Abstract
Although hypothalamic-pituitary-adrenal axis activation is generally considered to be the hallmark of the stress response, many of the same stimuli that initiate this response also activate the locus coeruleus-norepinephrine system. Given its functional attributes, the parallel engagement of the locus coeruleus-norepinephrine system with the hypothalamic-pituitary-adrenal axis serves to coordinate endocrine and cognitive limbs of the stress response. The elucidation of stress-related afferents to the locus coeruleus and the electrophysiological characterization of these inputs are revealing how the activity of this system is fine-tuned by stressors to facilitate adaptive cognitive responses. Emerging from these studies, is a picture of complex interactions between the stress-related neuropeptide, corticotropin-releasing factor (CRF), endogenous opioids and the excitatory amino acid neurotransmitter, glutamate. The net effect of these interactions is to adjust the activity and reactivity of the locus coeruleus-norepinephrine system such that state of arousal and processing of sensory stimuli are modified to facilitate adaptive behavioral responses to stressors.
This review begins with an introduction to the basic anatomical and physiological characteristics of locus coeruleus neurons. The concept that locus coeruleus neurons operate through two activity modes, i.e., tonic vs. phasic, that determine distinct behavioral strategies is emphasized in light of its relevance to stress. Anatomical and physiological evidence are then presented suggesting that interactions between stress-related neurotransmitters that converge on locus coeruleus neurons regulate shifts between these modes of discharge in response to the challenge of a stressor. This review focuses specifically on the locus coeruleus because it is the major source of norepinephrine to the forebrain and has been implicated in behavioral and cognitive aspects of stress responses.
Keywords: corticotropin-releasing factor, opioids, norepinephrine, locus coeruleus
1. Attributes of the locus coeruleus-norepinephrine system
1.1 Anatomy: a broad-reaching system
The unique anatomical characteristics of the locus coeruleus have been described in detail and are the subject of several reviews (Aston-Jones et al., 1995; Foote et al., 1983; Grzanna and Molliver, 1980; Swanson, 1976; Swanson and Hartman, 1975; Waterhouse et al., 1998). A distinguishing feature is the broad-reaching, highly collateralized projection system that arises from this tightly clustered nucleus of norepinephrine-containing neurons in the pons. The locus coeruleus-norepinephrine system innervates the entire neuraxis. It is a primary source of norepinephrine in the forebrain and the sole source of norepinephrine in the cortex and hippocampus, regions that govern cognition, memory and complex behaviors, as well as the cerebellum. A certain topographical organization of neurons within the locus coeruleus has been described with respect to neurochemicals that are co-localized with norepinephrine, as well as efferent targets (Loughlin et al., 1986; Mason and Fibiger, 1979). Nonetheless, it is relatively homogeneous in that all neurons synthesize norepinephrine and many have collateralized projections to functionally diverse targets. This feature, along with the homogeneous physiological characteristics of locus coeruleus neurons (described below) gives the system the potential to broadly influence neuronal activity throughout the brain.
1.2 Physiology: Dual modes of activity
Locus coeruleus neurons exhibit both tonic and phasic activity and the mode of activity may function to determine behavioral strategy in particular situations or environments (Aston-Jones and Cohen, 2005). Locus coeruleus tonic discharge rate is positively correlated to the state of arousal (Aston-Jones and Bloom, 1981a; Foote et al., 1980). However, more than a correlation, selective pharmacological manipulation of tonic locus coeruleus discharge is sufficient to change forebrain electroencephalographic indices of arousal, underscoring the influence of this system on forebrain neuronal activity (Berridge and Foote, 1991; Berridge et al., 1993). Selective locus coeruleus activation desynchronizes cortical electroencephalographic activity and induces hippocampal theta rhythm, consistent with active waking. Conversely, selective inactivation has opposing effects, resulting in a shift to synchronous high amplitude, low frequency activity resembling sleep. This physiological attribute has suggested a role for the locus coeruleus in promoting and maintaining arousal. Indeed, increases in tonic locus coeruleus discharge rate are required for forebrain electroencephalographic activation in response to certain physiological stressors (Page et al., 1993).
Locus coeruleus neurons are phasically activated by discrete sensory stimuli of many modalities (Aston-Jones and Bloom, 1981b; Foote et al., 1980). The pattern of elicited discharge is characterized by a brief excitatory component followed by a longer duration of inhibition (e.g., Fig. 1). This is consistent with evidence for a role of excitatory amino acid neurotransmission in locus coeruleus sensory responses (Ennis et al., 1992), as these agents produce a robust, but brief activation of locus coeruleus neurons. Microdialysis studies correlating locus coeruleus discharge rate and pattern to extracellular forebrain norepinephrine predict that norepinephrine release in terminal fields is more efficient when locus coeruelus neurons are in a phasic mode of discharge (Florin-Lechner et al., 1996). In the phasic mode, locus coeruleus neurons discharge in a synchronous manner, i.e., en masse, and this has been attributed to electrotonic coupling through gap junctions between dendrites outside of the nucleus, in the peri-coerulear region (Ishimatsu and Williams, 1996). In contrast to phasic discharge, the tonic mode of discharge is favored by uncoupling and this has been verified in computational models (Usher et al., 1999).
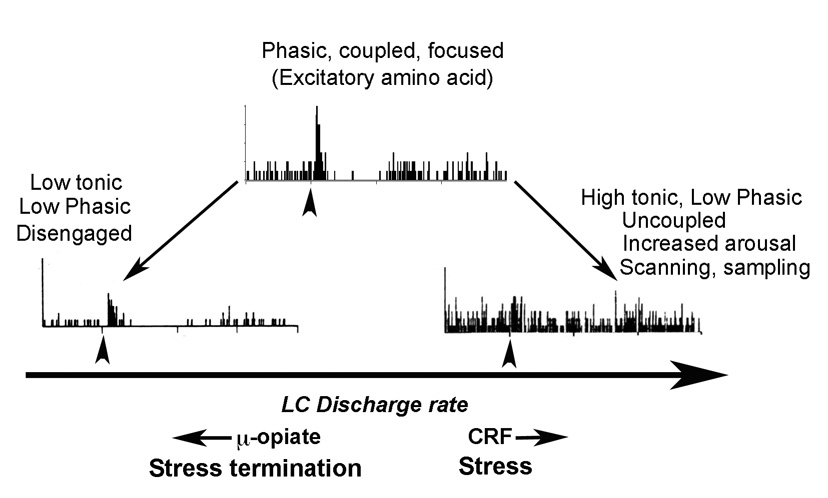
Figure 1.
Schematic depicting different modes of locus coeruleus activity and the relationship between tonic and phasic activity. Representative poststimulus time histograms (PSTHs) are shown that indicate neuronal activation (ordinates) in response to repeated presentations of a brief sensory stimulus (arrowhead). The abscissae of the histograms indicate time before and after the stimulus. When locus coeruleus tonic activity is low (left), the response to sensory stimuli is relatively low and this is associated with drowsiness and disengagement from the environment. There is an optimal level of tonic locus coeruleus discharge rate at which the response to sensory stimuli is high (Phasic). This is associated with electrotonic coupling of locus coeruleus neurons, focused attention and the maintenance of ongoing behavior. Increases in tonic discharge rate above this optimal level result in a loss of selective sensory responses. This is associated with uncoupling, increased arousal, scanning attention and sampling of behaviors. By increasing tonic discharge, CRF shifts the mode of locus coeruleus activity towards the right of this spectrum. This is predicted to occur during stress. During stress termination, endogenous opioids acting at μ–opiate receptors in the locus coeruleus shift the mode of activity towards the left. Excitatory amino acid neurotransmission in the locus coeruleus may favor the phasic mode.
The two patterns of locus coeruleus discharge (i.e., tonic vs. phasic) favor different modes of signal processing and/or behaviors and which is operative depends on predominant afferent drive as well as the degree of electrotonic coupling (Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003) (Fig. 1). The magnitude of phasic activity is related to the level of ongoing tonic activity such that it is optimal at moderate levels of tonic activity and is diminished either when tonic activity is low (as in slow wave sleep) or when tonic activity exceeds a moderate level (as in stress) (Aston-Jones et al., 2000; Usher et al., 1999). This has supported the idea that the locus coeruleus promotes and maintains an appropriate level of arousal that is necessary for optimally processing sensory information in the environment. During times of elevated tonic locus coeruleus activity, as in stress, the loss of the phasic sensory response may signify a shift towards scanning diverse stimuli in the environment, a response that may be most adaptive in a challenging environment. Recently this sensory-based interpretation of locus coeruleus operation has evolved to one that is more behaviorally based (Aston-Jones and Cohen, 2005). Thus, locus coeruleus recordings from monkeys during the performance of specific tasks suggest that locus coeruleus phasic activity is more closely linked to behavioral outcome in response to task-related stimuli, rather than the sensory component of the stimuli (Aston-Jones and Cohen, 2005). These studies suggest that phasic locus coeruleus discharge facilitates behavioral responses to specific tasks. In contrast, a shift towards higher tonic activity and diminished phasic activity is suggested to promote searching for alternative tasks that may provide better solutions in the midst of a changing environment or when the present behavior is not optimally adaptive. The ability of locus coeruleus neurons to readily switch between modes of neuronal activity would be advantageous for rapidly adjusting behavior in response to a stressor or after stressor termination.
2. The locus coeruleus-norepinephrine system and stress
The locus coeruleus-norepinephrine system is consistently activated by diverse stressors, as indicated by many different endpoints. Early studies using shock as a stressor demonstrated stress-induced increases in norepinephrine metabolites and turnover in locus coeruleus forebrain targets that were abolished by locus coeruleus lesions (Cassens et al., 1981; Cassens et al., 1980; Korf et al., 1973; Thierry et al., 1968). Importantly, unpredictable shock was more effective than shock that was signaled by stimuli (Tsuda et al., 1989). That immunological challenge produced by interleukin-2 injection similarly increased norepinephrine utilization in brain regions targeted by locus coeruleus projections, underscored the generalized nature of the activation (Lacosta et al., 2000). More direct indices of norepinephrine release in locus coeruleus target regions, such as microdialysis, have yielded results that agree with turnover studies. Thus, restraint, tailshock, auditory stress and hypotensive stress all increase extracellular norepinephrine levels in locus coeruleus terminal regions (Abercrombie et al., 1988; Britton et al., 1992; Smagin et al., 1994).
Additional endpoints of activation of the locus coeruleus-norepinephrine system, including expression of tyrosine hydroxylase mRNA and protein or c-fos mRNA or protein, consistently suggest that this system is activated by a variety of stressors, including restraint, shock, hypotension, swim, immune challenge, water avoidance stress and social stress (Beck and Fibiger, 1995; Bonaz and Tache, 1994; Campeau and Watson, 1997; Chan and Sawchenko, 1995; Chang et al., 2000; Dun et al., 1995; Duncan et al., 1993; Funk and Amir, 2000; Graham et al., 1995; Ishida et al., 2002; Kollack-Walker et al., 1997; Makino et al., 2002; Rusnak et al., 2001; Sabban and Kvetnansky, 2001; Smith et al., 1992; Smith et al., 1991).
3. The locus coeruleus and CRF
3.1 CRF innervation of the locus coeruleus
Among the neurochemically-identified afferents to the locus coeruleus, CRF has stood out as a likely mediator of stress-elicited locus coeruleus activation. CRF was originally characterized as the hypothalamic neurohormone that initiates the cascade of responses linked to corticosteroid secretion (Rivier et al., 1982; Vale et al., 1981). However, the widespread distribution of CRF-containing axon terminals and CRF binding sites, as well as the diverse behavioral and physiological effects produced by intracerebral CRF administration support the concept that it serves as a modulator of neuronal activity to mediate a host of behavioral and physiological effects (Chalmers et al., 1996; Owens and Nemeroff, 1991). A confluence of evidence supports a neurotransmitter role for CRF in the locus coeruleus and moreover suggests that this mediates locus coeruleus activation by stressors and serves to facilitate cognitive and behavioral responses to stress.
CRF-immunoreactive fibers sparsely innervate the nuclear core of the locus coeruleus but densely innervate the peri-coerulear regions into which locus coeruleus dendrites extend (Valentino et al., 1992). Electron microscopic analyses of this region identified anatomical substrates for both direct and indirect actions of CRF on locus coeruleus neurons (Van Bockstaele et al., 1996). Thus, identified synaptic specializations between CRF-immunoreactive axon terminals and locus coeruleus dendrites, the majority of which are asymmetric (excitatory type), indicate a direct modulation of locus coeruleus activity. Additionally, CRF terminals that are apposed to unlabeled terminals that form synaptic specializations with locus coeruleus dendrites suggest an indirect modulation of locus coeruleus activity via presynaptic actions. This may be an anatomical substrate for the regulation of locus coeruleus sensory responses (described below).
3.2 Co-localization with other neurochemicals
Consistent with the majority of CRF axon terminals forming excitatory-type synaptic specializations with locus coeruleus dendrites, there is a substantial degree of co-localization of CRF and glutamate in axon terminals in the locus coeruleus region (Valentino et al., 2001). In addition to co-localization, individual CRF- and glutamate-immunoreactive terminals were often apposed to each other or found to converge onto common dendrites in the rostrolateral locus coeruleus dendritic zone. CRF is also co-localized with enkephalin in a population of axon terminals in the locus coeruleus region and there is evidence for convergence of individual CRF and enkephalin-containing axon terminals onto common locus coeruleus dendrites, providing an anatomical substrate for co-regulation of the locus coeruleus-norepinephrine system as described below (Tjoumakaris et al., 2003).
3.3 CRF receptors in the locus coeruleus
An on-going controversy regarding CRF regulation of the locus coeruleus relates to the inability of in situ hybridization studies to detect CRF receptor mRNA in locus coeruleus neurons (Van Pett et al., 2000). One explanation for this is that CRF interacts presynaptically with receptors on terminals within the locus coeruleus region whose cell bodies are distant from the locus coeruleus, although in vitro electrophysiological studies argue for a direct action of CRF on locus coeruleus neurons (Jedema and Grace, 2004). In contrast to in situ hybridization studies, electron microscopic studies have identified CRF receptor-immunoreactivity in locus coeruleus dendrites and demonstrated agonist-induced trafficking of the immunolabeled receptor (Reyes et al., 2006). Moreover, stress elicited cellular trafficking of the CRF receptor in locus coeruleus soma and dendrites that was attenuated by prior treatment with a selective CRF1 receptor antagonist, making it highly unlikely that the antibody was detecting a protein other than a CRF receptor (Reyes Soc Neurosci. Abstr. 2007). Thus, the difficulty in reconciling in situ hybridization studies of CRF receptor mRNA with other anatomical and functional evidence remains.
3.4 Circuitry linking CRF to the locus coeruleus
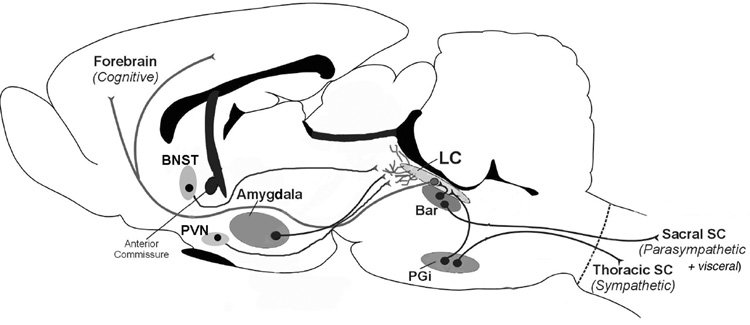
CRF afferents to the locus coeruleus include the central nucleus of the amygdala, bed nucleus of stria terminalis, paraventricular nucleus of the hypothalamus, Barrington’s nucleus and the nucleus paragigantocellularis (Reyes et al., 2005; Valentino et al., 1996; Valentino et al., 1992; Van Bockstaele et al., 1998; Van Bockstaele et al., 1999). The terminals from these afferents are topographically organized within the locus coeruleus region, such that CRF neurons from the nucleus paragigantocellularis and Barrington’s nucleus terminate in the core, whereas the central nucleus of the amygdala and bed nucleus of the stria terminalis terminate outside of the locus coeruleus core, in the rostrolateral peri-locus coeruleus, a locus coeruleus dendritic zone. CRF neurons of the paraventricular hypothalamic nucleus may project to both regions (Fig. 2).
Figure 2.
Schematic depicting the localization of CRF afferents to the locus coeruleus and sites of termination in the locus coeruleus region. CRF neurons from Barrington’s nucleus (Bar) and the nucleus paragigantocellularis (PGi) terminate in the nuclear core of the locus coeruleus. These nuclei also project to preganglionic parasympathetic and sympathetic neurons, respectively and thereby may coordinate autonomic activity with cognitive functions. The central nucleus of the amygdala, bed nucleus of the stria terminalis (BNST) and paraventricular nucleus of the hypothalamus (PVN) terminate outside of the nuclear core in the peri-coerulear region where locus coeruleus dendrites extend.
Combining retrograde labeling from the locus coeruleus with another target of a CRF-containing nucleus has provided information on the role of the locus coeruleus in certain complex functions. Studies such as this revealed that many CRF neurons in Barrington’s nucleus diverge to simultaneously innervate the locus coeruleus and preganglionic column of the lumbosacral spinal cord which provides parasympathetic input to the pelvic viscera (Valentino et al., 1996). Because it is positioned to co-regulate the locus coeruleus-norepinephrine system with the lumbosacral spinal parasympathetic system, this particular CRF-containing circuit may serve to coordinate central with visceral responses to pelvic visceral stimuli and may underlie the co-morbidity of pelvic visceral and behavioral symptoms observed in many stress-related disorders (e.g., functional bowel syndrome) (Valentino et al., 1999).
Using a similar dual retrograde tracing approach, it was determined that CRF neurons of the paraventricular nucleus of the hypothalamus that project to the locus coeruleus are distinct from those that project to the median eminence to initiate adrenocorticotropin release in response to stress. This indicated that the neurohormone and neuromodulator actions of CRF are independently engaged (Reyes et al., 2005).
Different sources of CRF are engaged by distinct stimuli to activate the locus coeruleus. For example, locus coeruleus neurons are activated by non-noxious levels of colon distention and this is mediated by CRF release from Barrington’s nucleus neurons that project to the locus coeruleus (Kosoyan et al., 2005; Lechner et al., 1997; Rouzade-Dominguez et al., 2001). By engaging CRF-containing neurons in Barrington’s nucleus, pelvic visceral stimuli such as colon distention can initiate a coordinated response composed of visceral and cognitive components. In contrast to colon distention, hypotensive stress activates the locus coeruleus-norepinephrine system through CRF afferents from the central nucleus of the amygdala (Curtis et al., 2002). The central nucleus of the amygdala coordinates autonomic and behavioral responses to emotional stimuli (e.g., conditioned fear) via projections to the lateral hypothalamus and central gray, respectively (LeDoux et al., 1988). CRF-containing projections from the central nucleus of the amygdala to locus coeruleus dendrites in the rostrolateral peri-locus coeruleus may serve as a cognitive limb of this fear response. In addition to its role in fear responses, the central nucleus of the amygdala coordinates information from the cardiovascular system and other viscera with behavior. Thus, it is not surprising that this nucleus plays a critical role in activation of the locus coeruleus-norepinephrine system by hypotension.
3.5 Effects of CRF administration
CRF increases tonic locus coeruleus discharge rate and it is approximately 300 times more potent when administered into the locus coeruleus vs. intracerebroventricularly (Curtis et al., 1997; Valentino and Foote, 1988; Valentino et al., 1983). Studies of the effects of CRF on locus coeruleus neurons in vitro in slice preparations demonstrating that CRF increases discharge rates of locus coeruleus neurons even when synaptic activity is blocked are consistent with in vivo studies and support the idea that this is a direct effect on locus coeruleus neurons (Jedema and Grace, 2004). Activation of locus coeruleus neurons by CRF is mediated by CRF1 receptors (Schulz et al., 1996). Consistent with the ability of CRF to increase locus coeruleus neuronal discharge, it also increases c-fos expression in the locus coeruleus and norepinephrine release in terminal fields (Page and Abercrombie, 1999; Rassnick et al., 1998; Rassnick et al., 1994).
Locus coeruleus activation by CRF differs qualitatively from that produced by excitatory amino acids in that the maximum magnitude of activation is less (approximately a two-fold increase in discharge rate), the onset slower and the duration substantially longer. These qualitative differences in the response of locus coeruleus neurons to CRF vs. excitatory amino acids suggest differential regulation by these two prominent influences on locus coeruleus activity. Specifically, CRF would bias locus coeruleus activity towards the tonic mode and away from the phasic mode that is elicited by excitatory amino acid afferents (Fig. 1). Consistent with this, CRF attenuates locus coeruleus sensory responses by elevating baseline discharge and decreasing evoked discharge, thereby reducing the signal-to-noise ratio of the locus coeruleus sensory response (Valentino and Foote, 1987; Valentino and Foote, 1988). Given the evidence for co-localization of CRF and glutamate in axon terminals in the locus coeruleus and convergence onto common locus coeruleus dendrites, there are multiple potential mechanisms for this interaction. It is also possible that CRF changes the state of electrotonic coupling, perhaps through modification of connexins. Importantly, differential effects of these two major inputs to the locus coeruleus provide a mechanism for rapidly changing the mode of locus coeruleus activity and thereby behavior. It is tempting to speculate that glutamate inputs engaged by sensory stimuli put the locus coeruleus in a phasic mode that facilitates focused attention and engagement in a specific behavioral task. In contrast, CRF inputs activated by stressors would increase tonic activity, disable the influence of glutamate, disrupt selective responding to sensory stimuli and favor sampling of alternate behaviors in the environment. The net effect of this switch in neuromodulator influence on the locus coeruleus would be adaptive in a threatening environment (Fig. 1).
3.6 Release of endogenous CRF in the locus coeruleus
The locus coeruleus-norepinephrine system is not under tonic regulation by endogenous CRF because administration of CRF receptor antagonists have no effect on locus coeruleus discharge rate or norepinephrine release in targets in unstressed rats (Page and Abercrombie, 1999). In contrast, evidence for tonic CRF release in the locus coeruleus is apparent in adrenalectomized rats, which have higher locus coeruleus discharge rates compared to sham-operated rats (Pavcovich and Valentino, 1997). Administration of CRF receptor antagonists into the locus coeruleus normalizes this difference, suggesting that CRF release within the locus coeruleus is normally restrained by basal levels of corticosteroids. This has important implications for conditions in which corticosteroid receptors may be dysfunctional (e.g., depression), in which case locus coeruleus activity would be predicted to be tonically elevated.
There is substantial evidence for endogenous CRF release in the locus coeruleus during stress. Thus, hypotensive challenge, which activates the hypothalamic-pituitary-adrenal axis, mimics the effects of CRF on tonic and phasic locus coeruleus discharge (Valentino and Wehby, 1988a). As blood pressure decreases, locus coeruleus discharge rate increases and this is temporally correlated to forebrain electroencephalographic activation, underscoring the functional relevance of this effect. Sensory evoked activity is inhibited at this time, presumably keeping the locus coeruleus in an elevated tonic mode that would promote heightened arousal and scanning attention. These effects are completely prevented by prior administration of a CRF receptor antagonist into the locus coeruleus (Curtis et al., 2001; Valentino et al., 1991; Valentino and Wehby, 1988a). Taken with the finding that factors that determine locus coeruleus sensitivity to CRF (e.g., prior stress history, opiate administration, sex) similarly affect sensitivity to hypotensive challenge, the results support the idea that hypotensive stress engages CRF release into the locus coeruleus to affect the activity of this system and thereby forebrain neuronal activity (Curtis et al., 2006; Curtis et al., 1995; 1999; Xu et al., 2004). CRF-containing neurons of the central nucleus of the amygdala are integral to locus coeruleus activation by hypotensive challenge (Curtis et al., 2002).
Studies using endpoints of locus coeruleus activation that do not rely on electrophysiological recordings, also provide evidence that CRF is released into the locus coeruleus during stress to activate the locus coeruleus-norepinephrine system. For example, microinfusion of CRF receptor antagonists into the locus coeruleus prevented stress-induced increases in tyrosine hydroxylase expression in locus coeruleus neurons (Melia and Duman, 1991) and stress-induced increases in cortical norepinephrine extracellular levels (Kawahara et al., 2000; Smagin et al., 1996). CRF receptor internalization into locus coeruleus neurons provides another endpoint of CRF effects on these cells. Microinfusion of CRF into the locus coeruleus in doses that increase discharge rates of locus coeruleus neurons causes a relatively long lasting receptor internalization in locus coeruleus dendrites (Reyes et al., 2006). This is also observed 1 and 24 h after swim stress and is significantly attenuated by administration of a selective CRF1 receptor antagonists prior to swim stress, providing evidence that CRF is released by swim stress to impact on this system (Reyes et al., 2007). This endpoint may be useful for determining whether endogenous CRF impacts on the locus coeruleus system during a particular challenge in studies that preclude neuronal recordings.
3.7 Factors governing the sensitivity of locus coeruleus neurons to CRF
Sensitivity of locus coeruleus neurons to CRF is not static, but can be influenced by a number of factors. For example, in male rats with a history of stress, the CRF dose-response curve for locus coeruleus activation is shifted to the left with a decreased maximum response (Curtis et al., 1995; 1999). The decreased maximum response may be attributed to receptor internalization. The stress-induced sensitization to low doses of CRF is functionally relevant because it allows low levels of CRF, that would typically have no effect, to activate the brain norepinephrine system by a magnitude that produces arousal. This stress-related sensitization of locus coeruleus neurons to CRF may play a role in the hypervigilance seen in post-traumatic stress disorder. Like prior stress, chronic administration of opiates also sensitizes locus coeruleus neurons to CRF and this has important consequences for behavior (see below).
Sex is also an important determinant of locus coeruleus sensitivity to CRF and stress. Regardless of hormonal status, locus coeruleus neurons of female rats are more sensitive to CRF as indicated by a shift to the left in the CRF dose-response curve (Curtis et al., 2006). Moreover, whereas prior stress alters locus coeruleus sensitivity to CRF in male rats, it does not do so for female rats. This sex specific regulation of locus coeruleus sensitivity to CRF suggests sex differences in CRF receptor structure or signaling that may be very relevant to sex differences in vulnerability to stress-related disorders. Importantly, the results suggest that potential sex differences in CRF receptors or signaling need to be taken into account in the development of CRF receptor antagonists for the treatment of stress-related disorders that are more prevalent in females.
3.8 Structural Effects of CRF on locus coeruleus neurons
In addition to CRF effects on locus coeruleus physiology that have relatively short-term adaptive consequences, recent evidence suggests that CRF impacts on locus coeruleus dendritic structure, an effect with potentially long term consequences (Swinny and Valentino, 2006). Exposure of locus coeruleus explants to CRF increased dendritic length into the surrounding peri-coerulear region. This effect was mediated by CRF1 receptors and involved activation of protein kinase A and mitogen activated protein kinase. Importantly, the Rho GTPases that regulate dendritic growth were identified as molecules linking CRF receptor signaling and actin cytoskeletal dynamics. Because different afferents terminate in the locus coeruleus core vs. the peri-coerulear region, the degree of dendritic extension outside of the core is relevant to which afferents will have predominant influence over this brain norepinephrine system. Dendritic extension into the peri-coerulear region would increase the probability of communicating with limbic afferents that relay emotionally-related information to the locus coeruleus. Thus, by governing the extension of locus coeruleus dendrites into the peri-coerulear region, CRF may determine the level of emotional arousal (Fig. 3).
Figure 3.
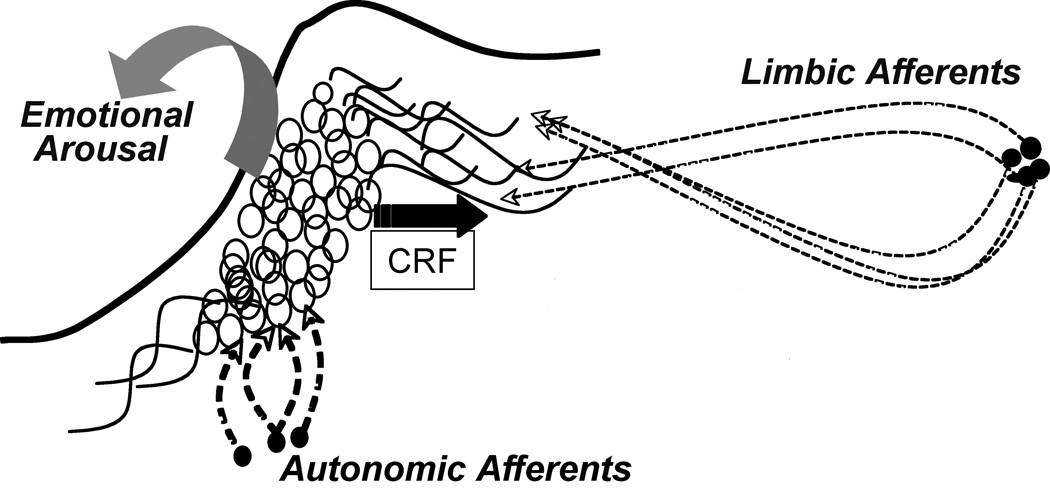
Schematic depicting potential consequences of CRF morphological effects on locus coeruleus dendrites. In contrast to autonomically-related CRF afferents to the locus coeruleus that terminate in the nuclear core, limbic sources of CRF terminate outside of the core in the peri-coerulear region where locus coeruleus dendrites extend. By promoting the extension of locus coeruleus dendrites into this region, CRF potentially increases the probability that these dendrites will synapse with limbic afferents that convey affective information and enhance the influence of these afferents on locus coeruleus activity. Through this mechanism, CRF can determine the structural basis for emotional arousal.
4. Endogenous opioids and the locus coeruleus: countering the effects of stress
4.1 Anatomical Considerations
Endogenous opioids are another class of stress-related neuromodulators that are poised to regulate activity of the locus coeruleus-norepinephrine system. β-endorphin, one of the first endogenous opioids identified in brain, derives from pre-proopiomelanocortin, the same precursor as that which gives rise to adrenocorticotropin (Hughes et al., 1980). Moreover, the most consistent means for eliciting endogenous opioid release in brain is through stressor or pain presentation (Drolet et al., 2001). The potential for afferent regulation of the locus coeruleus by endogenous opioids is supported by anatomical evidence and the effects of opiates on locus coeruleus neuronal activity. Enkephalin densely innervates the nuclear core of locus coeruleus neurons and is robust in peri-coerulear dendritic zones, particularly at the level of the rostral locus coeruleus where its distribution overlaps that of CRF (Tjoumakaris et al., 2003; Van Bockstaele et al., 1995; Van Bockstaele and Chan, 1997; Van Bockstaele et al., 2000). Enkephalin-immunoreactive terminals form synaptic specializations with tyrosine hydroxylase-immunoreactive dendrites (Van Bockstaele et al., 1995; Van Bockstaele et al., 1998). Enkephalin and CRF are co-localized in a small population of axon terminals in the locus coeruleus region and electron microscopic visualization of triple labeled tissue demonstrates that these contact tyrosine hydroxylase-immunoreactive dendrites (Tjoumakaris et al., 2003). Individual enkephalin and CRF-immunolabeled axon terminals also converge onto locus coeruleus dendrites, offering another mechanism by which these two peptides can interact.
Whereas, the central nucleus of the amygdala is a primary source of CRF afferents to the locus coeruleus, this is not a source of enkephalin afferents (Tjoumakaris et al., 2003). Rather, these derive in part from the nucleus paragigantocellaris and potentially other regions (Drolet et al., 1992).
4.2 Electrophysiological Considerations
Immunohistochemical and receptor binding studies demonstrate a high density of μ-opiate receptors in the locus coeruleus and mRNA for this receptor is expressed in locus coeruleus neurons (Mansour et al., 1994; Moriwaki et al., 1996; Pert et al., 1975; Tempel and Zukin, 1987). The electrophysiological effects associated with μ-opiate receptor activation in locus coeruleus neurons have been extensively studied in vitro (Aghajanian and Wang, 1987; North and Williams, 1983; 1985; Williams et al., 1982; Williams and North, 1984). Opiate agonists activate an outward potassium current resulting in hyperpolarization and this is mediated through μ-opioid receptors that are G-protein coupled to potassium channels. Evidence argues against a role for adenyl cyclase in this particular cellular response. However, an additional effect of μ-opiates that has been suggested to contribute to inhibition of locus coeruleus discharge rate involves a sodium-dependent inward current and requires inhibition of adenylate cyclase (Duman et al., 1988; Nestler et al., 1994).
In vivo, μ-opiate receptor activation in the locus coeruleus produces effects that are directly opposite those of CRF. The predominant action is inhibition of tonic activity and at certain doses, tonic discharge is selectively decreased and phasic activity is unaffected, resulting in an increased signal-to-noise of the sensory evoked response (Valentino and Wehby, 1988b). The net effect of μ-opiate receptor activation in the locus coeruleus is a shift in the mode of activity in a direction opposite to CRF (Fig. 1). If baseline tonic activity is relatively high, engaging μ-opiate receptors would favor a phasic mode of activity. Consistent with this, multichannel recordings in locus coeruleus demonstrate that in addition to decreasing locus coeruleus discharge rate, morphine induces synchronous oscillations in locus coeruleus neurons that are prevented by both naloxone and excitatory amino acid receptor antagonists (Zhu and Zhou, 2001; 2005). Thus, whereas CRF biases locus coeruleus activity towards a tonic mode to promote heightened arousal, scanning attention and a behavioral sampling, μ-opiates bias activity towards the phasic mode to promote focused attention and maintenance of ongoing behavior.
Like CRF, opioids are not tonically released to regulate locus coeruleus activity because pure opiate receptor antagonists have no effect on locus coeruleus discharge of previously untreated rats (Valentino and Wehby, 1989). This is in contrast to the dramatic activation of locus coeruleus neurons produced by systemic administration of opiate receptor antagonists to opiate-dependent animals, which involves excitatory amino acid afferents to the locus coeruleus (Akaoka and Aston-Jones, 1991; Rasmussen and Aghajanian, 1989). However, stress induces release of endogenous opioids in the locus coeruleus that is unmasked by naloxone administration. This was first reported by Abercrombie et al. (1988) who demonstrated that systemic naloxone increased locus coeruleus discharge rates of opiate-naive, stressed rats, at doses that had no effect in unstressed rats (Abercrombie and Jacobs, 1988). Because naloxone was administered systemically in this study, it was not clear whether the effects were due to antagonism of opioids within the locus coeruleus or elsewhere in the brain. More recent studies, using hypotensive stress, provided evidence for endogenous opioid release in the locus coeruleus and revealed that an important function of this is to reset the level and mode of neuronal activity upon stressor termination (Curtis et al., 2001). With the termination of hypotensive stress, locus coeruleus discharge rapidly declines to levels that are below baseline for up to 15 min, prior to returning to pre-stress levels. Microinfusion of naloxone into the locus coeruleus had no effect on locus coeruleus activation during the stress, but prevented post-stressor inhibition. In naloxone-pretreated rats, locus coeruleus discharge remained elevated for an extended time after the termination of the hypotensive episode and did not decrease below baseline levels. These findings suggested that if the opioid influence is compromised, the brain noradrenergic system would be elevated for a more prolonged period of time after stress termination. Taken with findings that CRF receptor antagonists selectively prevent locus coeruleus activation during hypotensive stress, but not post-stress inhibition, the studies suggest that stress engages both CRF and opioid afferents to the locus coeruleus, which have opposing influences on activity. CRF release during stress should shift locus coeruleus activity towards a tonic mode and promote arousal, scanning of the environment and sampling behavioral strategies. Opioid influence should shift the mode of locus coeruleus activity away from the high tonic mode and towards a phasically driven mode. The post-stress inhibitory opioid influence may serve to counterbalance the excitatory effects of CRF in an effort to facilitate termination of the brain noradrenergic stress response. (Curtis et al., 2002; Tjoumakaris et al., 2003).
An implication of the opposing influences of endogenous opioids and CRF on locus coeruleus activity is that changes in the sensitivity to one neuromodulator will alter sensitivity to the other if these are in balance. A test of this hypothesis revealed that chronic opiate administration selectively increased the sensitivity of locus coeruleus neurons to CRF (Xu et al., 2004). This was apparent as an increased magnitude of activation produced by microinfused CRF, as well as by stress. More importantly, sensitization of locus coeruleus neurons to CRF in chronic morphine rats translated to a change in the behavioral repertoire in response to a challenge (Xu et al., 2004). Thus, exposure of morphine-dependent rats to swim stress resulted in excessive climbing at times when control rats would adopt a passive coping strategy of immobility. Climbing behavior in this model has been linked to activation of the brain norepinephrine system (Detke et al., 1995). This represents an example of how the level and mode of locus coeruleus activity is the result of a balance of neuromodulators that are engaged and active at a particular time. The mechanism by which chronic opioids induce postsynaptic sensitization to CRF in locus coeruleus neurons is currently unknown, although many changes in intracellular signaling systems within locus coeruleus neurons have been documented to occur as a result of chronic opiate administration and these could contribute to altered postsynaptic sensitivity to CRF (Nestler et al., 1999).
5. Conclusions and Synthesis
As an adaptive response to a perceived life-threatening challenge, arousal should be elevated and maintained for the duration of the challenge and attention should be directed towards diverse stimuli in the environment, rather than being focused on specific stimuli. This favors sampling of behavioral strategies that can be adopted, given the environmental constraints, so that the organism has the highest probability for survival. Moreover, these changes should subside when the stressor is no longer present. Locus coeruleus neurons with their widely distributed network of axons, promote these aspects of the cognitive and behavioral limbs of the stress response through changes in their discharge rate and pattern. In turn, the mode of locus coeruleus activity is finely tuned by the convergence of afferents, particularly excitatory amino acids, CRF and endogenous opioids onto locus coeruleus neurons. By biasing the activity of locus coeruleus neurons towards a particular mode of discharge, these afferents can govern predominant behavioral strategies in specific situations or environments. In addition to acute physiological effects, CRF impacts on the morphology of locus coeruleus neurons. By determining the degree to which certain afferents communicate with locus coeruleus neurons, this can have enduring consequences on behavior or emotional expression. The emerging knowledge of how the locus coeruleus-norepinephrine system is regulated by stressors and determinants of sensitivity of locus coeruleus neurons to stress-related neurotransmitters is an important step in understanding and alleviating diverse stress-related psychiatric disorders.
Acknowledgements
This work was supported by PHS Grants MH40008, DA09082 and a Distinguished Investigator Award from NARSAD (RJV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Jacobs BL. Systemic naloxone administration potentiates locus coeruleus noradrenergic neuronal activity under stressful but not non-stressful conditions. Brain Res. 1988;441:362–366. doi: 10.1016/0006-8993(88)91415-1. [DOI] [PubMed] [Google Scholar]
- Abercrombie ED, Keller RW, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. doi: 10.1016/0306-4522(88)90192-3. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang Y-Y. Common alpha2 and opiate effector mechanisms in the locus coeruleus: intracellular studies in brain slices. Neuropharmacology. 1987;26:793–399. doi: 10.1016/0028-3908(87)90054-2. [DOI] [PubMed] [Google Scholar]
- Akaoka H, Aston-Jones G. Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J. Neurosci. 1991;11:3830–3839. doi: 10.1523/JNEUROSCI.11-12-03830.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981a;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J. Neurosci. 1981b;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Ann. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Locus coeruleus and regulation of behavioral flexibility and attention. Prog. Brain Res. 2000;126:165–182. doi: 10.1016/S0079-6123(00)26013-5. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Grzanna R. The locus coeruleus, A5 and A7 noradrenergic cell groups. In: Paxinos G, editor. The Rat Brain. Academic Press; 1995. pp. 183–213. [Google Scholar]
- Beck CHM, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 1995;15:709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Foote SL. Effects of locus coeruleus activation on electroencephalographic activity in the neocortex and hippocampus. J. Neurosci. 1991;11:3135–3145. doi: 10.1523/JNEUROSCI.11-10-03135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus. Neuroscience. 1993;55:381–383. doi: 10.1016/0306-4522(93)90507-c. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotropin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral response and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Cassens G, Kuruc A, Roffman M, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism and behavior induced by environmental stimuli previously paired with inescapable shock. Brain Res. 1981;2:387–407. doi: 10.1016/0166-4328(81)90020-6. [DOI] [PubMed] [Google Scholar]
- Cassens G, Roffman M, Kuruc A, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism induced by environmental stimuli previously paired with inescapable shock. Science. 1980;209:1138–1139. doi: 10.1126/science.7403874. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, Grigoriadis DE, Behan DP, De Souza EB. Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends in Pharmacological Science. 1996;17:166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- Chan RKW, Sawchenko PE. Hemodynamic regulation of tyrosine hydroxylase messenger RNA in medullary catecholamine neurons: a c-fos-guided hybridization histochemical study. Neuroscience. 1995;66:377–390. doi: 10.1016/0306-4522(94)00600-a. [DOI] [PubMed] [Google Scholar]
- Chang MS, Sved AF, Zigmond MJ, Austin MC. Increased transcription of the tyrosine hydroxylase gene in individual locus coeruleus neurons following footshock stress. Neuroscience. 2000;101:131–139. doi: 10.1016/s0306-4522(00)00352-3. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Connally KR, Valentino RJ. Corticotropin-releasing factor neurons of the central nucleus of the amygdala mediate locus coeruleus activation by cardiovascular stress. J. Neuroendocrinol. 2002;14:667–682. doi: 10.1046/j.1365-2826.2002.00821.x. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Bello NT, Valentino RJ. Endogenous opioids in the locus coeruleus function to limit the noradrenergic response to stress. J. Neurosci. 2001;21:RC152. doi: 10.1523/JNEUROSCI.21-13-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotrophin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J. Pharmacol. Exp. Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Previous stress alters corticotrophin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Long term regulation of locus coeruleus sensitivity to corticotropin-releasing factor by swim stress. J. Pharmacol. Exp. Ther. 1999;289:1211–1219. [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Drolet G, Dumont EC, Gosselin I, Kinkead R, Laforest S, Trottier JF. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuropsychopharmacol. Biol. Psych. 2001;25:729–741. doi: 10.1016/s0278-5846(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Drolet G, Van Bockstaele EB, Akaoka H, et al. Robust enkephalin innervation of the locus coeruleus from the rostral medulla. J. Neurosci. 1992;12:3162–3174. doi: 10.1523/JNEUROSCI.12-08-03162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Tallman JF, Nestler EJ. Acute and chronic opiate-regulation of adenylate cyclase in brain: specific effects in locus coeruleus. J. Pharmacol. Exp. Ther. 1988;246:1033–1039. [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Shen E, Tang H, Huang R, Chiu TH. c-fos expression as a marker of central cardiovascular neurons. Biol. Signals. 1995;4:117–123. doi: 10.1159/000109431. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GR. Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of fos-like immunoreactivity. J. Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M, Aston-Jones G, Shiekhattar R. Activation of locus coeruleus neurons by nucleus paragigantocellularis or noxious sensory stimulation is mediated by intracoerulear excitatory amino acid neurotransmission. Brain Res. 1992;598:185–195. doi: 10.1016/0006-8993(92)90182-9. [DOI] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan J, Aston-Jones G, Valentino RJ. Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res. 1996;742:89–97. doi: 10.1016/s0006-8993(96)00967-5. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G. Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol. Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- Funk D, Amir S. Circadian modulation of fos resonses to odor of the red fox, a rodent predator, in the rat olfactory system. Brain Res. 2000;866:262–267. doi: 10.1016/s0006-8993(00)02249-6. [DOI] [PubMed] [Google Scholar]
- Graham JC, Hoffman GE, Sved AF. c-fos expression in brain in response to hypotension and hypertension in conscious rats. J. Auton. Nerv. Sys. 1995;55:92–104. doi: 10.1016/0165-1838(95)00032-s. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- Hughes J, Beaumont A, Fuentes JA, Malfroy B, Unsworth C. Opioid peptides: aspects of their origin, release and metabolism. J. Exp. Biol. 1980;89:239–255. doi: 10.1242/jeb.89.1.239. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Hashiguchi H, Takeda R, Ishizuka Y, Mitsuyama Y, Kannan H, Nishimori T, Nakahara D. Conditioned-fear stress increases Fos expression in monoaminergic and GABAergic neurons of the locus coeruleus and dorsal raphe nuclei. Synapse. 2002;45:46–51. doi: 10.1002/syn.10086. [DOI] [PubMed] [Google Scholar]
- Ishimatsu M, Williams JT. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J. Neurosci. 1996;16:5196–5204. doi: 10.1523/JNEUROSCI.16-16-05196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J. Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Kawahara Y, Westerink BH. The role of afferents to the locus coeruleus in the handling stress-induced increase in the release of norepinephrine in the medial prefrontal cortex: a dual-probe microdialysis study in the rat brain. Eur. J. Pharmacol. 2000;387:279–286. doi: 10.1016/s0014-2999(99)00793-1. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J. Neurosci. 1997;17:8842–8865. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korf J, Aghajanian GK, Roth RH. Increased turnover of norepinephrine in the rat cerebral cortex during stress: role of the locus coeruleus. Neuropharmacology. 1973;12:933–938. doi: 10.1016/0028-3908(73)90024-5. [DOI] [PubMed] [Google Scholar]
- Kosoyan HP, Grigoriadis DE, Tache Y. The CRF(1) receptor antagonist, NBI-35965, abolished the activation of locus coeruleus neurons induced by colorectal distension and intracisternal CRF in rats. Brain Res. 2005;1056:85–96. doi: 10.1016/j.brainres.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lacosta S, Merali Z, Anisman H. Central monoamine activity following acute and repeated systemic interleukin-2 administration. Neuroimmunomodulation. 2000;8:83–90. doi: 10.1159/000026457. [DOI] [PubMed] [Google Scholar]
- Lechner S, Curtis A, Brons R, Valentino R. Locus coeruleus activation by colon distention: role of corticotropin-releasing factor and excitatory amino acids. Brain Res. 1997;756:114–124. doi: 10.1016/s0006-8993(97)00116-9. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Regulatory role of glucocorticoids and glucocorticoid receptor mRNA levels on tyrosine hydroxylase gene expression in the locus coeruleus during repeated immobilization stress. Brain Res. 2002;943:216–223. doi: 10.1016/s0006-8993(02)02647-1. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- Mason ST, Fibiger HC. Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J. Comp. Neurol. 1979;187:703–724. doi: 10.1002/cne.901870405. [DOI] [PubMed] [Google Scholar]
- Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc. Natl. Acad. Sci. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki A, Wang JB, Svingos A, van Bockstaele E, Cheng P, Pickel V, Uhl GR. mu Opiate receptor immunoreactivity in rat central nervous system. Neurochem. Res. 1996;21:1315–1331. doi: 10.1007/BF02532373. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res. Bull. 1994;35:521–528. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol. Psychiatry. 1999;46:1131–1139. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- North RA, Williams JT. Opiate activation of potassium conductance inhibits calcium action potentials in rat locus coeruleus neurons. Br. J. Pharmac. 1983;80:225–228. doi: 10.1111/j.1476-5381.1983.tb10023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Williams JT. On the potassium conductance increase by opioids in rat locus coeruleus neurons. J. Physiol. (Lond.) 1985;364 doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol. Rev. 1991;43:425–474. [PubMed] [Google Scholar]
- Page ME, Abercrombie ED. Discrete local application of corticotropin-releasing factor increases locus coeruleus discharge and extracellular norepinephrine in rat hippocampus. Synapse. 1999;33:304–313. doi: 10.1002/(SICI)1098-2396(19990915)33:4<304::AID-SYN7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Page ME, Berridge CW, Foote SL, Valentino RJ. Corticotropin-releasing factor in the locus coeruleus mediates EEG activation associated with hypotensive stress. Neurosci. Lett. 1993;164:81–84. doi: 10.1016/0304-3940(93)90862-f. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Valentino RJ. Regulation of a putative neurotransmitter effect of corticotropin-releasing factor: effects of adrenalectomy. J. Neurosci. 1997;17:401–408. doi: 10.1523/JNEUROSCI.17-01-00401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Autoradiographic localization of the opiate receptor in rat brain. Life. Sci. 1975;16:1849–1854. doi: 10.1016/0024-3205(75)90289-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–350. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Hoffman GE, Rabin BS, Sved AF. Injection of corticotropin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience. 1998;85:259–268. doi: 10.1016/s0306-4522(97)00574-5. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Sved AF, Rabin BS. Locus coeruleus stimulation by corticotrophin-releasing hormone suppresses in vitro cellular immune responses. J. Neurosci. 1994;14:6033–6040. doi: 10.1523/JNEUROSCI.14-10-06033.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur. J. Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur. J. Neurosci. 2005;22:93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- Rivier C, Rivier J, Vale W. Inhibition of adrenocorticotropic hormone secretion in the rat by immunoneutralization of corticotropin-releasing factor. Science. 1982;218:377–378. doi: 10.1126/science.6289439. [DOI] [PubMed] [Google Scholar]
- Rouzade-Dominguez M-L, Curtis AL, Valentino RJ. Role of Barrington's nucleus in the activation of rat locus coeruleus neurons by colonic distension. Brain Res. 2001;917:206–218. doi: 10.1016/s0006-8993(01)02917-1. [DOI] [PubMed] [Google Scholar]
- Rusnak M, Kvetnansky R, Jelokova J, Palkovits M. Effect of novel stressors on gene expression of tyrosine hydroxylase and monoamine transporters in brainstem noradrenergic neurons of long-term repeatedly immobilized rats. Brain Res. 2001;899:20–35. doi: 10.1016/s0006-8993(01)02126-6. [DOI] [PubMed] [Google Scholar]
- Sabban EL, Kvetnansky R. Stress-triggered activation of gene expression in catecholaminergic systems: dynamics of transcriptional events. Trends Neurosci. 2001;24:91–98. doi: 10.1016/s0166-2236(00)01687-8. [DOI] [PubMed] [Google Scholar]
- Schulz DW, Mansbach RS, Sprouse J, Braselton JP, Collins J, Corman M, Dunaiskis A, Faraci S, Schmidt AW, Seeger T, Seymour P, Tingley FDI, Winston EN, Chen YL, Heym J. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10477–10482. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Harris RB, Ryan DH. Corticotropin-releasing factor antagonist infused into the locus coeruleus attenuates immobilization stress-induced defensive withdrawal in rats. Neurosci. Lett. 1996;220:167–170. doi: 10.1016/s0304-3940(96)13254-7. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Swiergiel AH, Dunn AJ. Sodium nitroprusside infusions activate cortical and hypothalamic noradrenergic systems in rats. Neurosci. Res. Comm. 1994;14:85–91. [Google Scholar]
- Smith MA, Banerjee S, Gold PW, Glowa J. Induction of c-fos mRNA in rat brain by conditioned and unconditioned stressors. Brain Res. 1992;578:135–141. doi: 10.1016/0006-8993(92)90240-a. [DOI] [PubMed] [Google Scholar]
- Smith MA, Brady LS, Glowa J, Gold PW, Herkenham M. Effects of stress and adrenalectomy on tyrosine hydroxylase mRNA levels in the locus ceruleus by in situ hybridization. Brain Res. 1991;544:26–32. doi: 10.1016/0006-8993(91)90881-u. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Hartman BK. The central adrenergic system. An immunofluorescence study of the location of cell bodies and their efferent connections in the rat using dopamine-B-hydroxylase as a marker. J. Comp. Neurol. 1975;163:467–506. doi: 10.1002/cne.901630406. [DOI] [PubMed] [Google Scholar]
- Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur. J. Neurosci. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- Tempel A, Zukin RS. Neuroanatomical patterns of the μ, d and k opioid receptors of rat brain as determined by quantitative in vitro autoradiography. Proc. Natl. Acad. Sci. U. S. A. 1987;84:4308–4312. doi: 10.1073/pnas.84.12.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Javoy F, Glowinski J, Kety SS. Effects of stress on the metabolism of norepinephrine, dopamine and serotonin in the central nervous system of the rat: modification of norpinephrine turnover. J. Pharmacol. Exp. Ther. 1968;163:163–171. [PubMed] [Google Scholar]
- Tjoumakaris SI, Rudoy C, Peoples J, Valentino RJ, Van Bockstaele EJ. Cellular interactions between axon terminals containing endogenous opioid peptides or corticotropin-releasing factor in the rat locus coeruleus and surrounding dorsal pontine tegmentum. J. Comp. Neurol. 2003;466:445–456. doi: 10.1002/cne.10893. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Ida Y, Satoh H, Tsujimaru S, Tanaka M. Stressor predictability and rat brain noradrenaline metabolism. Pharmacol. Biochem. Behav. 1989;32:569–572. doi: 10.1016/0091-3057(89)90198-6. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G. The role of locus coeruleus in the regulation of cognitive performance. Science. 1999;283:549–554. doi: 10.1126/science.283.5401.549. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Chen S, Zhu Y, Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington's nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732:1–15. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. Corticotropin-releasing factor increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J. Neurosci. 1988;8:1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL, Aston-Jones G. Corticotropin-releasing factor activates noradrenergic neurons of the locus coeruleus. Brain Res. 1983;270:363–367. doi: 10.1016/0006-8993(83)90615-7. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Miselis RR, Pavcovich LA. Pontine regulation of pelvic viscera: Pharmacological target for pelvic visceral dysfunctions. Trends Pharmacol. Sci. 1999;20:253–266. doi: 10.1016/s0165-6147(99)01332-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Curtis AL. Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res. 1991;555:25–34. doi: 10.1016/0006-8993(91)90855-p. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Page ME, Van Bockstaele E, Aston-Jones G. Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience. 1992;48:689–705. doi: 10.1016/0306-4522(92)90412-u. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Rudoy C, Saunders A, Van Bocstaele EJ. Corticotropin-releasing factor is preferentially colocalized with glutamate in terminals in the locus coeruleus region. Neuroscience. 2001;106:375–384. doi: 10.1016/s0306-4522(01)00279-2. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Corticotropin-releasing factor: Evidence for a neurotransmitter role in the locus coeruleus during hemodynamic stress. Neuroendocrinology. 1988a;48:674–677. doi: 10.1159/000125081. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Morphine effects on locus coeruleus neurons are dependent on the state of arousal and availability of external stimuli: Studies in anesthetized and unanesthetized rats. J. Pharmacol. Exp. Ther. 1988b;244:1178–1186. [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. Locus coeruleus discharge characteristics of morphine-dependent rats: Effects of naltrexone. Brain Res. 1989;488:126–134. doi: 10.1016/0006-8993(89)90701-4. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Branchereau P, Pickel VM. Morphologically heterogeneous metenkephalin terminals form synapses with tyrosine hydroxylase containing dendrites in the rat nucleus locus coeruleus: an immuno-electron microscopic analysis. J. Comp. Neurol. 1995;363:423–438. doi: 10.1002/cne.903630307. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Chan J. Electron microscopic evidence for coexistence of leucine5-enkephalin and gamma-aminobutyric acid in a subpopulation of axon terminals in the rat locus coeruleus region. Brain Res. 1997;746:171–182. doi: 10.1016/s0006-8993(96)01194-8. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J. Comp. Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EEO, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. J. Neuroendocrinol. 1998;10:743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Valentino RJ. Differential regulation of the rostrolateral peri-locus coeruleus region by limbic afferents. Biol. Psych. 1999;46:1352–1363. doi: 10.1016/s0006-3223(99)00213-9. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Saunders A, Commons KG, Liu XB, Peoples J. Evidence for coexistence of enkephalin and glutamate in axon terminals and cellular sites for functional interactions of their receptors in the rat locus coeruleus. J. Comp. Neurol. 2000;417:103–114. doi: 10.1002/(sici)1096-9861(20000131)417:1<103::aid-cne8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Devilbiss D, Fleischer D, Sessler FM, Simpson KL. New perspectives on the functional organization and postsynaptic influences of the locus ceruleus efferent projection system. Adv. Pharmacol. 1998;42:749–754. doi: 10.1016/s1054-3589(08)60856-x. [DOI] [PubMed] [Google Scholar]
- Williams JT, Egan TM, North RA. Enkephalin opens potassium channels on mammalian central neurons. Nature (Lond.) 1982;299:74–77. doi: 10.1038/299074a0. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA. Opiate receptor interactions on single locus coeruleus neurons. Mol. Pharm. 1984;26:489–497. [PubMed] [Google Scholar]
- Xu GP, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ. Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J. Neurosci. 2004;24:8193–8197. doi: 10.1523/JNEUROSCI.1657-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhou W. Morphine induces synchronous oscillatory discharges in the rat locus coeruleus. J. Neurosci. 2001;21:RC179. doi: 10.1523/JNEUROSCI.21-21-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Zhou W. Excitatory amino acid receptors are involved in morphine-induced synchronous oscillatory discharges in the locus coeruleus of rats. Eur. J. Pharmacol. 2005;528:73–78. doi: 10.1016/j.ejphar.2005.10.048. [DOI] [PubMed] [Google Scholar]