Abstract
The function of the immune system is highly dependent on cellular differentiation and clonal expansion of antigen-specific lymphocytes. However, little is known about mechanisms that may have evolved to protect replicative potential in actively dividing lymphocytes during immune differentiation and response. Here we report an analysis of telomere length and telomerase expression, factors implicated in the regulation of cellular replicative lifespan, in human B cell subsets. In contrast to previous observations, in which telomere shortening and concomitant loss of replicative potential occur in the process of somatic cell differentiation and cell division, it was found that germinal center (GC) B cells, a compartment characterized by extensive clonal expansion and selection, had significantly longer telomeric restriction fragments than those of precursor naive B cells. Furthermore, it was found that telomerase, a telomere-synthesizing enzyme, is expressed at high levels in GC B cells (at least 128-fold higher than those of naive and memory B cells), correlating with the long telomeres in this subset of B cells. Finally, both naive and memory B cells were capable of up-regulating telomerase activity in vitro in response to activation signals through the B cell antigen receptor in the presence of CD40 engagement and/or interleukin 4. These observations suggest that a novel process of telomere lengthening, possibly mediated by telomerase, functions in actively dividing GC B lymphocytes and may play a critical role in humoral immune response by maintaining the replicative potential of GC and descendant memory B cells.
A hallmark of the immune system is its ability to respond effectively to the constantly changing antigenic and pathogenic challenges in its environment. In the case of antibody-mediated humoral responses, this ability is achieved by the existence of a diverse repertoire of naive B cells and by an adaptive process that allows extensive receptor diversification by somatic hypermutation, clonal expansion, and selection to generate optimized binding affinity of antibody to antigen (1). B lymphocytes derive from bone marrow progenitor cells and undergo an ordered sequence of differentiation with phenotypically distinct stages during lineage development and during activation of an immune response (2). During T cell-dependent immune responses, mature antigen-naive B lymphocytes differentiate in a unique germinal center (GC) environment into GC B cells, then to memory B cells or plasma cells (3). In the GC, substantial cell division occurs during a differentiation process that comprises several important processes, including somatic hypermutation of variable domains of immunoglobulin (Ig) genes (4–6), clonal selection of mutated antibody-producing B cells with high antigen-binding affinity (7), and Ig isotype switching (8). In recent years, substantial progress has been made in understanding the process of Ig variable gene rearrangement, the structure and function of GCs, and the phenotypic changes that occur in the transition of immature to mature B cells, and of naive B cells to GC to memory or plasma cells. However, less is known about the mechanisms underlying the replicative lifespan of lymphocytes and immunologic memory.
Telomerase and the regulation of telomere length have recently drawn considerable attention for their potential roles in critical biological functions, including the control of cellular replication (9). Telomeres are specialized terminal chromosomal structures that consist of tandem hexanucleotide repeats (TTAGGG)n in vertebrates (10, 11) and telomere binding proteins (12, 13). The primary function of telomeres appear to be in maintaining the integrity of chromosomes and completing chromosomal replication. Telomeres have also been implicated in determining cellular replicative capacity. Due to the inability of DNA polymerase to replicate the ends of eukaryotic chromosomes completely (14, 15), each cell division results in a loss of 50–200 bp of telomere repeats in normal human somatic cells (16–21). If a minimal length of telomeres is essential for chromosomal integrity and replication, telomere length could limit the replicative lifespan of cells. Telomerase is a ribonucleoprotein enzyme that is capable of synthesizing telomeric repeats (22, 23). It has been proposed that telomerase is expressed in germ-line and malignant cells but not in most normal human somatic cells (24–26), and this selective expression of telomerase has been hypothesized as a basis for the immortality of the germ line and of malignant cells (27, 28). However, recent studies indicate that telomerase activity is also detected in normal human somatic cells that have self-renewal capability, such as hematopoietic stem cells (29–31), lymphocytes (29, 32–34), and skin epithelial cells (35–37). Moreover, the expression of telomerase in T lymphocytes is highly regulated during development and activation (33, 38, 39).
We report here the analysis of telomere length and telomerase expression in tonsil B cell subsets. Telomere length and telomerase activity were found to be closely regulated in the course of B cell differentiation. In contrast to previously characterized models of telomere shortening during somatic cell division, telomeres are significantly longer in GC B cells than in either precursor naive or descendant memory B cells. Telomerase activity was markedly increased in GC B cells relative to these other B cell populations, correlating with increased telomere length. Furthermore, high levels of telomerase activity are induced in naive and memory B cells by in vitro activation. These results suggest that telomerase may play a critical role in immune responses by elongating telomeres and thus preserving the replicative lifespan of GC and progeny memory B cells.
MATERIALS AND METHODS
Antibodies.
Monoclonal antibodies used for immunomagnetic cell separations: anti-CD3 (OKT-3), CD4 (0516), CD8 (B9.8), CD14 (63D3), CD16 (3G8), CD44 (NIH44–1), platelet (37F9), and erythrocyte (10F7) antibodies were gifts from Steve Shaw (National Institutes of Health). Purified anti-IgD, IgG, and IgA antibodies, fluorescein isothiocyanate (FITC)-conjugated anti-CD20, and phycoerythrin (PE)-conjugated anti-IgG were purchased from PharMingen. Purified anti-CD38 and CD40, FITC-conjugated anti-CD23, CD38, and IgD; PE-conjugated anti-CD38, CD40, CD44, and IgD; and Tri-color (TC)-conjugated anti-CD19, CD38, and CD2 antibodies were purchased from Caltag (South San Francisco, CA). Purified anti-CD77 was purchased from Immunotech (Westbrook, ME). FITC-conjugated mouse anti-rat IgM was purchased from Jackson ImmunoResearch.
Isolation of B Cell Subsets from Tonsils.
Human tonsils were obtained from tonsillectomies for chronic tonsillitis on children and adults aged 3–31 years and mononuclear cells were isolated by Ficoll gradient centrifugation (Organon Teknika–Cappel). B cells were isolated by binding to anti-CD19 beads (Dynal, Great Neck, NY) and subsets of B cells were further isolated by negative immunomagnetic cell separation as described (40). Monoclonal antibodies against CD38, IgG, and IgA were used for isolation of naive B cells (CD19+IgD+IgG−IgA−CD38−); antibodies against CD44 and IgD, for isolation of GC B cells (CD19+IgD−CD38+CD44−), and antibodies against CD38 and IgD for isolation of memory B cells (CD19+IgD−CD38−). For telomerase activity analysis, subsets of B cells were isolated by fluorescent cell sorting (FACStar; Becton Dickinson). Bm1 (CD19+IgD+CD38−CD23−) and Bm2 (CD19+IgD+CD38−CD23+) subsets were isolated from enriched naive B cells based on CD23 expression; Bm2′ (CD19+IgD+CD38+) and Bm5 (CD19+IgD−CD38−) were directly sorted from CD19+ B cells; Bm3 (centroblast, CD19+IgD−CD38+CD77+) and Bm4 (centrocyte, CD19+IgD−CD38+CD77−) were isolated from enriched GC B cells based on CD77 expression. Plasma cells (CD3−CD14−CD38hiCD20lo) were isolated directly either from mononuclear cells after depleting T cells (CD3+, CD4+ and CD8+) and monocytes (CD14+) by immunomagnetic beads, or from CD19+ B cells (41). The purity of isolated B cells was analyzed by flow cytometry as described previously (33), and it was generally 90–95% for cells isolated from cell sorting, and 80–90% for cells isolated by immunomagnetic cell separation.
Measurement of Telomere Length.
Mean telomeric restriction fragment (TRF) length was analyzed by hybridization in dried gels with a telomeric repeat oligonucleotide probe as previously described (42). ImageQuant software was used to measure mean TRF length with compensation for the telomere length effect on the intensity of the signals by using DNA standards as previously described (17). The statistical analysis of TRF lengths difference was done by t test using software (Statistica, StatSoft, Tulsa, OK) for analysis of difference between dependent samples.
PCR-Based Telomerase Assay.
The telomerase assay used here was modified from the telomeric repeats amplification protocol (TRAP) (24) described previously (33). Cell extracts were prepared from 2 × 105 to 2 × 106 FACS-sorted cells of each of seven B cell subsets at a concentration of 100 μl of 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS; Calbiochem) lysis buffer per 106 cells. Telomere synthesis was carried out starting with 5 × 104 cell equivalents, and PCR amplification was carried out starting with 1 × 104 cell equivalents in a separate tube. The amplified products from 3.3 × 103 cell equivalents were loaded as the original concentration on a 12% acrylamide gel (NOVEX, San Diego), and the results were analyzed on a PhosphorImager (Molecular Dynamics). Telomerase activity was quantitated by serial dilution of cell extracts, and an internal competitive control employed to monitor the reproducibility of the assay and to serve as a reference for comparing results between different experiments (43). Controls for the TRAP assay included cell lysates from 293 cells as a positive control, DNase-free RNase (Boehringer Mannheim) treatment of cell extracts, single primer, Ts or Cx alone, (Genosys, Woodlands, TX), and lysate buffer as template for PCR.
Activation of Naive and Memory Tonsil B Cells in Vitro.
Freshly isolated naive and memory tonsil B cells were stimulated as follows: Cells were suspended at 2–5 × 106 cells per ml in RPMI 1640 (BioWhittaker) with 10% fetal bovine serum (Biofluids, Rockville, MD) in the presence of one of the following: 0.2 μg/ml (1:10,000 dilution) of Staphylococcus aureus Cowan strain (SAC; Pansorbin cell, Calbiochem), or 12 μg/ml purified goat F(ab′)2 fragment against human IgM Fc5μ (Jackson ImmunoResearch) (44), or 0.5 μg/ml anti-CD40 (45), or 10 units/ml recombinant interleukin 4 (rIL-4; Genzyme), or combinations of anti-IgM and rIL-4, or anti-CD40. Cells were collected at day 2 after stimulation for the analysis of telomerase activity and hTR expression. Cell proliferation was assayed by [3H]thymidine incorporation as previously described (46). In brief, 1–3 × 105 naive and memory B cells were cultured in triplicate samples in flat-bottomed 96-well microtiter plates in RPMI 1640 medium containing 10% fetal bovine serum. Cells were incubated for 24 hr, 1 μCi (1 μCi = 37 kBq) of [3H]thymidine was added, and incubation was continued for another 24 hr before harvest. [3H]Thymidine (New England Nuclear) incorporation was measured by liquid scintillation counting.
Northern Blot Analysis of Telomerase RNA Template (hTR) Expression.
Total RNA was isolated and probed with hTR and 7SK as described previously (47). In brief, 10 μg of total RNA was used for each sample. The membranes were hybridized first with hTR probe in Quickhyb solution (Stratagene) and then with 7SK after removal of hTR probe. The washed membranes were then exposed to PhosphorImager screens and the results were analyzed using ImageQuant (Molecular Dynamics).
RESULTS
Telomere Length in Naive, GC, and Memory B Cells.
The process of somatic hypermutation and subsequent clonal selection in GC occurs in the context of substantial cell division and clonal expansion (5, 7, 48–50). In the absence of compensating mechanisms, clonal expansion would be expected to result in significant loss of telomere repeats; based on the telomere hypothesis, such reduction of telomere length during B cell differentiation would in turn decrease the replicative potential of memory or effector B cells. To examine the effect of differentiation and cell division on telomere length in B cell subsets, genomic DNA was isolated from naive, GC, and memory B cells of five tonsils, and TRF length was measured (Fig. 1A). It was found that mean TRF was longest in GC B cells, followed by memory and naive B cells (Fig. 1B). The mean difference in TRF between GC and naive B cells was statistically significant (GC longer than naive cell TRF by 0.8 ± 0.2 kb, P = 0.001), with a less significant difference between GC and memory B cells (GC longer by 0.6 ± 0.4 kb, P = 0.05), and no significant difference between memory and naive B cells (0.2 ± 0.56 kb, P = 0.4). Thus, in a model in which naive B cells differentiate into GC cells and GC cells into memory cells, a significant increase in telomere length occurred in the transition from naive to GC B cells, and significant shortening in the transition from GC to memory B cells. This represents a unique model in which telomere lengthening occurs in the course of B cell differentiation concurrent with substantial cell division.
Figure 1.
Telomere length in tonsil B cell subsets. (A) TRF blot of naive (N), GC (G), and memory (M) B cells from three tonsils. One microgram of digested genomic DNA from each sample was loaded in a 0.6% agarose gel and separated by electrophoresis. Hybridization was carried out in the dried gel with a 32P-labeled (CCCTAA)3 oligonucleotide at 43°C overnight. (B) Comparison of mean TRF length of naive, GC, and memory B cells from five patients. The mean TRF was calculated by converting the intensity of signals to molecular size based on DNA molecular weight markers, using ImageQuant (software of PhosphorImager). Telomere size effect on the signal intensity was taken into consideration in calculation as previously described (17).
Telomerase Activity in Tonsil B Cell Subsets.
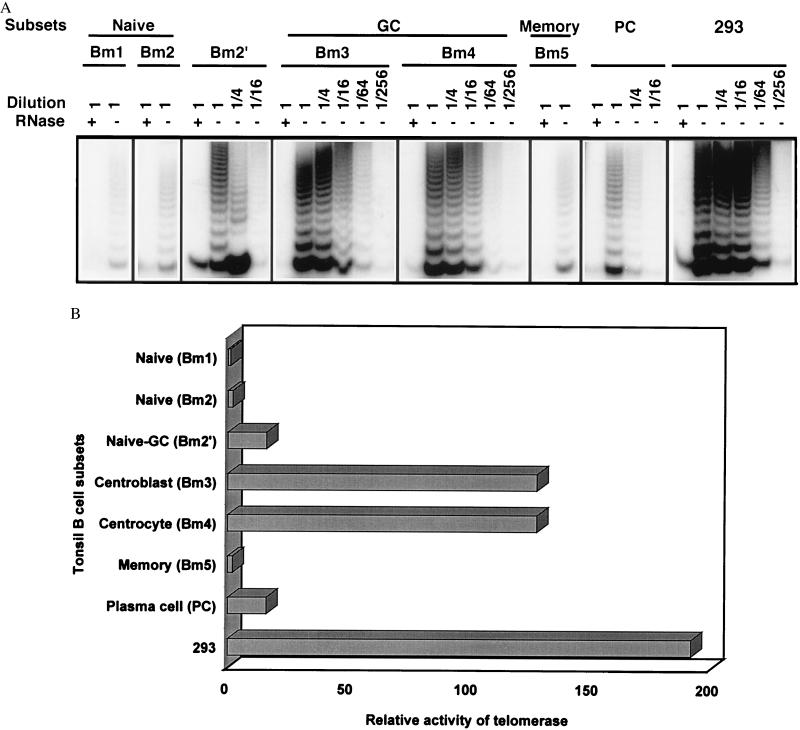
The increased telomere length found in GC B cells relative to other B cell populations could reflect the existence of a mechanism capable of elongating telomeres in these B cells. A known candidate for such activity is telomerase, which is capable of extending telomeric repeats (22, 23). To determine whether telomerase expression is regulated during B cell differentiation and is related to telomere length in B cell subsets, we analyzed telomerase activity in tonsil B cell subsets. Analysis revealed that telomerase activity was tightly regulated during B cell differentiation: the highest levels of telomerase activity were found in centrocytes (Bm4) and centroblasts (Bm3), followed by the naive to GC transition population (Bm2′); and low to undetectable levels of telomerase were found in memory B cells (Bm5), activated naive B cells (Bm2), and virgin naive B cells (Bm1) (Fig. 2). Based on serial dilution analysis and competition of an internal PCR standard (42), and compared with the level of telomerase activity in Bm1 cells, telomerase activity was at least 128-fold higher in Bm3 and Bm4, 16-fold higher in Bm2′, and 2-fold higher in Bm2 and Bm5 (Fig. 2B). Plasma cells, which represent terminally differentiated B cells, were also isolated and were found to express telomerase activity 16-fold higher than that in resting naive and memory B cells, and 16-fold lower than GC B cells (Fig. 2).
Figure 2.
Telomerase activity in tonsil B cell subsets. (A) Representative results of telomerase activity in B cell subsets. Seven subsets of B cells [Bm1–5 and plasma cell (PC)] were isolated from two donors by a combination of immunomagnetic cell separation and fluorescent cell sorting, and three subsets (naive, GC, and memory) were isolated from five donors by immunomagnetic cell separation alone. The relative activity of telomerase was consistent and reproducible among subsets of B cells from these different donors and cell separations. A transformed cell line, 293, was used as a positive control and standard for quantitation of relative levels of telomerase activity. (B) Comparison of the levels of telomerase activity in tonsil B cell subsets. The relative levels of telomerase activity were determined by serial dilution and are minimal estimates, compared with the levels of telomerase activity of Bm1 B cell subsets. The internal standard was used to monitor the reproducibility of the assay and served as a reference for comparing results among experiments.
Induction of Telomerase Activity in Vitro in Naive and Memory B Cells.
The GC B cells, populations in which the highest levels of telomerase activity are expressed, consist of activated and dividing cells as well as cells undergoing apoptosis (51, 52). To ascertain whether telomerase activity is induced by signals that induce activation and proliferation, and/or by the signals that induce apoptosis, freshly isolated naive and memory tonsil B cells, which expressed low levels of telomerase, were cultured under various conditions for 2 days and collected for telomerase analysis. In both naive and memory B cells, SAC treatment resulted in strong induction of telomerase (32-fold increase), as did treatment with (anti-IgM + anti-CD40 + rIL-4) (16-fold), (anti-IgM + anti-CD40) (12-fold), and (anti-IgM + rIL-4) (8-fold). In contrast, treatment with anti-CD40, or rIL-4 alone, or (anti-CD40 + rIL-4) did not induce telomerase activity (Fig. 3). Treatment with anti-IgM alone induced low levels of telomerase in naive (4-fold) but not memory B cells (Fig. 3). The induction of telomerase in naive and memory B cells generally correlated well with induction of cell proliferation as measured by [3H]thymidine incorporation under these stimulation conditions (Table 1).
Figure 3.
In vitro activation induces telomerase in naive and memory tonsil B cells. (A) The results shown are representative of telomerase induction in naive and memory tonsil B cells with in vitro activation. Similar results were obtained in three independent experiments. (B) Relative levels of telomerase activity in naive and memory tonsil B cells induced by different stimulation conditions. The relative levels of telomerase activity were determined as described in the text and in the legend of Fig. 2.
Table 1.
DNA synthesis by naive and memory B cells in vitro
| Conditions* | [3H]Thymidine incorporation, cpm
|
|
|---|---|---|
| Naive | Memory | |
| Medium | 443 ± 48 | 495 ± 19 |
| Anti-IgM | 4,694 ± 285 | 1,667 ± 184 |
| rIL-4 | 847 ± 52 | 884 ± 88 |
| Anti-CD40 | 782 ± 49 | 996 ± 142 |
| Anti-IgM + rIL-4 | 11,072 ± 2,208 | 6,157 ± 120 |
| Anti-IgM + anti-CD40 | 14,977 ± 1,376 | 9,061 ± 816 |
| rIL−4 + anti-CD40 | 3,197 ± 70 | 2,986 ± 442 |
| Anti-IgM + rIL−4 + anti-CD40 | 15,355 ± 722 | 11,055 ± 2,728 |
| SAC | 15,002 ± 345 | 11,330 ± 339 |
The results are expressed as mean ± SD.
Cells were incubated at 37°C for 24 hr under various stimulation conditions. After addition of 1 μCi of [3H]thymidine, cells were incubated for another 24 hr and harvested for DNA synthesis analysis.
Because apoptotic death is a common phenomenon occurring in GC B cells, where telomerase activity is highest, it was determined whether telomerase is induced by apoptosis-inducing signals in vitro. Although dexamethasone and γ-irradiation induced substantial apoptosis in naive and memory B cells, neither treatment induced telomerase (data not shown).
hTR Expression in B Cell Subsets.
To assess the regulation of telomerase activity in B cell subsets at a molecular level, we examined the expression of hTR (53) in naive, GC, and memory B cells. It was found that the level of hTR is higher in GC B cells than in naive and memory B cells (Fig. 4A). When the level of hTR expression was normalized to a constitutively expressed 7SK gene (54), hTR in GC B cells was twice as abundant as in naive and memory B cells (Fig. 4B). This result indicates that regulation of hTR correlates with the levels of telomerase activity in these B cell subsets. Up-regulation of hTR expression was also observed in both naive and memory B cells after 2-day in vitro activation with either (anti-IgM + anti-CD40 + rIL-4) or SAC (data not shown).
Figure 4.
Telomerase RNA template expression in B cell subsets. (A) Representative Northern blot of hTR expression in naive (N), GC (G), and memory (M) B cells. (B) Abundance of hTR expression in tonsil B cell subsets. The abundance of hTR was analyzed by normalization to 7SK level, presented as in arbitrary units (a.u.), and the results were summarized from B cells of three tonsils.
DISCUSSION
The T cell-dependent response of mature B cells to antigenic stimulation is marked by a complex set of differentiation events coupled to proliferation, somatic hypermutation, Ig class switching, clonal selection, and clonal expansion. It has been estimated that antigen-naive B cells undergo at least 4 cell divisions in the transition to GC B cell phenotype (51) and that GC B cells undergo at least 20 cell divisions to generate effector or memory B cells (55). Memory B cells are believed to be generated predominantly from a GC-dependent pathway, whereas plasma cells are probably derived from both GC-dependent and -independent paths (3). The persistence of memory B cells ensures the capacity to generate an accelerated response to a subsequent encounter with the same antigen, with production of antibody previously selected for high affinity and isotype (56). However, the mechanisms underlying many of these striking events are poorly understood, including the mechanisms involved in regulating the capacity for clonal expansion. In this report, we have assessed telomere length and telomerase activity in B cell subsets in human tonsils to evaluate the potential role of telomere regulation in B cell differentiation and activation.
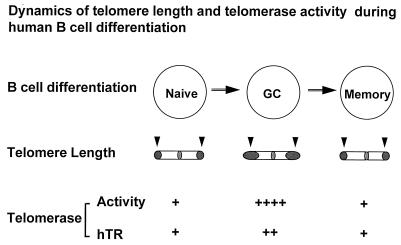
The loss of telomeric DNA in vivo as a function of age and in vitro with cell division has been well documented in many types of normal human somatic cells over the past several years, including fibroblasts (17, 19), endothelial cells (21), lymphocytes (41, 57, 58), and hematopoietic stem cells (20). If telomere length serves as a mitotic clock as proposed (19), the capacity for cellular replication will be restricted by the telomere length. Evidence supporting this model has included the observation that the replication potential of human fibroblasts is better correlated with the initial length of telomeres than with other parameters such as the age of the donors (19). An additional observation consistent with telomere shortening during in vivo cell division is the finding that human CD4+ memory T cells have telomeres that are consistently 1.4 kb shorter than the CD4+ naive T cells from which they appear to differentiate and that memory T cells have correspondingly less replicative capacity than naive T cells (42). Here we demonstrate that telomere length is also regulated during B cell differentiation. However, the longest telomeres are found not in precursor naive B cells but rather in GC B cells that are the descendants of naive B cells. In principal, the long telomeres found in GC B cells either could reflect their selective derivation from precursor naive B cells, which have long telomeres, or could reflect an actual elongation of telomeres by mechanisms operating in GC B cells. Although it is difficult to rule out the first possibility, it is not apparent that there would be an initial selective advantage to those naive B cells that have relatively longer telomeres. It has previously been shown in dividing yeast that telomerase not only is essential for preventing telomere shortening (59) but also can function to dramatically lengthen telomeres when specific mutations are introduced into telomerase RNA template (60). Thus, the expression of strikingly high levels of telomerase activity in GC B cells is consistent with the possibility that telomeres have been elongated in GC B cells by telomerase, serving to preserve the replicative lifespan of GC and their memory B cell descendants (Fig. 5). These results provide unique evidence that telomere length can be increased during the course of B cell differentiation, and they suggest that telomerase may play a role in regulating telomere length in these normal human somatic cells in vivo.
Figure 5.
Model of coordinated regulation of telomere length and telomerase activity in peripheral B cell differentiation. Arrows indicate telomeres of chromosomes and the shaded end reflects the length of telomeres.
Substantial evidence now indicates that telomerase can be expressed in normal human somatic cells (29–37). The expression of telomerase in these somatic cells appears to be generally down-regulated during in vivo development and differentiation, as demonstrated in hematopoietic progenitor cells (30) and T cells (33) and during the induction of differentiation in the promyelocytic cell line HL60 in vitro (61–63). A low to undetectable level of telomerase activity has been reported in peripheral blood B cells (29, 32), and a high level in tonsil GC cells (31, 34). Our results provide a detailed picture of regulation of telomerase during B cell differentiation from which several interesting points emerge. Telomerase activity is low in naive B cells but present at high levels in the transition from naive B cells to GC centroblasts and centrocytes. Telomerase then decreases as GC cells transition to memory cells. Plasma cells, which are terminally differentiated cells, expressed moderate levels of telomerase, thus differing from previous observations of other terminally differentiated cells (61–63). The telomerase RNA component was regulated in parallel with telomerase activity in tonsil B cell subsets both in vivo and in vitro.
The results presented here indicate that naive and memory tonsil B cells can be induced to express telomerase after the engagement of the B cell antigen receptor together with costimulatory signals and cytokines such as IL-4 in vitro. The induction of telomerase in vitro is generally correlated with the entry into the cell cycle and proliferation. However, high levels of telomerase activity were also observed in CD4+CD8+ thymocytes (33), and tonsil centrocytes (ref. 34 and present report), which are principally noncycling cells. The identity of the stimuli that regulate telomerase activity in vivo is unclear. It is notable that the stimuli which induce telomerase in naive B cells in vitro, including engagement of the surface IgM receptor together with engagement of CD40 and/or exposure to IL-4, are all equivalents of signals that may be delivered to B cells in the GC environment. Because a high proportion of GC B cells undergo apoptotic death, we determined whether telomerase was activated by stimuli that induce apoptotic death; neither dexamethasone nor irradiation, both of which induced apoptosis in naive and memory B cells, induced telomerase expression. Additional studies will be necessary to define the precise signaling requirement for induction of telomerase and their relationship to regulation of cell cycle progression.
The mechanisms that control replicative lifespan of lymphocytes remain to be elucidated. The analysis of telomere length and telomerase expression in B cell differentiation provides one approach to analysis of this regulation, and the use of lymphocytes as a model system may provide insights into how telomeres and telomerase function in normal somatic cells.
Acknowledgments
We thank Holy Cross Hospital (Silver Spring, MD) for providing tonsils; Drs. Garnett Kelsoe, Alfred Singer, Bruce Levine, and Karen Hathcock for critically reading this manuscript; Dr. Dimiter Dimitrov for stimulating discussion and help in telomere measurement; Drs. Apurva Sarin and Mark Williams for stimulating discussion and assistance in carrying out the analysis of apoptosis; David Winkler for synthesizing oligonucleotides; and Larry Palmer for technical assistance.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: GC, germinal center; TRF, telomeric restriction fragment; SAC, Staphylococcus aureus Cowan strain.
References
- 1.Tonegawa S. Nature (London) 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Rajewsky K. Nature (London) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y J, Banchereau J. The Immunologist. 1996;4(2):55–66. [Google Scholar]
- 4.Neuberger M S, Milstein C. Curr Opin Immunol. 1995;7:248–254. doi: 10.1016/0952-7915(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 5.Kelsoe G. Semin Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- 6.Pascual V, Liu Y J, Magalski A, de Bouteiller O, Banchereau J, Capra J D. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kepler T B, Perelson A S. Immunol Today. 1993;14:412–415. doi: 10.1016/0167-5699(93)90145-B. [DOI] [PubMed] [Google Scholar]
- 8.Coffman R L, Lebman D A, Rothman P. Adv Immunol. 1996;54:229–270. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 9.Greider C W, Harley C B. In: Cellular Aging and Cell Death. Holbrook N J, Martin G R, Lockshin R A, editors. New York: Wiley–Liss; 1996. pp. 123–138. [Google Scholar]
- 10.Blackburn E H. Nature (London) 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 11.Zakian V A. Science. 1995;270:1601–1607. doi: 10.1126/science.270.5242.1601. [DOI] [PubMed] [Google Scholar]
- 12.Fang G, Cech T R. In: Telomeres. Blackburn E H, Greider C W, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. pp. 69–105. [Google Scholar]
- 13.Chong L, van Steensel B, Broccoli D, Erdjument-Bromage H, Hanish J, Tempst P, de Lange T. Science. 1995;270:1663–1667. doi: 10.1126/science.270.5242.1663. [DOI] [PubMed] [Google Scholar]
- 14.Watson J. Nat New Biol. 1972;239:197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- 15.Olovnikov A M. J Theor Biol. 1973;41:181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 16.Cooke H J, Smith B A. Cold Spring Harb Symp Quant Biol. 1986;51:213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Harley C B, Futcher A B, Greider C W. Nature (London) 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 18.de Lange T, Shiue L, Myers R M, Cox D R, Naylor S L, Killery A M, Varmus H E. Mol Cell Biol. 1990;10:518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allsopp R C, Vaziri H, Patterson C, Goldstein S, Younglai E V, Futcher A B, Greider C W, Harley C B. Proc Natl Acad Sci USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaziri H, Dragowska W, Allsopp R C, Thomas T E, Harley C B, Lansdorp P M. Proc Natl Acad Sci USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang E, Harley C B. Proc Natl Acad Sci USA. 1995;92:11190–11194. doi: 10.1073/pnas.92.24.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackburn E H. Annu Rev Biochem. 1992;61:113–129. doi: 10.1146/annurev.bi.61.070192.000553. [DOI] [PubMed] [Google Scholar]
- 23.Greider C W. In: Telomeres. Blackburn E H, Greider C W, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1995. pp. 35–68. [Google Scholar]
- 24.Kim N W, Piatyszek M A, Prowse K R, Harley C B, West M D, Ho P L, Coviello G M, Wright W E, Weinrich S L, Shay J W. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 25.Counter C M, Hirte H W, Bacchetti S, Harley C B. Proc Natl Acad Sci USA. 1994;91:2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright W E, Piatyszek M A, Rainey W E, Byrd W, Shay J W. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.de Lange T. Proc Natl Acad Sci USA. 1994;91:2882–2885. doi: 10.1073/pnas.91.8.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shay J W. Molecular Medicine Today. 1995;1:378–384. doi: 10.1016/s1357-4310(95)93872-9. [DOI] [PubMed] [Google Scholar]
- 29.Hiyama K, Hirai Y, Kyoizumi S, Akiyama M, Hiyama E, Piatyszek M A, Shay J W, Ishioka S, Yamakido M. J Immunol. 1995;155:3711–3715. [PubMed] [Google Scholar]
- 30.Chiu C P, Dragowska V, Kim N W, Vaziri H, Yui J, Thomas T E, Harley C B, Lansdorp P M. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 31.Morrison S J, Prowse K R, Ho P, Weissman I L. Immunity. 1996;5:207–216. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 32.Broccoli D, Young J W, de Lange T. Proc Natl Acad Sci USA. 1995;92:9082–9086. doi: 10.1073/pnas.92.20.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weng N, Levine B L, June C H, Hodes R J. J Exp Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norrback K F, Dahlenborg K, Carlsson R, Roos G. Blood. 1996;88:222–229. [PubMed] [Google Scholar]
- 35.Yasumoto S, Kunimura C, Kikuchi K, Tahara H, Ohji H, Yamamoto H, Ide T, Utakoji T. Oncogene. 1996;13:433–439. [PubMed] [Google Scholar]
- 36.Harle-Bachor C, Boukamp P. Proc Natl Acad Sci USA. 1996;93:6476–6481. doi: 10.1073/pnas.93.13.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor R S, Ramirez R D, Ogoshi M, Chaffins M, Piatyszek M A, Shay J W. J Invest Dermatol. 1996;106:759–765. doi: 10.1111/1523-1747.ep12345811. [DOI] [PubMed] [Google Scholar]
- 38.Buchkovich K J, Greider C W. Mol Biol Cell. 1996;7:1443–1454. doi: 10.1091/mbc.7.9.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igarashi H, Sakaguchi N. Biochem Biophys Res Commun. 1996;219:649–655. doi: 10.1006/bbrc.1996.0288. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y J, Barthelemy C, de Bouteiller O, Arpin C, Durand I, Banchereau J. Immunity. 1995;2:239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 41.Merville P, Dechanet J, Desmouliere A, Durand I, de Bouteiller O, Garrone P, Banchereau J, Liu Y J. J Exp Med. 1996;183:227–236. doi: 10.1084/jem.183.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weng N, Levine B L, June C H, Hodes R J. Proc Natl Acad Sci USA. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright W E, Shay J W, Piatyszek M A. Nucleic Acids Res. 1995;23:3794–3795. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mond J J, Brunswick M. In: Current Protocols in Immunology. Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. New York: Wiley; 1995. pp. 3.10.1–3.10.5. [Google Scholar]
- 45.Lagresle C, Mondiere P, Bella C, Krammer P H, Defrance T. J Exp Med. 1996;183:1377–1388. doi: 10.1084/jem.183.4.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.June C H, Ledbetter J A, Gillespie M M, Lindsten T, Thompson C B. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng N, Levine B L, June C H, Hodes R J. J Immunol. 1997;158:3215–3220. [PubMed] [Google Scholar]
- 48.Hodgkin P D, Lee J, Lyons A B. J Exp Med. 1996;184:277–281. doi: 10.1084/jem.184.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacLennan I C. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 50.Leanderson T, Kallberg E, Gray D. Immunol Rev. 1992;126:47–61. doi: 10.1111/j.1600-065x.1992.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 51.Kelsoe G. Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y J, Arpin C, de Bouteiller O, Guret C, Banchereau J, Martinez-Valdez H, Lebecque S. Semin Immunol. 1996;8:169–177. doi: 10.1006/smim.1996.0021. [DOI] [PubMed] [Google Scholar]
- 53.Feng J, Funk W D, Wang S S, Weinrich S L, Avilion A A, Chiu C P, Adams R R, Chang E, Allsopp R C, Yu J. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 54.Murphy S, Altruda F, Ullu E, Tripodi M, Silengo L, Melli M. J Mol Biol. 1984;177:575–590. doi: 10.1016/0022-2836(84)90038-x. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, MacLennan I C, Liu Y J, Lane P. Immunol Lett. 1988;18:297–300. doi: 10.1016/0165-2478(88)90178-2. [DOI] [PubMed] [Google Scholar]
- 56.Vitetta E S, Berton M T, Burger C, Kepron M, Lee W T, Yin X. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- 57.Vaziri H, Schachter F, Uchida I, Wei L, Zhu X, Effros R, Cohen D, Harley C B. Am J Hum Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- 58.Slagboom P E, Droog S, Boomsma D I. Am J Hum Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 59.Singer M S, Gottschling D E. Science. 1994;266:404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 60.McEachern M J, Blackburn E H. Nature (London) 1995;376:403–409. doi: 10.1038/376403a0. [DOI] [PubMed] [Google Scholar]
- 61.Holt S E, Wright W E, Shay J W. Mol Cell Biol. 1996;16:2932–2939. doi: 10.1128/mcb.16.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savoysky E, Yoshida K, Ohtomo T, Yamaguchi Y, Akamatsu K, Yamazaki T, Yoshida S, Tsuchiya M. Biochem Biophys Res Commun. 1996;226:329–334. doi: 10.1006/bbrc.1996.1356. [DOI] [PubMed] [Google Scholar]
- 63.Bestilny L J, Brown C B, Miura Y, Robertson L D, Riabowol K T. Cancer Res. 1996;56:3796–3802. [PubMed] [Google Scholar]