Abstract
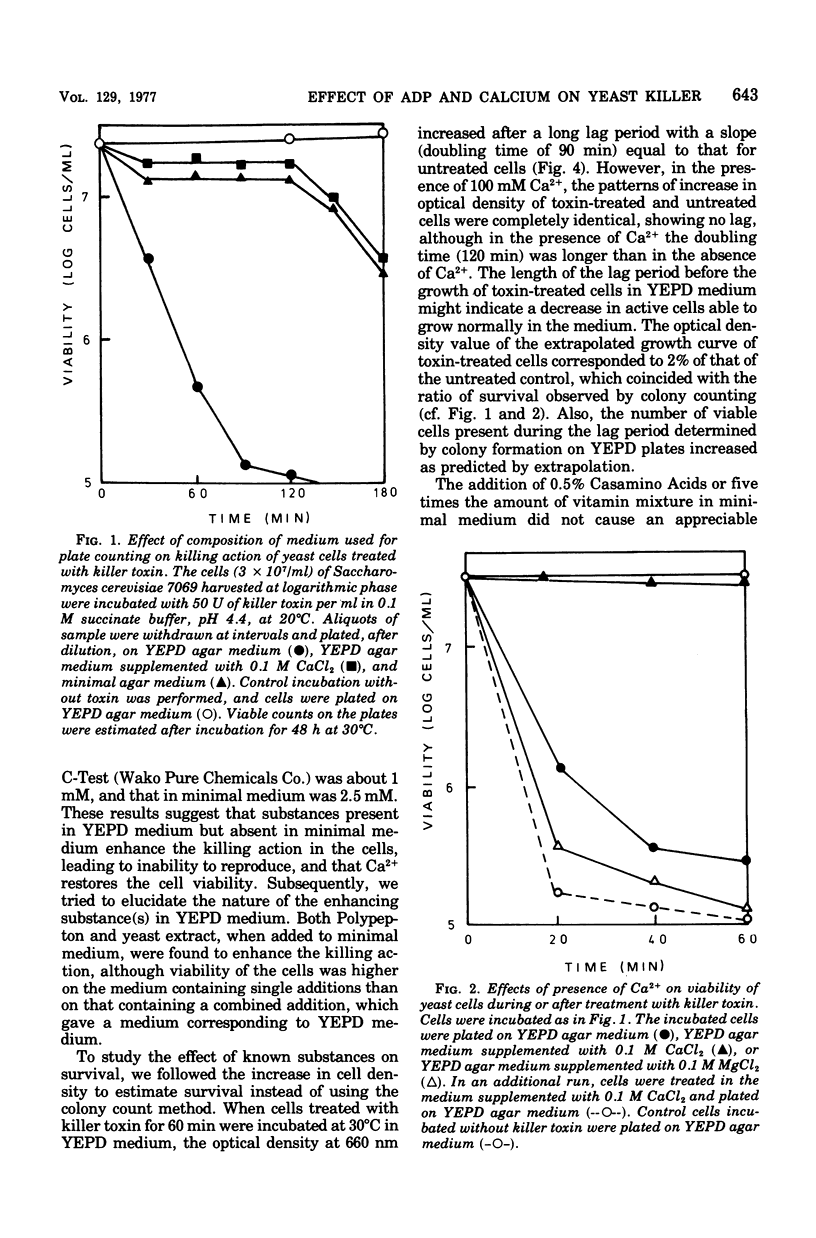
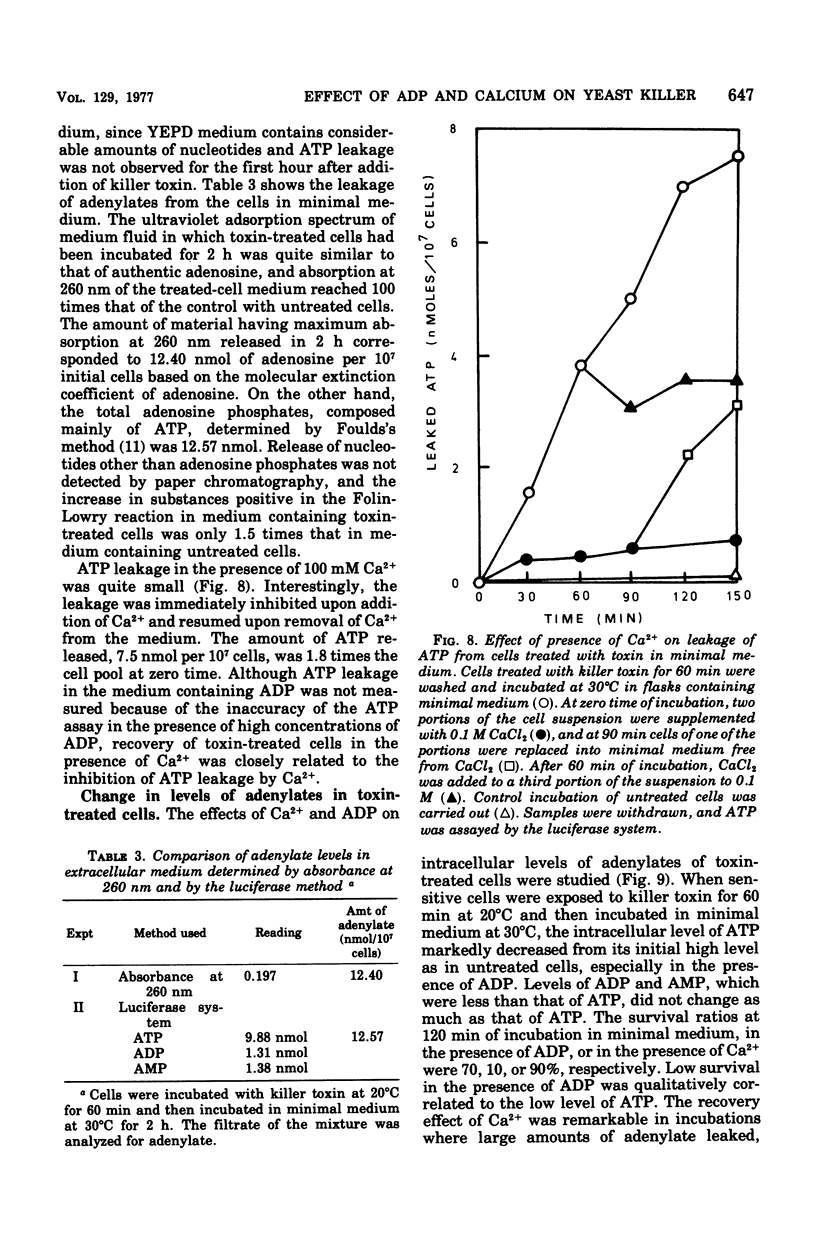
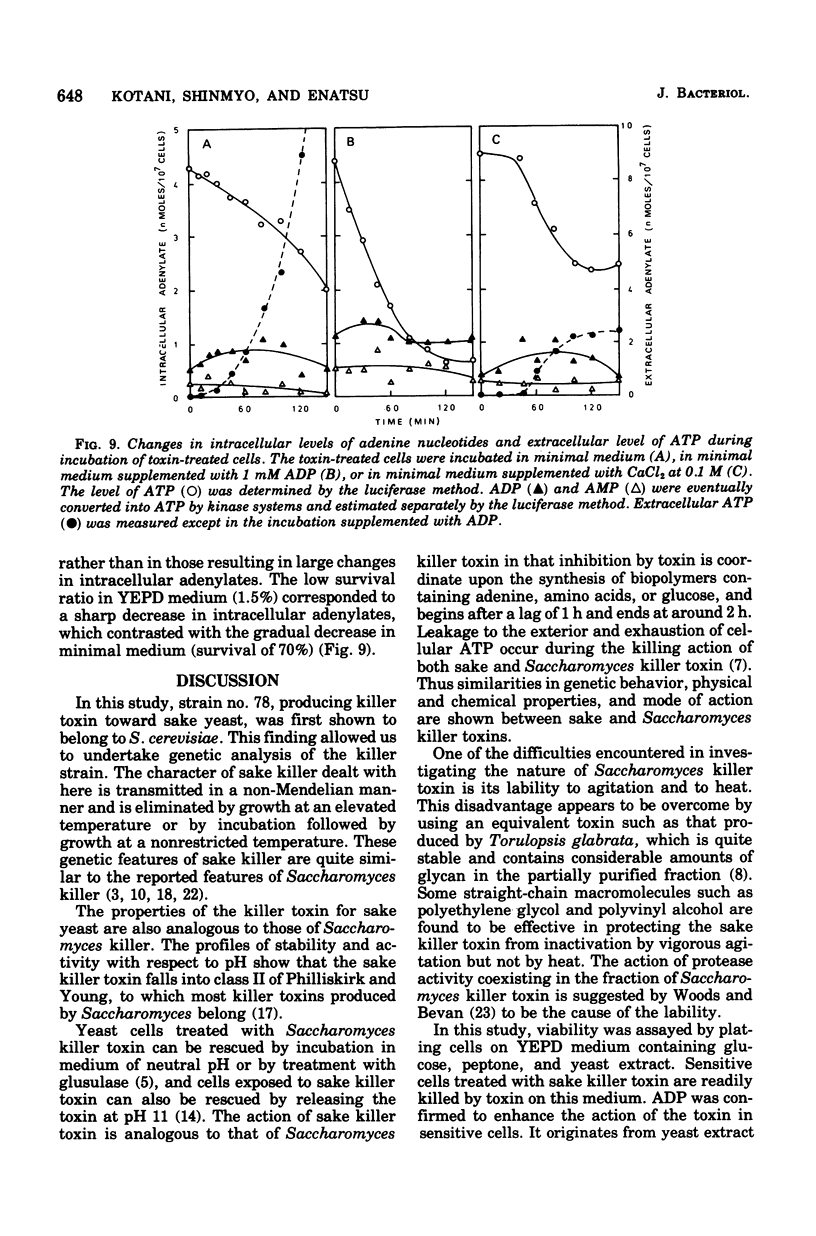
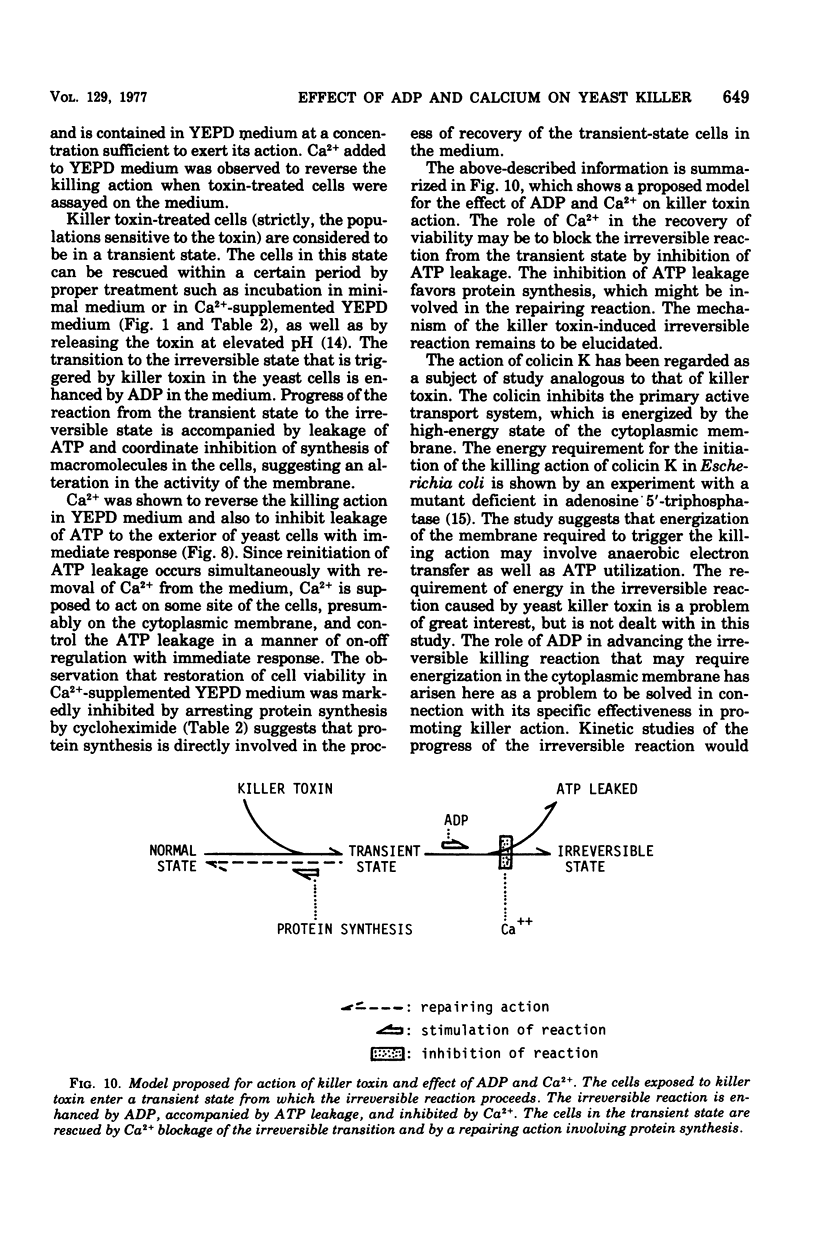
The killer character of strain isolated from the main mash of sake brewing which produces a killer substance for sake yeast was transmitted to hybrids of the strain and a standard strain of Saccharomyces cerevisiae through a cytoplasmic determinant. The character was eliminated at 41 degrees C by incubation followed by growth at 30 degrees C. The killer strain produced the killer toxin in a growth-associated manner. A preparation of crude killer toxin extract showed first-order inactivation and a linear Arrhenius plot between 25 and 40 degrees C, with an activation of energy of 55.0 kcal/mol. Addition of 1% of synthetic polymer protected the toxin from inactivation by agitation but not by heat. Enhancement of the killer action toward sensitive yeast cells by only the nucleotide adenosine 5'-diphosphate (ADP) was observed after plating on agar medium as well as after incubation in liquid medium. The addition of CaCl2 reversed the enhancing effect of ADP on killing activity. This action of CaCl2 was inhibited by cycloheximide, suggesting that protein synthesis is required for recovery of toxin-induced cells in the presence of CaCl2. Further, CaCl2 overcame the decrease in the intracellular level of adenosine 5'-triphosphate (ATP) enhanced by ADP in killer-treated cells and also inhibited leakage of ATP from the cells with immediate response. The mode of killing action is discussed in terms of a transient state of the cells and the action of ADP and CaCl2.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball W. J., Jr, Atkinson D. E. Adenylate energy charge in Saccharomyces cerevisiae during starvation. J Bacteriol. 1975 Mar;121(3):975–982. doi: 10.1128/jb.121.3.975-982.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan E. A., Herring A. J., Mitchell D. J. Preliminary characterization of two species of dsRNA in yeast and their relationship to the "killer" character. Nature. 1973 Sep 14;245(5420):81–86. doi: 10.1038/245081b0. [DOI] [PubMed] [Google Scholar]
- Bevan E. A., Somers J. M. Somatic segregation of the killer (k) and neutral (n) cytoplasmic genetic determinants in yeast. Genet Res. 1969 Aug;14(1):71–77. doi: 10.1017/s0016672300001865. [DOI] [PubMed] [Google Scholar]
- Bussey H. Effects of yeast killer factor on sensitive cells. Nat New Biol. 1972 Jan 19;235(55):73–75. doi: 10.1038/newbio235073a0. [DOI] [PubMed] [Google Scholar]
- Bussey H., Sherman D. Yeast killer factor: ATP leakage and coordinate inhibition of macromolecular synthesis in sensitive cells. Biochim Biophys Acta. 1973 Apr 16;298(4):868–875. doi: 10.1016/0005-2736(73)90391-x. [DOI] [PubMed] [Google Scholar]
- Bussey H., Skipper N. Membrane-mediated killing of Saccharomyces cerevisiae by glycoproteins from Torulopsis glabrata. J Bacteriol. 1975 Oct;124(1):476–483. doi: 10.1128/jb.124.1.476-483.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman A. G., Fall L., Atkinson D. E. Adenylate energy charge in Escherichia coli during growth and starvation. J Bacteriol. 1971 Dec;108(3):1072–1086. doi: 10.1128/jb.108.3.1072-1086.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Curing of a killer factor in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2846–2849. doi: 10.1073/pnas.69.10.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Mode of action of a bacteriocin from Serratia marcescens. J Bacteriol. 1971 Sep;107(3):833–839. doi: 10.1128/jb.107.3.833-839.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Bevan E. A. Virus-like particles associated with the double-stranded RNA species found in killer and sensitive strains of the yeast Saccharomyces cerevisiae. J Gen Virol. 1974 Mar;22(3):387–394. doi: 10.1099/0022-1317-22-3-387. [DOI] [PubMed] [Google Scholar]
- Jetten A. M., Jetten M. E. Energy requirement for the initiation of colicin action in Escherichia coli. Biochim Biophys Acta. 1975 Apr 14;387(1):12–22. doi: 10.1016/0005-2728(75)90048-1. [DOI] [PubMed] [Google Scholar]
- Philliskirk G., Young T. W. The occurrence of killer character in yeasts of various genera. Antonie Van Leeuwenhoek. 1975;41(2):147–151. doi: 10.1007/BF02565046. [DOI] [PubMed] [Google Scholar]
- Somers J. M., Bevan E. A. The inheritance of the killer character in yeast. Genet Res. 1969 Feb;13(1):71–83. doi: 10.1017/s0016672300002743. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Fink G. R. A nucleic acid associated with a killer strain of yeast. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1069–1072. doi: 10.1073/pnas.70.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodkin M., Katterman F., Fink G. R. Yeast killer mutants with altered double-stranded ribonucleic acid. J Bacteriol. 1974 Feb;117(2):681–686. doi: 10.1128/jb.117.2.681-686.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. "Killer character" of Saccharomyces cerevisiae: curing by growth at elevated temperature. J Bacteriol. 1974 Mar;117(3):1356–1357. doi: 10.1128/jb.117.3.1356-1357.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Chromosomal and nonchromosomal mutations affecting the "killer character" of Saccharomyces cerevisiae. Genetics. 1974 Mar;76(3):423–432. doi: 10.1093/genetics/76.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. R., Bevan E. A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J Gen Microbiol. 1968 Apr;51(1):115–126. doi: 10.1099/00221287-51-1-115. [DOI] [PubMed] [Google Scholar]


