Abstract
Background and Aims
Glial Derived Neurotrophic factor (GDNF) promotes the survival and proliferation of enteric neurons. Neuropeptide Y (NPY) is an important peptide regulating gastrointestinal motility. The role of NPY on the survival and proliferation of enteric neurons is not known. We examined the effects of GDNF on the expression and release of NPY from enteric neurons and the role of NPY in promoting enteric neuronal proliferation and survival.
Methods
Studies were performed in primary enteric neuronal cultures and NPY knock out mice (NPY−/−). GDNF-induced expression of NPY was assessed by RT-PCR, immunocytochemistry and ELISA. Using NPY-siRNA and NPY- Y1 receptor antagonist we examined the role of NPY in mediating the survival and proliferation effects of GDNF. Gastrointestinal motility was assessed by measuring gastric emptying, intestinal transit and isometric muscle recording from intestinal muscle strips.
Results
GDNF induced a significant increase in NPY mRNA and protein expression in primary enteric neurons, and the release of NPY into the culture medium. NPY (1 μM) significantly increased proliferation of neurons and reduced apoptosis. In the presence of NPY-siRNA and NPY-Y1 receptor antagonist or in enteric neurons cultured from NPY−/− mice, GDNF-mediated neuronal proliferation and survival was reduced. NPY increased the phosphorylation of Akt, a downstream target of the PI-3-Kinase pathway. In NPY−/− mice there were significantly fewer nNOS-containing enteric neurons compared to WT mice. NPY−/− mice had accelerated gastric emptying and delayed intestinal transit compared to WT mice.
Conclusion
We demonstrate that NPY acts as an autocrine neurotrophic factor for enteric neurons.
Keywords: Enteric neurons, NPY, apoptosis, GDNF, proliferation, survival
Introduction
The enteric nervous system (ENS) is a network of neurons that controls the motility and secretion of the gastrointestinal tract. Several growth factors influence the proliferation and survival of the ENS, including glial cell line-derived neurotrophic factor (GDNF) and neurturin 1,2. GDNF binds to its receptor Ret, a transmembrane tyrosine kinase receptor, which requires one of the four glycosylphosphatidylinositol (GPI)-linked co-receptors (GFRα1–4) for its activation. GDNF/GFRα1/Ret signaling is essential for the migration, proliferation, maturation and survival of ENS precursors during embryonic development 3–8. GDNF-deficient mice, GFRα1-deficient mice or Ret −/− mice have renal agenesis, total intestinal aganglionosis and defects in the sympathetic, parasympathetic and sensory nervous system 9–14.
Neuropeptide Y (NPY) is a neurotransmitter consisting of 36 amino acid residues, and is a member of the peptide family that also includes peptide YY (PYY) and pancreatic polypeptide (PP). PYY and PP are primarily synthesized and released from endocrine cells of the gut and pancreas, respectively, whereas NPY is widely distributed in the central and peripheral nervous system during development and adulthood 15–18. By embryonic day E-13, the entire length of the fetal murine bowel contains NPY-staining neurons19. NPY regulates gastrointestinal motility, feeding behavior and stimulation of cellular growth, including hypertrophy of cardiomyocytes and proliferation of vascular smooth muscle cells20–26. In neural tissue, NPY promotes proliferation of neuroblasts in the olfactory epithelium, post-natal and adult dentate gyrus, and is protective against methamphetamine-induced neurotoxicity in the striatum27–29. The biological effects of NPY are mediated by five G-protein coupled receptors (Y1, Y2, Y4, Y5 and Y6)30. The NPY receptor subtypes are restricted to tissue-specific expression and account for the diverse action of NPY in different tissues. NPY/Y1 double labeling showed that NPY and its Y1 receptor subtype are co-localized within ganglion cells of the submucosal and myenteric plexes31. In addition, Y1-expressing neurons co-localize with the nNOS-expressing neurons. The Y1 receptor may act as an autoreceptor within the colonic gut wall31.
Certain growth factors like brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) can modulate NPY expression32–35. The effects of growth factors on the synthesis and release of peptide neurotransmitters such as NPY have not been studied in the ENS. In the present study, isolated embryonic enteric neuronal cultures were used to examine the effect of GDNF on NPY mRNA and protein expression and the release of NPY into the culture medium. The role of NPY in meditating GDNF-induced proliferation and survival of enteric neurons was further confirmed by using NPY-specific siRNA and a NPY-Y1 receptor antagonist, as well as neurons from NPY−/− mice. Using NPY−/− mice, we assessed the effects of NPY on gastric emptying and intestinal transit in whole animals. We also evaluated the expression of neuronal subtypes in the myenteric plexus and enteric neuronal-mediated intestinal muscle relaxation in longitudinal and circular intestinal muscle strips.
Materials and Methods
Reagents
Collagenase and dispase were obtained from Worthington Biochemical. Tyramide signal amplification kits were obtained (TSA-FITC or TSA-Cy3) from NEN Life Science Products). NPY and BIBP3226 (NPY-Y1 receptor antagonist) from BACHEM, Omniscript Reverse Transcription Kit from Qiagen, Oligo (dT) 12–18 primers and Lipofectamine 2000 from Invitrogen, NPY and GAPDH primers from IDT, Taq DNA polymerase from Promega, SYBR Green Supermix from Bio-Rad, control Cyclophilin B and NPY-specific siRNA from Dharmacon, and methylene blue from Mayne Pharma. All chemicals not included above were purchased from Sigma. GDNF was produced as previously described 36.
Antibodies
The antibodies to p75NTR, NPY, peripherin, nNOS (neuronal Nitric Oxide Synthase), ChAT (Choline Acetyl transferase) were obtained from Chemicon International. Antibodies to Cleaved caspase-3, phospho-Akt and Akt from Cell Signaling, antibody to BrdU from Molecular Probes; antibody to NPY monoclonal (NPY02 and NPY05) and monoclonal anti-C flanking peptide of NPY (CPON01) were from Dr. Eric Grouzmann, antibody to PYY was a gift from Dr. Roger Corder, William Harvey Research Institute.; antibody to β-actin from Sigma; Secondary antibodies including donkey anti-rabbit biotin, donkey anti-mouse biotin and peroxidase-conjugated streptavidin were from Jackson ImmunoResearch Laboratories, Inc.
Primary culture and magnetic bead immunoselection of p75 expressing cells
p75-expressing cells were isolated from stomach, small and large bowel of E14.5 Sprague-Dawley rat embryos (10–12 embryos) or E13.5 mouse embryos (WT and NPY−/− mice) by magnetic bead immunoselection with a monoclonal antibody to low affinity NGF receptor (p75NTR, 10 μg/mL) as previously described1,37. Following immunoselection, 12,500 trypan blue-excluding cells were resuspended in 125 μl of modified N2 medium 37 and plated into a single well of an 8-well slide coated with poly-D-lysine and laminin. The N2 medium contains DMEM/F12 with the addition of sodium selenite (30 nM), putrescine dihydrocholoride (100 μM), progesterone(20 nM), insulin (5 μg/mL), transferrin (10 μg/mL), fetuin (0.1 mg/mL), and bovine serum albumin (1mg/mL). An additional 125 μl of medium were added to each well with the desired factors. Cells were grown in this medium at 37°C in a humidified tissue culture incubator containing 5% CO2. Unless otherwise stated, cells were grown in the presence of GDNF (100 ng/mL). As described by other investigators38, our immunoselection method yielded a population of 80% Ret-positive cells after 24 h of culture. The p75- negative fraction had 2–3% Ret-positive cells. To investigate the effect of NPY on neuronal cell survival and proliferation, cells were grown in the presence of NPY (1 μM) for 24 or 48 h.
RT-PCR
Immunoselected rat embryonic enteric neurons were cultured for 24 h in N2 medium in the presence or absence of recombinant rat GDNF (100 ng/mL) in poly-D-lysine/laminin- coated 6-well plates. Total RNA was isolated using the RNeasy Mini Kit (Qiagen). 0.92 μg of total RNA was used to synthesize first strand cDNA using the Omniscript Reverse Transcription Kit and Oligo (dT)12–18 primers according to recommended procedure. The NPY and GAPDH primers used for semi-quantitative and real-time PCR quantification of the NPY and GAPDH messages have been described previously 39. For semi-quantitative PCR, 25 μl reactions were set up comprising of 1x Taq DNA polymerase buffer, 1.5 mM MgCl2, 200 μM dNTP mix, 0.4 μM each of the NPY or GAPDH oligonucleotide primers, 1.25 U Taq DNA polymerase and 1 μl first-strand cDNA. The reactions were heated for 3 min at 95°C, and subjected to 30 cycles of PCR amplification, each cycle consisting of 30 sec at 95°C, 30 sec at 59°C and 45 sec at 72°C. A final extension for 7 min at 72°C was performed before cooling to 4°C. Two thirds of each reaction were analyzed on a 2% agarose gel stained with ethidium bromide and the amplified products visualized by UV translumination.
Real-time PCR
For real time PCR, amplifications were detected using SYBR Green I in reactions performed with the iCycler iQ real-time PCR Detection system (Bio-Rad). Each 25 μl PCR reaction contained 1x iQ SYBR Green Supermix, 0.1 μM of each primer pair and 1 μl of each cDNA. PCR reactions were started with an initial denaturation at 95°C for 5 min followed by 45 cycles each consisting of denaturation at 95°C for 30 sec, annealing at 59°C for 30 sec and extension at 72°C for 45 sec. A melting curve analysis was performed after amplification was completed. NPY mRNA was then normalized to the respective control GAPDH amounts and presented as a ratio under different conditions.
Neuropeptide Y ELISA
Enteric neurons were cultured in 6-well plates for 48 h in N2 medium in the presence or absence of GDNF (100 ng/mL). The medium was aspirated from the cells and stored in minisorp tubes containing 1 μl of Tween 20 per mL of medium. The medium was stored at −80°C until the measurement of NPY or PYY.
Microplates (Minisorp, Nunc) were coated for 16 h at 4°C with 50 ng of the anti-NPY monoclonal antibody NPY02 or 150 ng of protein A-purified anti-PYY polyclonal antibody diluted in 50 mM Tris pH 7.5. The plates were washed four times with Tris buffer containing 0.08% Tween-20, 5% lactose, and 0.5% casein. NPY/PYY standards were added to the wells. After 16 h incubation at room temperature, the plates were washed with Tris buffer containing 0.1% Tween-20, followed by 100 μl of alkaline phosphatase-conjugated NPY-05 diluted 1/4000 with Tris buffer containing 0.1% Tween-20 and 5% non-fat dry milk. The plates were then incubated for 7 h at room temperature and washed four times with Tris buffer containing 150 mM NaCl before the addition of 50 μl of substrate. Forty-five minutes later, 50 μl of the amplifier were added to each well according to the immunoselect kit (Gibco, MD) recommendations. Optical densities were measured kinetically at 492 nm using a Molecular Devices microplate reader (Sunnyvale, CA). For NPY the limit of quantification of the assay is 25 attomoles/well or 0.25 pM. The assay is specific for amidated NPY and does not cross-react with PYY and PP at concentrations up to 10 nM40. For PYY the limit of quantification of the assay is 0.5 fmole/well or 5 pM. The assay is specific for amidated PYY and does not cross-react with PYY (13–36) and NPY at concentrations up to 1 nM.
Immunocytochemistry
Enteric neurons were cultured according to the conditions described in individual experiments. Cells were fixed (4% p-formaldehyde, 30 min), permeabilized (0.1% Triton X-100, 0.1% sodium citrate in PBS, 2 min on ice), blocked (10% NDS or 5% BSA) and incubated with primary antibody in 1% NDS or 1% BSA: NPY, 1:1000; cleaved caspase-3, 1:2500; peripherin, 1:100; nNOS, 1:500; ChAT, 1:100. Secondary detection was performed with either a FITC-conjugated donkey anti-mouse antibody (ChAT) or in conjunction with Cy3-TSA/FITC-TSA tyramide amplification kit in certain cases. As a negative control, the primary antibody was omitted in certain wells. At least four wells (Two hundred cells/well) were scored in a blinded fashion for each condition in each of the experiments, and the experiment was repeated three times.
BrdU staining
Enteric neurons treated with vehicle, NPY or GDNF in the presence or absence of either NPY-Y1 receptor antagonist (BIBP3226, 1 μM) or LY294002 (15 μM) were maintained in culture for 48 h. After 24 h of culture, BrdU (10 μM) was added to the medium. Twenty-four hours later the cells were fixed with ethanol-fixative (3 volumes of 50 mM glycine, pH 2.0, and 7 volumes of absolute ethanol) for 45 min at RT followed by PBS wash. Peripherin staining was performed in-conjunction with TSA-Cy3 kit. Cells were denatured with 4M HCl for 15 min at RT and washed with PBS to neutralize the pH. Blocking with 0.5% BSA and 0.1% Tween 20 in PBS for 30 min at RT was followed by incubation with anti-BrdU (FITC-linked) antibody in blocking buffer for 1 h at 37°C in a humid chamber. Two hundred peripherin+ enteric neurons/well were scored in a blinded fashion to determine the percentage of BrdU+/peripherin+ cells. At least four wells were scored for each condition and the experiment was repeated four times. To determine the total number of cells and neurons, we counted the number of DAPI+ and peripherin+ cells per well in a blinded fashion.
TUNEL staining
To visualize apoptotic cells, slides were washed in PBS and then incubated with TUNEL reaction mixture (Roche) in a humidified chamber for 1 hr at 37°C in the dark. Two hundred TUNEL+ neurons/well were scored in a blinded fashion to determine the percentage of apoptotic cells. At least four wells were scored for each condition and the experiment was repeated three times.
Western Blotting
Western blotting was performed according to standard methods as previously described41. A semi-quantitative measurement of the band intensity was performed using the computer software Scion Image (Maryland, USA) and expressed as a ratio of band intensity with respect to the loading control.
Cleaved caspase-3
Cell lysates were obtained from vehicle and NPY-treated cells and approximately 15 μg of protein/lane were loaded onto a 12% SDS-PAGE gel. β-actin was used as a loading control. Western blotting was performed using an antibody specific for cleaved caspase-3.
pAkt
Isolated enteric neurons cultured for 24 h in the presence of vehicle and washed with Krebs buffer for 1 h were treated with vehicle, NPY (1 μM) or GDNF (100 ng/ml) in the presence or absence of LY294002 (15 μM) for 30 min in Krebs buffer. Cell lysates were obtained and 15 μg of protein/lane were loaded onto a 12% SDS-PAGE gel. Phospho-Akt was probed by using phospho-Akt (Ser473) polyclonal antibody. As a measure of equal protein loading, total Akt was probed using Akt polyclonal antibody.
nNOS
Tissue homogenates were obtained from the stomach and colon of WT and NPY −/− mice using lysis buffer containing protease inhibitors. Twenty micrograms of total protein were loaded onto a 4–20% SDS-PAGE gel. Western blot was performed using an nNOS specific antibody. β-actin was used as a loading control.
siRNA Transfection
Enteric neurons were transfected with control Cyclophilin B/NPY-specific siRNA (5 pmol/well) (Dharmacon) and cultured for 24 h in the presence of vehicle, followed by 24 h in the presence of vehicle or GDNF. Transfection was performed as per manufacturer’s recommendation. Cells were fixed and assessed for apoptosis by the TUNEL/Peripherin double-labeling method. For the assessment of proliferation BrdU (10 μM) was added to the medium 24 h prior to fixation of the cells. Proliferation was quantitated by the Peripherin/BrdU double-labeling method. To assess the percentage of nNOS and ChAT neurons we stained the transfected slides for peripherin/nNOS or peripherin/ChAT and determined the number of nNOS+ or ChAT+ neurons in two hundred peripherin+ neurons per well. Knock down of NPY by NPY siRNA was verified by staining the cells for NPY/Peripherin. Cells were scored for the percentage of NPY+ neurons as a semi-quantitative measure of siRNA knock down.
NPY knock out mice (NPY−/−)
All animal studies were approved by the Emory University Institutional Review Board. WT and NPY−/− mice were obtained from Charles River. Experiments were performed in four week old male mice. The WT and NPY−/− mice were maintained on a 129S1/SvImJ background. The WT mice were litter and age matched. In the NPY−/− mice, the NPY gene was disrupted in embryonic stem cells by homologous recombination using a targeting vector in which exon 2, encoding the signal peptide and 35 amino acids of NPY, was replaced by the lacZ reporter gene and a neomycin-resistance cassette42. Mutant mice are born at approximately the expected frequency and are viable through adulthood. The mice do not have gross anomalies in the brain or other organs. Growth of mutant mice occurs at a normal rate, and both male and female mutants are fertile. A subset of young adult NPY-deficient mice had mild seizures (usually at 8 weeks of age), and these seizures do not occur as the mice age. No other behavioral modifications were obvious in NPY-deficient mice42.
Whole Mount staining
For assessment of neuronal and fiber density, proximal colons (1 cm) were obtained from four week old WT and NPY−/− mice. The colon was cut along the mesentery and pinned down in a silicone dish. Care was taken that the segment was not stretched to more than 1.1 cm. Fixation and careful peeling of myenteric plexus and the longitudinal muscle layer and NADPH Diaphorase was done as described43. For ChAT staining the sections were incubated with goat anti ChAT (1:100) overnight. The positive cells were visualized using the ABC kit (Vector labs) in conjunction with the DAB stain. Peripherin staining was performed using a rabbit anti-peripherin polyclonal antibody (1:500) and donkey anti-rabbit IgG-Alexa Fluor 594 (1:500). Myenteric fiber counts were performed on both NADPH-diaphorase and ChAT-stained intestines by counting fibers crossing a 0.1 mm2 grid with ten horizontal and ten vertical lines44. Ten randomly selected fields per mouse were evaluated from six animals of each genotype. The neurons were counted within the 0.1 mm2 grid and the total number of neurons with each stain was calculated. Data were analyzed using the Prism Software in conjunction with ANOVA.
Measurement of gastric emptying and intestinal transit
Wild type and NPY−/− mice received 0.1 mL methylene blue-labeled 10% dextrose solution using oral gavage and sacrificed at the indicated times (5 or 15 min). Gastric emptying and intestinal transit were determined as described previously 43. The intestinal transit was measured from the pylorus to the most distal point of migration of methylene blue.
Isometric muscle recording with electrical field stimulation
Longitudinal muscle strips (with myenteric plexus) and circular muscle strips were obtained from the proximal colon of WT and NPY−/− mice by careful dissection using a dissecting microscope. Settings for recording were done as described previously43. Colonic relaxation was measured in response to transmural stimulation and was recorded by electrical field stimulation (EFS) (100 V/48 V, 20 Hz, 5 msec pulse for a duration of 30 s) in the presence or absence of atropine and guanethidine45. We chose 20 Hz as we get our most reproducible relaxations at this frequency. For quantitative determination of relaxation longitudinal and circular intestinal strips from at least three WT and NPY−/− mice were used and the average of relaxations was obtained from EFS. The NO dependence of the relaxation was confirmed by incubation with L-NAME prior to EFS.
Statistical Analysis
Neuronal staining and motility measurements between the WT and NPY−/− mice were compared with the one-way analysis of variance (ANOVA) (1999 Graph Pad software Inc). Repeated-measures analyses were used to compare animals under each of the 3 treatment conditions for apoptosis, proliferation and differences in specific neuronal staining in cultured enteric neurons. Multiple comparisons between treatment conditions were made with Tukey’s Honestly Significant Difference (HSD) procedure. Repeated-measures analyses of apoptosis and proliferation were done with a means model with SAS Proc Mixed (version 8) providing separate estimates of the means by age (E12.5, E14.5 and E16.5) and treatment condition. A compound symmetry variance-covariance form in the repeated measurements was assumed for each outcome, and robust estimates of the standard errors of parameters were used to do statistical tests and construct confidence intervals. Statistical tests were 2-sided. A P value 0.05 was considered statistically significant.
Results
GDNF increased NPY mRNA and protein expression
The effect of growth factors on the synthesis and release of peptide neurotransmitters such as NPY has not been studied in the enteric nervous system. GDNF is known to promote enteric neuronal proliferation and suppress apoptosis2,37. We examined the effect of GDNF on the expression of NPY. Enteric neurons obtained from E14.5 rat embryos were cultured in the presence of GDNF or vehicle for 24 h and assessed for NPY mRNA and protein expression. The presence of GDNF resulted in a significant increase in NPY mRNA expression assessed by semi-quantitative PCR (Fig. 1a), and real-time PCR compared to vehicle (Fig. 1b). GDNF induced an increase in the number of NPY-expressing neurons (Fig. 1c, d), assessed using an antibody specific for NPY. Using a sensitive immunoassay for NPY, we determined the effects of GDNF on the release of NPY and a closely related peptide, PYY, into the culture medium. GDNF (48 h) induced a significant release of NPY into the culture medium compared to vehicle and had no effect on PYY release (Fig. 1 e).
Figure 1.
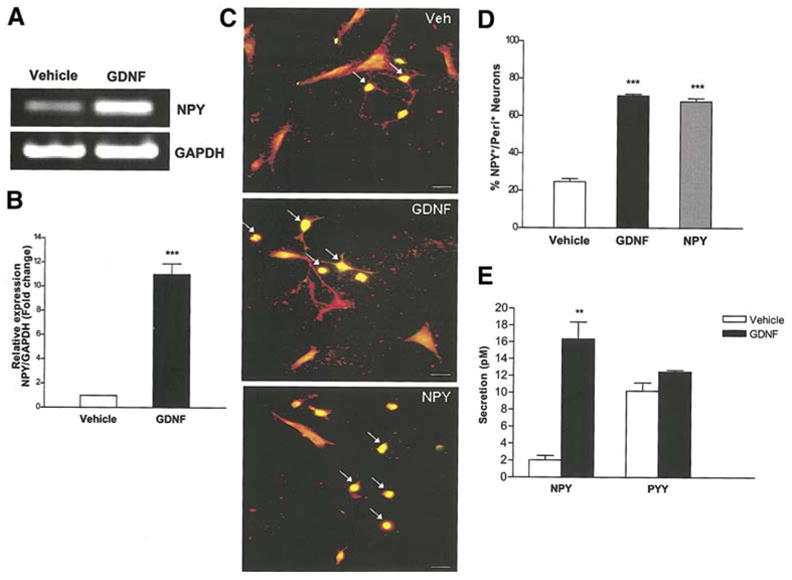
GDNF increases NPY mRNA and protein expression. (a) RNA was isolated from enteric neurons cultured in the presence or absence of GDNF for 24 h and amplified for GAPDH (loading control) and NPY. The presence of GDNF causes an increase in NPY mRNA. (b) Quantitative real-time PCR showing the relative level of expression of NPY (GDNF vs. vehicle) after normalization to GAPDH expression. Results are mean + S.E., n=9, *** = p<0.001. (c, d) Enteric neurons cultured in the presence of vehicle, GDNF or NPY for 24 h were assessed for peripherin (red)/NPY (green) and the results expressed as % NPY positive neurons. GDNF induced an increase in the number of NPY+ neurons (arrows pointing at yellow neurons). Results are mean + S.E., n=3, *** = p<0.001. Scale bar: 20 μm. (e) Enteric neurons were cultured in the presence or absence of GDNF (48 h) and medium was assayed for NPY and PYY by ELISA. GDNF induced a significant release of NPY but not PYY into the culture medium compared to vehicle. Results are mean + S.E., n=3, ** = p<0.01. P value is with respect to neurons cultured in the presence of vehicle.
GDNF-induced enteric neuronal proliferation and survival is mediated through NPY
We next examined the effect of knock down of NPY on GDNF mediated enteric neuronal survival and proliferation. Enteric neurons were transfected with control Cyclophilin B or NPY-specific siRNA for 24 h in the presence of vehicle. Neurons were subsequently cultured for 24 h in the presence or absence of GDNF and fixed to assess proliferation and apoptosis. The neurons were then stained for either Peripherin/BrdU or Peripherin/TUNEL. Treatment of enteric neurons with NPY siRNA, but not control Cyclophilin B siRNA, resulted in a reduction in proliferation and an increase in apoptosis in vehicle-treated and GDNF-treated enteric neurons. (Fig. 2a, b). Knock down of NPY with NPY siRNA was confirmed by staining for NPY using the NPY/Peripherin double- labeling method (Fig. 2c, d). These data suggest that NPY plays a significant role in GDNF-mediated survival and proliferation of enteric neurons.
Figure 2.
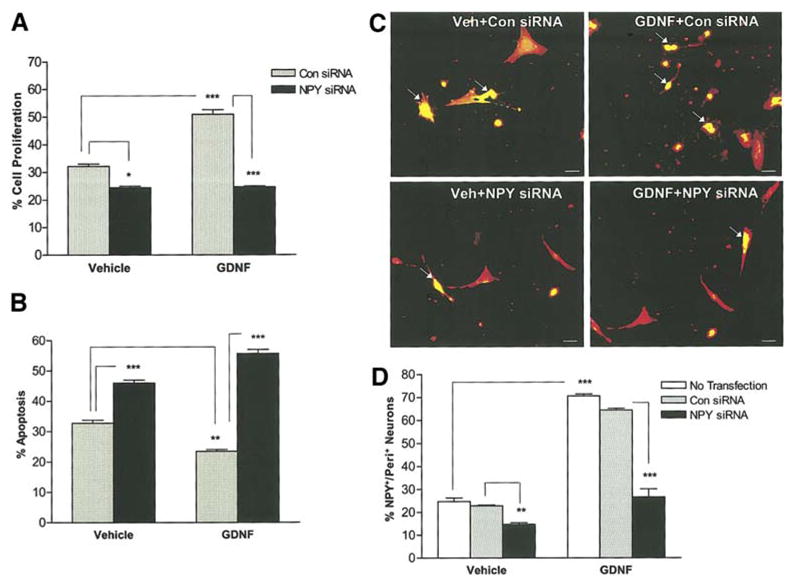
GDNF-induced proliferation and survival of enteric neurons is reduced in the presence of NPY siRNA. (a) Enteric neurons in the N2 medium were transfected with control Cyclophilin B or NPY-specific siRNA for 24 h and were subsequently cultured for 24 h in the presence or absence of GDNF. BrdU was added to the medium 24 h prior to the fixation of neurons and the % of BrdU+/peripherin+ neurons was determined to assess the proliferation, n=4. (b) Transfected enteric neurons were assessed for apoptosis using the double-labeling of TUNEL and peripherin, n=3. (c, d) To study the NPY siRNA specificity, transfected enteric neurons were stained for NPY (green)/Peripherin (red), n=3. Results are mean + S.E., *** = p<0.001, ** = p<0.01, * = p<0.05. Scale bar: 20 μm.
GDNF-induced proliferation and survival of enteric neurons is reduced in the presence of NPY-Y1 receptor antagonist and the PI-3-kinase inhibitor, LY294002
Since the GDNF-induced proliferation and survival of enteric neurons was mediated through NPY, we assessed the effects of NPY on enteric neuronal proliferation and survival by directly adding NPY to the culture medium. Embryonic enteric neurons treated with vehicle, NPY or GDNF in the presence or absence of either NPY-Y1 receptor antagonist (BIBP3226, 1 μM) or the PI-3-kinase inhibitor LY294002 (15 μM) were maintained in culture for 24 h, then fixed and assessed for apoptosis by the Peripherin/TUNEL method. Total cell number was determined through DAPI staining, and enteric neurons were specifically identified by peripherin staining. For the cell proliferation assessment, BrdU was added to the medium 24 h prior to fixation and was assessed by the BrdU/Peripherin method. Exposure of enteric neurons to NPY significantly increased proliferation, and this was inhibited in the presence of BIBP3226 and LY294002 (Fig. 3a, b). GDNF and NPY increased the total number of neurons per well (peripherin staining), and this was reflected in the total number of cells per well (DAPI staining) (Fig. 3c, d). NPY suppressed apoptosis similar to GDNF. This suppression of apoptosis was inhibited in the presence of BIBP3226 and LY294002 (Fig. 4 a, b). In the presence of the antagonist there is an increase in apoptosis in the vehicle treated conditions as the autocrine effects of NPY in the culture medium are inhibited. Addition of GDNF and NPY together did not have an additive effect on increasing the total number of neurons (number of neurons: Vehicle:1013 + 53; GDNF: 1663 +116; NPY:1466 + 62; GDNF + NPY: 1797 + 33, P>0.05 for GDNF + NPY vs. GDNF or NPY alone)or suppressing apoptosis over GDNF or NPY alone ( % apoptosis: Vehicle: 29.5 + 0.3; GDNF: 19.3 + 0.7%; NPY: 20.35 + 0.9 %; GDNF + NPY: 18.88 + 0.7 %, P>0.05 for GDNF +NPY vs. GDNF or NPY alone).
Figure 3.
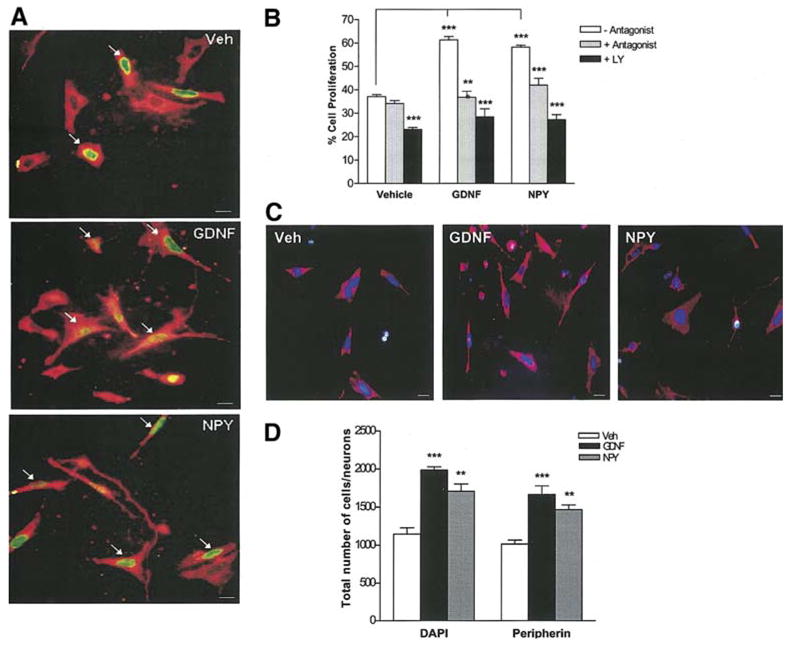
GDNF induces proliferation of enteric neurons through NPY. (a, b) Enteric neurons treated with vehicle, NPY or GDNF in the presence or absence of either the NPY-Y1 receptor antagonist (BIBP3226) or the PI-3-kinase inhibitor (LY294002) were maintained in culture for 48 h and fixed. BrdU was added to the medium 24 h prior to fixation. The % of BrdU+ (green)/peripherin+ (red) neurons was determined to assess proliferation. Arrows point to the BrdU+ neurons. Exposure of enteric neurons to NPY (48 h) significantly increased proliferation similar to GDNF. GDNF- and NPY- induced proliferation of enteric neurons was inhibited by both BIBP3226 and LY294002, n=4. Scale bar: 20 μm. (c, d) The total number of cells (DAPI-blue) and neurons (peripherin-red) per well was assessed under the stated culture conditions, n=2. Results are mean + S.E., *** = p<0.001, ** = p<0.01. P value is with respect to the neurons cultured in the absence of antagonist (b) or vehicle (d) in each respective condition.
Figure 4.
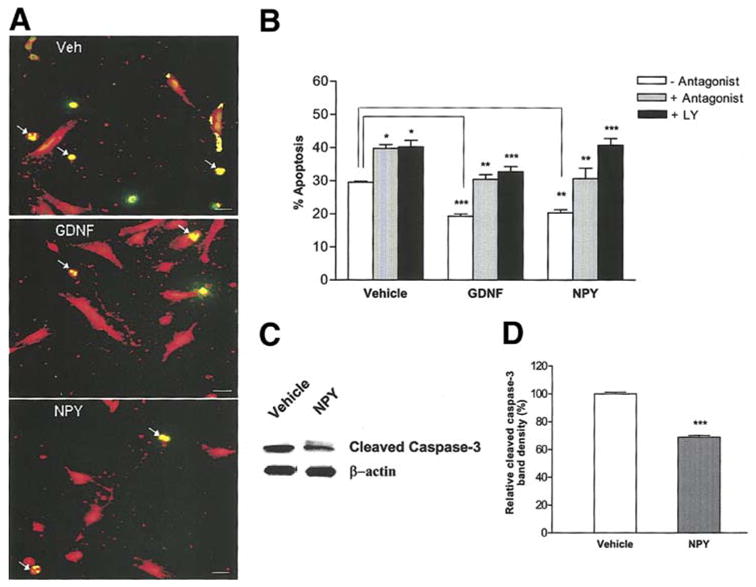
GDNF suppresses apoptosis of enteric neurons through NPY. (a, b) Enteric neurons treated with vehicle, NPY or GDNF in the presence or absence of BIBP3226 or LY 294002 for 24 h were assessed for apoptosis by the TUNEL (green)/peripherin (red) method. Exposure of enteric neurons to NPY or GDNF significantly reduced apoptosis compared to vehicle. This effect was inhibited by BIBP3226 and LY294002. Arrows show the apoptotic cells with condensed nuclei. Results are mean + S.E., n=3, *** = p<0.001, ** = p<0.01, * = p<0.05. Scale bar: 20 μm. P value is with respect to the neurons cultured in the absence of antagonist in each respective condition. (c, d) Western blot analysis of cleaved caspase-3 in enteric neurons cultured in the presence or absence of NPY for 24 h. β-actin was used as a loading control. There was a significant reduction in the expression of cleaved caspase-3 in NPY treated cells compared to vehicle. Results are ratio + S.E., n=3, *** = p<0.001.
As an additional method to measure apoptosis, caspase-3 cleavage was assessed. Enteric neurons were cultured in the presence or absence of NPY (1 μM) for 24 h and probed for cleaved caspase-3 by Western blotting. Western blot analysis showed less cleaved caspase-3 in NPY-treated cells compared to vehicle (Fig. 4c, d). These data suggest that GDNF induces the release of NPY, which mediates PI-3-kinase dependent survival and proliferation of enteric neurons, by acting in an autocrine manner through the Y1 receptor.
NPY increases pAkt levels in enteric neurons
The PI-3-kinase pathway is an important signal transduction pathway in GDNF-mediated enteric neuronal survival37. GDNF-induces phosphorylation of Akt, one of the major downstream targets of PI-3-kinase. We examined if this phosphorylation can by induced also be induced by NPY. Western blotting was performed using an antibody specific for pAkt. Figure 5 illustrates that NPY and GDNF significantly increased the phosphorylation of Akt compared to vehicle. This increased phosphorylation was lost when the cells were treated with the PI-3-Kinase inhibitor LY294002. These results suggest that NPY activates signal transduction pathways in a manner similar to GDNF.
Figure 5.
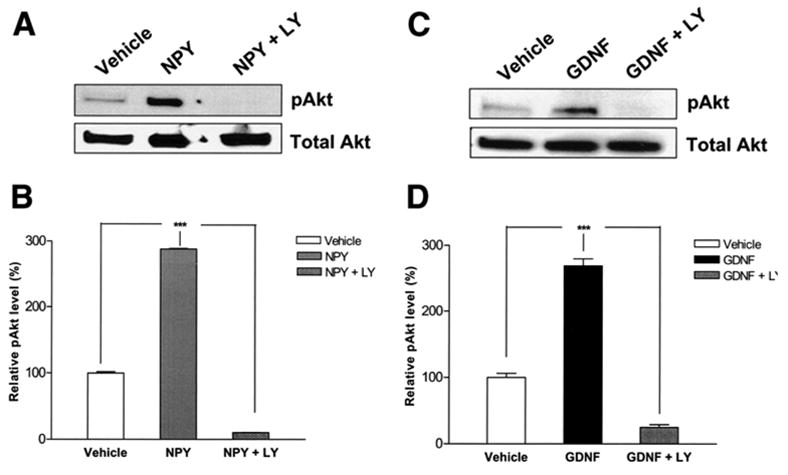
NPY activates the PI-3-kinase pathway. Cell lysates were obtained from enteric neurons treated with vehicle, NPY (a, b) or GDNF (c, d) with or without LY294002 for 30 min and Western blot was performed. GDNF and NPY significantly increased the phosphorylation of Akt compared to vehicle. This was inhibited in the presence of the PI-3-kinase inhibitor LY294002. Results are ratio (pAkt/Akt) ± S.E., n=3, *** = p<0.001.
Characterization of neurons induced by NPY
We next assessed the type of neurons that were induced by the addition of NPY to primary culture neurons. Primary neurons treated with vehicle, GDNF or NPY for 24 h were stained for nNOS or ChAT and co-stained with peripherin. The total number of ChAT+ and nNOS+ neurons per well were determined. We found that both GDNF and NPY induced a significant increase in the number of nNOS+ enteric neurons compared to vehicle-treated cells in a PI-3-kinase-dependent manner (Fig. 6a, b). No changes in ChAT+ cells were noted following either NPY or GDNF exposure for 24 h (Fig. 6c, d). In the presence of NPY siRNA there was a reduction in the percentage of nNOS+ neurons but not ChAT+ neurons (Fig. 6e, f). GDNF increases the peripherin+ neurons by approximately 70%. Approximately 40% of the neurons are nNOS+ and 45% are ChAT+. Thus we would predict that there is co-localization between the nNOS+ and ChAT+ neurons. We found that 17 + 3% of the nNOS+ neurons co-localize with ChAT+ neurons in the presence of GDNF.
Figure 6.
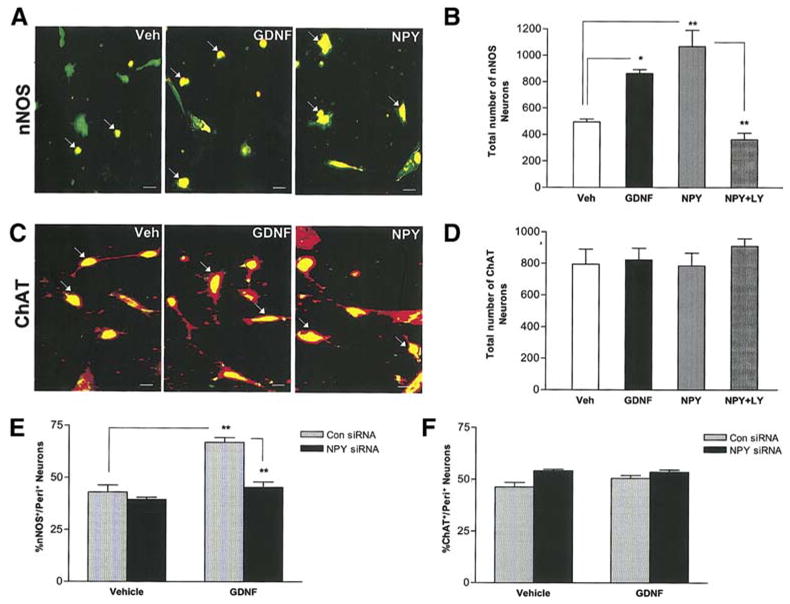
GDNF and NPY increase nNOS+ enteric neurons but not ChAT+ neurons. Primary enteric neurons were treated with vehicle, NPY, GDNF or NPY+LY294002 for 24 h, fixed and assessed for nNOS (red) and peripherin (green) (a, b) or ChAT (green) and peripherin (red) (c, d), n=4. Scale bar: 20 μm. (e, f) Enteric neurons were transfected with control Cyclophilin B siRNA or NPY specific siRNA for 24 h and then cultured for an additional 24 h in the presence or absence of GDNF, fixed and assessed for nNOS and ChAT, n=3. Results are mean ± S.E., ** = p<0.01, * = p<0.05.
NPY expressing neurons co-localize with nNOS staining neurons
We examined the co-localization of NPY with nNOS+ and ChAT+ neurons. We found that NPY co-localizes with about 77% of nNOS+ neurons and 36% of ChAT+ neurons (Fig. 7). These findings are consistent with our observations that NPY induces an increase in nNOS+ neurons.
Figure 7.
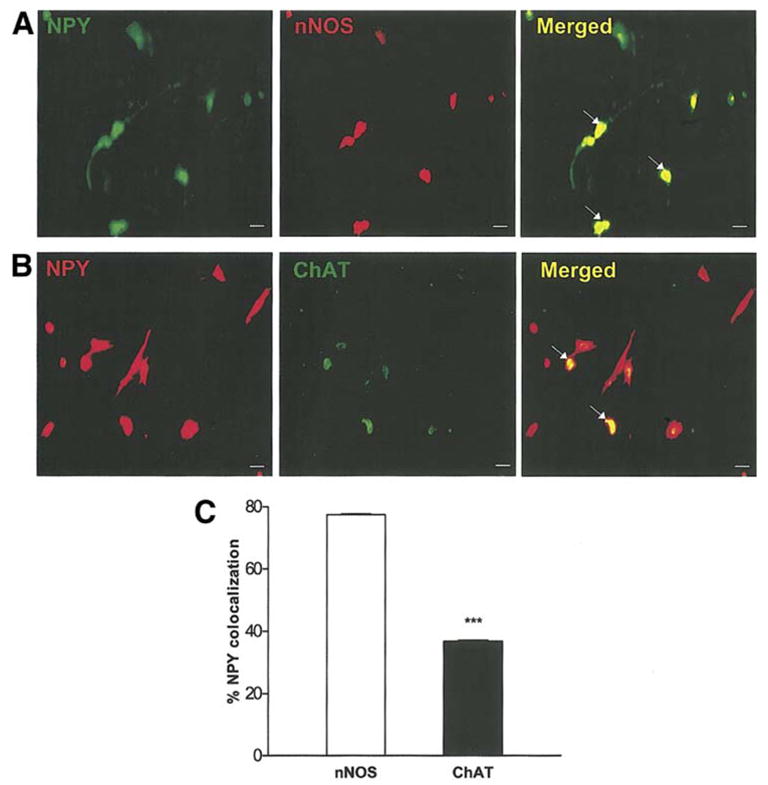
NPY co-localizes with nNOS. Primary enteric neurons were treated with GDNF for 24 h, fixed and assessed for nNOS (red) and peripherin (green) (a), or ChAT (green) and peripherin (red) (b). (c) Percent NPY colocalization with nNOS or ChAT is shown. Results are mean ± S.E., n=3, *** = p<0.001. Scale bar: 20 μm.
NPY has differential effects on proliferation and apoptosis at different developmental stages
The developmental expression of NPY was assessed in rat embryonic stages E12.5 and E16.5. As seen in Fig. 8a we observed the expression of NPY in enteric neurons of the proximal intestine as early as E12.5. We next assessed the effect of GDNF and NPY on apoptosis and proliferation from enteric neurons obtained at different developmental stages. In our culture system, we found that NPY affects proliferation in the early stages (E12.5) and apoptosis in the later embryonic stages (E16.5) (Fig 8 b, c). This effect of NPY is similar to the effect of GDNF noted by Chalazonitis et al 46 who demonstrated an effect of GDNF on proliferation at the earlier developmental stages and on apoptosis at the later developmental stages46. We found that both GDNF and NPY caused an increase in the number of nNOS neurons beginning at developmental stage E12.5 (Fig 8d).
Figure 8.
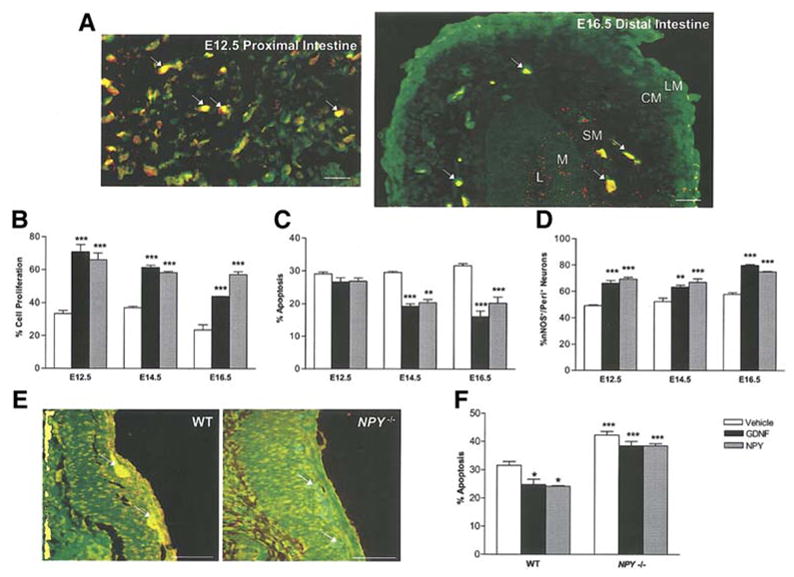
Expression and effects of NPY at different developmental stages. (a) NPY is expressed in the proximal intestine of E12.5 rat embryos and extends into the distal intestine in E16.5 rat embryos. NPY is stained with cy3 (red) and peripherin with FITC (green). Arrows show the NPY+ ganglia in E12.5 and E16.5 gut. In the E16.5 intestine the intestinal layers are labeled L (lumen), M (mucosa), SM (submucosal), CM (circular muscle layer), LM (longitudinal muscle layer). Scale bar for (a): 20 μm. (b) The effect of GDNF and NPY on apoptosis and proliferation was assessed in rat embryos at different developmental stages. NPY affects proliferation more in the early stages and apoptosis in the later embryonic stages. (d) nNOS neurons were assessed by immunostaining at different developmental stages. Both GDNF and NPY caused an increase in the number of nNOS+ neurons starting from developmental stage E12.5. (e) Immunohistochemistry for NPY was performed to demonstrate the absence of NPY expression in NPY −/− mice. The ganglia are stained with peripherin (green) and NPY (red). The overlap of red and green is seen in NPY+ neurons. (f) GDNF did not suppress the apoptosis in enteric neurons cultured from NPY −/− mice. The effect of GDNF and NPY on apoptosis in enteric neurons obtained from NPY−/− mice was assessed by the peripherin/TUNEL method. Results are mean + S.E., *** = p<0.001, ** = p<0.01, * = p<0.05. Scale bar for (e): 100 μm. P value is with respect to the neurons cultured in the presence of vehicle in each respective condition.
GDNF suppression of apoptosis is lost in enteric neurons isolated from NPY −/− mice
We compared the effects of GDNF on enteric neurons isolated from embryonic (E13.5), WT and NPY−/− mice. Lack of NPY expression in NPY−/− mice was demonstrated by immunohistochemistry using an antibody to NPY (Fig. 8e). We assessed the effect of GDNF and NPY on apoptosis in WT and NPY −/− mice enteric neurons. GDNF suppressed apoptosis in enteric neurons from WT mice, but not NPY −/− mice (Fig. 8 f).
NPY−/− mice have reduced inhibitory myenteric neurons in the small bowel and colon compared to WT mice
Having demonstrated the effects of NPY in primary enteric neuronal culture, we next assessed the effect of the lack of NPY expression on enteric neuronal numbers using the NPY −/− mouse model. Proximal colon from 4 week old mice was obtained from WT and NPY −/− mice, fixed and stained. The number of NADPH-diaphorase+ inhibitory neurons and large fibers were significantly decreased in NPY −/− mice compared to WT (Fig. 9a, b, c). No change was noted in the number of ChAT+ excitatory neurons and large fibers in the NPY −/− versus WT mice colon (Fig. 9d, e, f). Total number of neurons was assessed by peripherin staining. The number of peripherin+ neurons was decreased in the colon of NPY −/− mice compared to WT (Fig. 9g, h).
Figure 9.
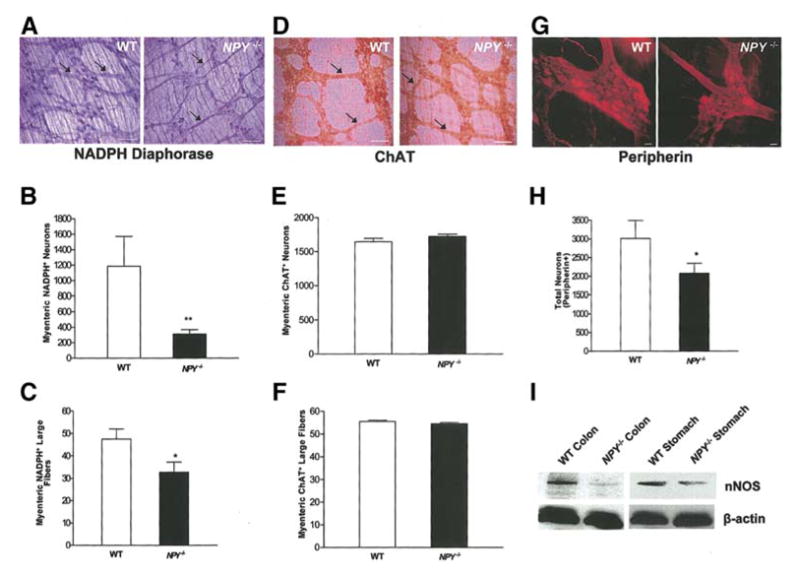
NPY−/− mice have reduced colonic nNOS+ containing neurons compared to WT mice. (a, b, c) Longitudinal muscle strips along with myenteric plexus from mouse colon were fixed and stained for nNOS+ neurons using the NADPH-diaphorase staining. The nNOS+ neurons and large fibers (arrows) of the myenteric plexus were significantly decreased in the colon of the NPY−/− mice compared to WT. (d, e, f) ChAT+ neurons and large fibers (arrows) of the myenteric plexus were similar in the NPY−/− and WT colon. Total number of neurons was assessed by peripherin staining (g, h). (i) nNOS expression in WT and NPY −/− mouse stomach and colon was assessed by Western blot. Scale bar: 100 μm (a, d) and 20 μm (g). Results are mean + S.E., n=6 in each group, ** = p<0.01, * = p<0.05.
We also assessed the expression of nNOS in the stomach and colon obtained from WT and NPY −/− mice by Western blot analysis. We found a reduction in nNOS expression in the colon and stomach of NPY −/− compared to the WT mice (Fig. 9i).
Gastric emptying, intestinal transit and colonic muscle relaxation in response to electrical field stimulation in WT and NPY−/− mice
Liquid gastric emptying and intestinal transit were assessed in WT and NPY−/− mice using methylene blue. Gastric emptying at 15 minutes after dye administration was significantly higher while intestinal transit was lower in NPY−/− mice compared to WT mice (Fig. 10 a, b). To assess the effects of lack of NPY expression in enteric neurons independent of the central effect of NPY, we performed isometric muscle relaxation in conjunction with electrical field stimulation. This technique allowed assessment of the numb−er and function of inhibitory neurons in the myenteric plexus. Percent relaxation was calculated by determining the difference between the maximal force generated in response to acetylcholine and the minimum force following electrical stimulation. Value was expressed as a percentage of the force generated in response to acetylcholine. Transmural stimulation (100 V/48 V, 20 Hz, 5 msec, 60 s) evoked relaxation of longitudinal muscle, and circular muscle (data not shown) strips of the proximal colon were significantly impaired in NPY−/− mice compared to WT (P<0.05, Fig. 10c, d). Pretreatment with L-NAME (10−4 mol/L, Fig. 10e) significantly reduced muscle relaxation (90.8 + 0.82 % reduction), indicating that muscle relaxation in our recording conditions were primarily mediated by nitric oxide production from the myenteric plexus. Relaxation induced by electrical field stimulation was significantly reduced (92 + 1.2 % reduction) in the presence of tetrodotoxin (10−7mol/L), indicating mediation through a neuronal pathway. These data support the presence of fewer numbers of inhibitory neurons in the NPY−/− mice.
Figure 10.
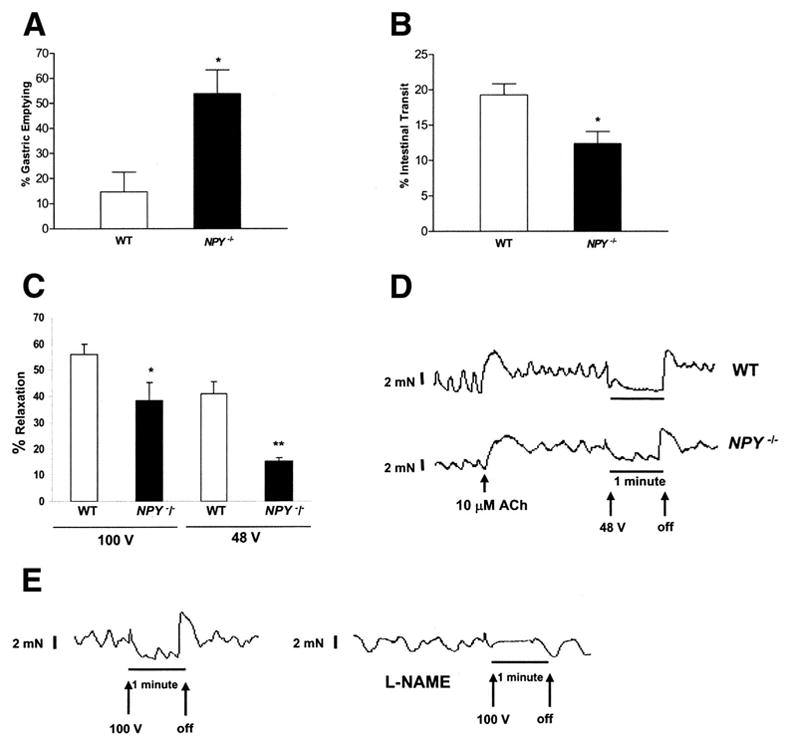
Altered motility in NPY−/− mice (a) Gastric emptying and intestinal transit was assessed in WT and NPY−/− mice. Gastric emptying was significantly higher in NPY−/− compared to WT mice. Results are mean + S.E., n=4, * = p<0.05. (b) Intestinal transit was significantly lower in NPY−/− compared to WT mice. Results are mean + S.E., n=4, * = p<0.05. (c) Assessment of colonic relaxation with electrical field stimulation in isolated colonic muscle strips containing the myenteric plexus. Transmural electrical field stimulation (100 V/48 V, 20 Hz, 5 msec, 60 s) evoked relaxation of the proximal colon was significantly impaired in NPY−/− compared to WT mice. Results are mean + S.E., n=4, * = p<0.05, ** = p<0.01. (d) Representative tracings of electrical field stimulation. The X-axis represents time and the Y-axis represents force in mN. The arrows represent when the electrical stimulation was turned on and off. (e). Effect of L-NAME on electrical field- induced stimulation. The isolated muscle strips from WT mice were pretreated with L-NAME or buffer alone, for 30 min and EFS was performed at 100V, 20 Hz, 5 msec, 60 s. The tracing shows the lack of relaxation in the presence of L-NAME.
Discussion
Growth factors like BDNF and NGF can modulate NPY expression32–35. BDNF-induces NPY production and secretion from neuronal cortical cells into the medium34. GDNF is an important growth factor for enteric neuronal survival and proliferation. The role of GDNF on modulating NPY expression has not been studied. In the present study, we have examined the effects of GDNF on NPY expression in enteric neurons. We characterized the effects of NPY on apoptosis and proliferation of enteric neurons. We observed that GDNF induced a significant increase in NPY mRNA expression and the release of NPY into the culture medium. Interestingly, PYY, a neuropeptide closely related to NPY, was unaffected by GDNF, suggesting a distinct regulation of NPY by GDNF. NPY is present both in the central and peripheral nervous system compared to PYY, which is mainly localized to the gastrointestinal tract 47, 48. This may explain the higher basal levels of PYY in our cultured enteric neurons.
Using NPY siRNA, as well as an antagonist to NPY-Y1 receptor, we show a role for NPY in mediating GDNF-induced enteric neuronal survival and proliferation. GDNF and NPY increase nNOS+ neurons in culture, but do not affect ChAT+ neurons. In NPY−/− mice we found a loss of nNOS+ myenteric neurons. Thus, we demonstrate a NPY-mediated autocrine mechanism to regulate enteric neuronal survival.
NPY exerts its action through several different receptor subtypes (Y1, Y2, Y4, Y5 and Y6). These are G-protein coupled receptors whose activation results in inhibition of adenylate cyclase activity. In order to delineate the involvement of a specific receptor, the Y1 receptor antagonist BIBP3226 was used in enteric neuronal cultures supplemented with NPY. The effects of NPY were abolished in the presence of the antagonist, confirming that the Y1 receptor plays an important role in mediating the effects of NPY on enteric neurons. NPY exerts hypertrophic effects on cardiomyocytes by the activating PI-3-kinase pathway25. The neuroproliferative effect of NPY in the dentate gyrus is Y1 receptor-mediated and involves extracellular signal-regulated kinase (ERK)1/2 activation28. The growth factor-induced expression of NPY can be dependent on either the PI-3-kinase or ERK-dependent pathways. GDNF is known to promote the survival of enteric neurons through the PI-3-kinase pathway37. The PI-3 kinase/Akt/FOXO signaling pathway plays a critical role in ENS precursor survival and neurite extension37. We now demonstrate that similar to GDNF, NPY also activates the PI-3-kinase pathway in enteric neurons. Although both GDNF and NPY were observed to act via the PI-3-kinase pathway, the addition of both compounds into the medium had little additive effect on survival/proliferation of enteric neurons in culture. The percent survival or proliferation rates for enteric neurons in the presence of GDNF or NPY alone were comparable to that in presence of both these growth factors. The percent survival rates for enteric neurons under our culture conditions were higher than what has been previously reported 8 and this is due to different plating density as well as different culture medium supplements.
Our findings are similar to those obtained with GDNF+/− mice studies2, suggesting that GDNF mediates the proliferation and survival of enteric neurons in part through NPY and the PI-3-kinase pathway, but other signaling pathways also contribute to enteric nervous system development. The reduction in nNOS+ neurons is modest and reflects the fact that other compensatory pathways might prevent a more dramatic reduction in nNOS+ neurons. Unlike the GDNF+/− mice, the NPY−/− mice do not have an obvious macroscopic bowel phenotype. A number of studies have identified increased NPY innervation in the aganglionic bowel of patients with Hirschsprung’s disease49–51. We believe this may be a compensatory response in the aganglionic segment, and this increased NPY may promote the extrinsic hyperinnervation that has been noted in Hirschsprung’s disease52. The GDNF−/− mice die at birth and these mice completely lack the enteric nervous system10. The NPY−/− mice have specific loss of the nNOS containing neurons.
NPY is broadly expressed in the developing nervous system and is maintained at moderately high levels in the adult, although its actions vary according to the target cell. The inhibitory influence of NPY neurons is consistent with the established ability of NPY to inhibit acetylcholine release in isolated nerve/muscle preparations53. Our in vivo study shows faster gastric emptying and impaired intestinal transit in NPY−/− mice compared to WT. These data reflect the role of NPY in the central nervous system. The peripheral effects of lack of NPY expression were the loss of inhibitory nNOS+ myenteric neurons. Even though the nNOS neurons were reduced in the NPY−/− mice, and the nNOS knockout mouse has delayed gastric emptying54–56, gastric emptying was accelerated in NPY−/− mice. We cannot fully explain this discrepancy but centrally administered NPY delays gastric emptying57 and so the phenotype could reflect a dominant effect of loss of central control by NPY compared with the effect of NPY on nNOS neurons.
The decrease in nNOS+ neurons in NPY −/− mice was further validated at the physiological level through isometric muscle recording experiments. Longitudinal and circular muscle strips from proximal colon of NPY −/− mice showed impaired /decreased relaxation on electrical field stimulation when compared to WT counterparts confirming the immunohistochemical studies. NPY co-localizes predominantly with nNOS neurons compared to ChAT neurons. Similar observations have been previously demonstrated 31,58. The immunostaining data was further confirmed by siRNA studies. We observed that NPY siRNA treatment resulted in significant decrease of nNOS neurons only. Further we have previously demonstrated that GDNF increases the number of nNOS+ neurons but not the ChAT+ neurons 43.
Analysis of NPY expression at various rat developmental stages showed NPY-expressing neurons as early as E12.5. NPY was present in the stomach and the proximal and distal gut. The effects of NPY were similar to the effects of GDNF as demonstrated by Chalazonitis et al 46. We found that NPY had profound effects on proliferation at early developmental stages and on survival at later developmental stages. Our findings demonstrate that GDNF, through NPY expression, can modulate enteric neuronal survival and proliferation and the specific neuronal subtypes in postnatal animals. However, the addition of both these trophic factors into the cell culture media had little additive effect, probably indicating a saturation of the common signaling pathway which is activated.
Recently, it was reported that NPY−/− mice were more sensitive than WT mice to methamphetamine-induced neuronal apoptosis in both enkephalin- and nitric oxide synthase-containing neurons, suggesting that NPY plays a general neuroprotective role within the striatum29. Reduced NPY content in the intestine has been demonstrated with aging, and this may contribute to age-associated gastrointestinal motility disorders. The ability of NPY to stimulate neuroproliferation in the ENS may be useful in fields such as stem cell therapy27. NPY analogs might be useful therapeutic agents against the neuronal losses29 that occur in the enteric nervous system, in disease conditions such as diabetes.
Acknowledgments
This work is supported by the NIH-KO8 DK067045 (S.S), DDRDC (DK064399), DK06411 (S.V.S.) and Crohn’s and Colitis Foundation of America Senior Research Award (S.V.S) and a Pilot and Feasibility Award from the Emory Center for Clinical and Molecular Nutrition. We would like to thank Kirk Easley, M.S., Associate Director, Biostatistics Consulting Center, Emory University for his assistance with statistical analysis. We would like to thank James Green, M.D, Ph.D for his advice on the ChAT immunostaining methods.
Abbreviations
- GDNF
Glial cell line-derived neurotrophic factor
- WT
Wild type
- NPY−/−
Neuropeptide Y knock-out
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J. Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol. 1998;200:116–29. doi: 10.1006/dbio.1998.8955. [DOI] [PubMed] [Google Scholar]
- 2.Gianino S, Grider JR, Cresswell J, Enomoto H, Heuckeroth RO. GDNF availability determines enteric neuron number by controlling precursor proliferation. Development. 2003;130:2187–2198. doi: 10.1242/dev.00433. [DOI] [PubMed] [Google Scholar]
- 3.Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–16. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 4.Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–60. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- 5.Barlow A, de Graaff E, Pachnis V. Enteric nervous system progenitors are coordinately controlled by the G protein-coupled receptor EDNRB and the receptor tyrosine kinase RET. Neuron. 2003;40:905–16. doi: 10.1016/s0896-6273(03)00730-x. [DOI] [PubMed] [Google Scholar]
- 6.Baloh RH, Enomoto H, Johnson EM, Jr, Milbrandt J. The GDNF family ligands and receptors - implications for neural development. Curr Opin Neurobiol. 2000;10:103–10. doi: 10.1016/s0959-4388(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 7.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 8.Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V. Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development. 1999;126:2785–97. doi: 10.1242/dev.126.12.2785. [DOI] [PubMed] [Google Scholar]
- 9.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–3. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 10.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–9. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 11.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–6. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–3. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 13.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM, Jr, Milbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–24. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 15.Allen YS, Adrian TE, Allen JM, Tatemoto K, Crow TJ, Bloom SR, Polak JM. Neuropeptide Y distribution in the rat brain. Science. 1983;221:877–9. doi: 10.1126/science.6136091. [DOI] [PubMed] [Google Scholar]
- 16.Danger JM, Tonon MC, Jenks BG, Saint-Pierre S, Martel JC, Fasolo A, Breton B, Quirion R, Pelletier G, Vaudry H. Neuropeptide Y: localization in the central nervous system and neuroendocrine functions. Fundam Clin Pharmacol. 1990;4:307–40. doi: 10.1111/j.1472-8206.1990.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 17.Wai SM, Kindler PM, Lam ET, Zhang A, Yew DT. Distribution of neuropeptide Y-immunoreactive neurons in the human brainstem, cerebellum, and cortex during development. Cell Mol Neurobiol. 2004;24:667–84. doi: 10.1023/B:CEMN.0000036404.39432.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YN, McDonald JK, Wyatt RJ. Immunocytochemical localization of neuropeptide Y-like immunoreactivity in adrenergic and non-adrenergic neurons of the rat gastrointestinal tract. Peptides. 1987;8:145–51. doi: 10.1016/0196-9781(87)90178-1. [DOI] [PubMed] [Google Scholar]
- 19.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–73. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 20.Fujimiya M, Itoh E, Kihara N, Yamamoto I, Fujimura M, Inui A. Neuropeptide Y induces fasted pattern of duodenal motility via Y(2) receptors in conscious fed rats. Am J Physiol Gastrointest Liver Physiol. 2000;278:G32–8. doi: 10.1152/ajpgi.2000.278.1.G32. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–16. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom PM. Mechanisms involved in colonic vasoconstriction and inhibition of motility induced by neuropeptide Y. Acta Physiol Scand. 1987;129:549–56. doi: 10.1111/j.1748-1716.1987.tb08096.x. [DOI] [PubMed] [Google Scholar]
- 23.Wager-Page SA, Ghazali B, Anderson W, Veale WL, Davison JS. The peripheral modulation of duodenal and colonic motility in rats by the pancreatic polypeptide-fold family: neuropeptide Y, peptide YY, and pancreatic polypeptide. Peptides. 1993;14:153–60. doi: 10.1016/0196-9781(93)90023-a. [DOI] [PubMed] [Google Scholar]
- 24.Sokolowski MB. NPY and the regulation of behavioral development. Neuron. 2003;39:6–8. doi: 10.1016/s0896-6273(03)00398-2. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg Y, Taimor G, Piper HM, Schluter KD. Intracellular signaling leads to the hypertrophic effect of neuropeptide Y. Am J Physiol. 1998;275:C1207–15. doi: 10.1152/ajpcell.1998.275.5.C1207. [DOI] [PubMed] [Google Scholar]
- 26.Zukowska-Grojec Z, Karwatowska-Prokopczuk E, Fisher TA, Ji H. Mechanisms of vascular growth-promoting effects of neuropeptide Y: role of its inducible receptors. Regul Pept. 1998;75–76:231–8. doi: 10.1016/s0167-0115(98)00073-1. [DOI] [PubMed] [Google Scholar]
- 27.Hansel DE, Eipper BA, Ronnett GV. Neuropeptide Y functions as a neuroproliferative factor. Nature. 2001;410:940–4. doi: 10.1038/35073601. [DOI] [PubMed] [Google Scholar]
- 28.Howell OW, Doyle K, Goodman JH, Scharfman HE, Herzog H, Pringle A, Beck-Sickinger AG, Gray WP. Neuropeptide Y stimulates neuronal precursor proliferation in the post-natal and adult dentate gyrus. J Neurochem. 2005;93:560–70. doi: 10.1111/j.1471-4159.2005.03057.x. [DOI] [PubMed] [Google Scholar]
- 29.Thiriet N, Deng X, Solinas M, Ladenheim B, Curtis W, Goldberg SR, Palmiter RD, Cadet JL. Neuropeptide Y protects against methamphetamine-induced neuronal apoptosis in the mouse striatum. J Neurosci. 2005;25:5273–9. doi: 10.1523/JNEUROSCI.4893-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–50. [PubMed] [Google Scholar]
- 31.Peaire AE, Krantis A, Staines WA. Distribution of the NPY receptor subtype Y1 within human colon: evidence for NPY targeting a subpopulation of nitrergic neurons. J Auton Nerv Syst. 1997;67:168–75. doi: 10.1016/s0165-1838(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 32.Takei N, Sasaoka K, Higuchi H, Endo Y, Hatanaka H. BDNF increases the expression of neuropeptide Y mRNA and promotes differentiation/maturation of neuropeptide Y-positive cultured cortical neurons from embryonic and postnatal rats. Brain Res Mol Brain Res. 1996;37:283–9. doi: 10.1016/0169-328x(95)00299-8. [DOI] [PubMed] [Google Scholar]
- 33.Barnea A, Cho G, Lu G, Mathis M. Brain-derived neurotrophic factor induces functional expression and phenotypic differentiation of cultured fetal neuropeptide Y-producing neurons. J Neurosci Res. 1995;42:638–47. doi: 10.1002/jnr.490420506. [DOI] [PubMed] [Google Scholar]
- 34.Barnea A, Roberts J. Induction of functional and morphological expression of neuropeptide Y (NPY) in cortical cultures by brain-derived neurotrophic factor (BDNF): evidence for a requirement for extracellular-regulated kinase (ERK)-dependent and ERK-independent mechanisms. Brain Res. 2001;919:57–69. doi: 10.1016/s0006-8993(01)02999-7. [DOI] [PubMed] [Google Scholar]
- 35.Barreto-Estrada JL, Medina-Ortiz WE, Garcia-Arraras JE. The morphological and biochemical response of avian embryonic sympathoadrenal cells to nerve growth factor is developmentally regulated. Brain Res Dev Brain Res. 2003;144:1–8. doi: 10.1016/s0165-3806(03)00129-9. [DOI] [PubMed] [Google Scholar]
- 36.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EMJ. Neurturin shares receptors and signal transduction pathways with glial cell line- derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivasan S, Anitha M, Mwangi S, Heuckeroth RO. Enteric neuroblasts require the phosphatidylinositol 3-kinase/Akt/Forkhead pathway for GDNF-stimulated survival. Mol Cell Neurosci. 2005;29:107–19. doi: 10.1016/j.mcn.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRα-1 in vitro and in vivo. Developmental Biology. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Goodchild AK, Pilowsky PM. Effect of haemorrhage on the expression of neurotransmitter-related genes in rat ventrolateral medulla: a quantitative real-time RT-PCR study. Brain Res Mol Brain Res. 2003;114:46–54. doi: 10.1016/s0169-328x(03)00131-1. [DOI] [PubMed] [Google Scholar]
- 40.Grouzmann E, Aubert JF, Waeber B, Brunner HR. A sensitive and specific two-site, sandwich-amplified enzyme immunoassay for neuropeptide Y. Peptides. 1992;13:1049–54. doi: 10.1016/0196-9781(92)90004-m. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasan S, Bernal-Mizrachi E, Ohsugi M, Permutt MA. Glucose promotes pancreatic islet beta-cell survival through a PI 3-kinase/Akt-signaling pathway. Am J Physiol Endocrinol Metab. 2002;283:E784–93. doi: 10.1152/ajpendo.00177.2002. [DOI] [PubMed] [Google Scholar]
- 42.Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–21. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- 43.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Jr, Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22:253–63. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–44. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 46.Chalazonitis A, Rothman TP, Chen J, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRalpha-1 in vitro and in vivo. Dev Biol. 1998;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- 47.Greeley GH, Jr, Hill FL, Spannagel A, Thompson JC. Distribution of peptide YY in the gastrointestinal tract of the rat, dog, and monkey. Regul Pept. 1987;19:365–72. doi: 10.1016/0167-0115(87)90178-9. [DOI] [PubMed] [Google Scholar]
- 48.El-Salhy M, Grimelius L, Wilander E, Ryberg B, Terenius L, Lundberg JM, Tatemoto K. Immunocytochemical identification of polypeptide YY (PYY) cells in the human gastrointestinal tract. Histochemistry. 1983;77:15–23. doi: 10.1007/BF00496632. [DOI] [PubMed] [Google Scholar]
- 49.Hamada Y, Bishop AE, Federici G, Rivosecchi M, Talbot IC, Polak JM. Increased neuropeptide Y-immunoreactive innervation of aganglionic bowel in Hirschsprung’s disease. Virchows Arch A Pathol Anat Histopathol. 1987;411:369–77. doi: 10.1007/BF00713383. [DOI] [PubMed] [Google Scholar]
- 50.Larsson LT, Malmfors G, Sundler F. Neuropeptide Y, calcitonin gene-related peptide, and galanin in Hirschsprung’s disease: an immunocytochemical study. J Pediatr Surg. 1988;23:342–5. doi: 10.1016/s0022-3468(88)80203-3. [DOI] [PubMed] [Google Scholar]
- 51.Larsson LT, Malmfors G, Ekblad E, Ekman R, Sundler F. NPY hyperinnervation in Hirschsprung’s disease: both adrenergic and nonadrenergic fibers contribute. J Pediatr Surg. 1991;26:1207–14. doi: 10.1016/0022-3468(91)90336-r. [DOI] [PubMed] [Google Scholar]
- 52.Koch TR, Roddy DR, Carney JA, Telander RL, Go VL. Distribution, quantitation, and origin of immunoreactive neuropeptide Y in the human gastrointestinal tract. Regul Pept. 1988;21:309–19. doi: 10.1016/0167-0115(88)90014-6. [DOI] [PubMed] [Google Scholar]
- 53.Grider JR, Langdon LE. Physiological role of neuropeptide Y in the regulation of the ascending phase of the peristaltic reflex. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1139–46. doi: 10.1152/ajpgi.00082.2003. [DOI] [PubMed] [Google Scholar]
- 54.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–73. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 55.Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, Ferris CD. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–84. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Micci MA, Kahrig KM, Simmons RS, Sarna SK, Espejo-Navarro MR, Pasricha PJ. Neural stem cell transplantation in the stomach rescues gastric function in neuronal nitric oxide synthase-deficient mice. Gastroenterology. 2005;129:1817–24. doi: 10.1053/j.gastro.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 57.Ishiguchi T, Amano T, Matsubayashi H, Tada H, Fujita M, Takahashi T. Centrally administered neuropeptide Y delays gastric emptying via Y2 receptors in rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1522–30. doi: 10.1152/ajpregu.2001.281.5.R1522. [DOI] [PubMed] [Google Scholar]
- 58.Elfvin LG, Holmberg K, Emson P, Schemann M, Hokfelt T. Nitric oxide synthase, choline acetyltransferase, catecholamine enzymes and neuropeptides and their colocalization in the anterior pelvic ganglion, the inferior mesenteric ganglion and the hypogastric nerve of the male guinea pig. J Chem Neuroanat. 1997;14:33–49. doi: 10.1016/s0891-0618(97)10010-2. [DOI] [PubMed] [Google Scholar]


