim Biophys Acta. 1970;196(2):336–350. doi: 10.1016/0005-2736(70)90021-0. [DOI] [PubMed] [Google Scholar]



