Abstract
The purpose of this review is to summarize the role that murine models of arthritis are playing in the understanding of human rheumatoid arthritis and how leukotriene B4 (LTB4) is emerging as an important target in this field. Both the collagen-induced arthritis (CIA) model and the K/BxN serum transfer arthritis model have contributed to outline the potential mechanisms involved in inflammatory arthritis. Indeed, the CIA model has contributed to the development of effective anti-TNF and anti-IL-1β based treatments for RA that are currently in the clinic. Many recent studies in mouse models have suggested a critical role for LTB4 and its receptors in the development of inflammatory arthritis. Inhibitors of LTB4 biosynthesis as well as LTB4 receptors are protective in mouse models of RA and mice deficient in the LTB4 biosynthetic enzymes or LTB4 receptors are resistant to disease development suggesting several promising targets for RA in this pathway.
Keywords: Rheumatoid Arthritis, Leukotriene B4 receptors, arthritis mouse models, FLAP
Take home message
Mouse models of RA have provided a great deal of information on the mechanisms involved in human RA and led to the development of effective therapies and are likely to uncover additional therapeutic opportunities
Chemoattractants and cytokines form important amplification loops for perpetual joint inflammation in RA
Inhibition of inflammatory cell recruitment to the rheumatoid synovium represents a potential target for RA treatment.
LTB4 and its receptors play a critical role in the recruitment of leucocytes to the inflammatory sites. Mice lacking either the enzymes involved in LTB4 biosynthesis or the LTB4 receptors are completely protected from the development of RA.
Unraveling the mechanisms of LTB4/BLT1 and LTB4/BLT2 axis and structure/function of BLT1 and BLT2 will provide important insights into identification of novel and dual antagonists for blocking their function.
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease that affects ~1% of the world population. Despite lack of consensus on what initiates RA in humans, many recent developments point to a role for the innate immune system in the pathogenesis of RA [1–3]. Many concepts developed in experimental animal models have begun yielding effective therapeutics for arthritis such as anti TNF-α and anti IL-1β based therapies that are currently leading the way[4, 5]. These therapies have been effective, but subgroups of RA patients do not respond to them. Another treatment regimen that showed great promise was the use of cyclooxygenase-2 (COX-2) inhibitors [6]. However, these had to be withdrawn due to increased risk of cardiovascular complications in patients taking the drug [7]. A clear understanding of the pathogenic mechanisms in mouse models will likely provide additional therapeutic targets for the treatment of RA. One of these potential leads involves leukotriene B4 (LTB4), a potent lipid inflammatory mediator and a strong chemoattractant for neutrophils. LTB4 mediates it’s effects through two G-protein coupled receptors (GPCRs), BLT1 (high affinity) and BLT2 (low affinity) [8, 9]. Recent data from animal models and arthritis patients demonstrate a critical role for LTB4 and its receptors in the progression of RA and suggests potential new targets for treatment. Here, we discuss briefly the available mouse models of RA and their use in the demonstration of an important role for LTB4 and its receptors in the development of RA.
Mouse Models of Arthritis
Collagen-induced arthritis (CIA] has been the most widely used model of arthritis initiated by intradermal immunization with type II collagen [10]. The immunization protocol varies due to a difference in susceptibility of the strains of mice, which is thought to be due to MHC class linking. DBA/1 (H-2q) and B10-RIII (H-2r) mice develop disease following immunization with collagen in Complete Freund’s Adjuvant (CFA) for the first injection and using incomplete Freund’s adjuvant (IFA) for the booster two to three weeks later. CIA in mice has been shown to have several features in common with human RA. In particular, the cytokine requirement and the effects of cytokines on the development and progression of the disease appear similar to human RA [11]. In this regard, TNF-α and IL-1β appear critical in the development and progression of RA[12]. IL-2, IL-6, IL-12, and IL-18 act as positive modulators and IL-4 and IL-10 are negative modulators of the disease activity [13–15].
Two other mouse models of RA have also attracted significant attention in the recent years [16, 17]. The K/BxN model based on a T-cell receptor (KRN] transgenic mouse which produces a T-cell repertoire that recognizes and makes auto antibodies to the ubiquitous glycolytic enzyme, glucose-6-phosphate isomerase (GPI) and develops an aggressive form of arthritis [18]. In this case disease may be transferred with serum or purified antibodies. Disease development in this model is T and B-cell independent while neutrophils and mast cells are required for joint inflammation [2, 19]. An interesting recent observation in this model suggests that histamine and serotonin control vascular leakage and incite joint inflammation by deposition of immune complexes formed against systemic antigens [20]. Modification of the immunization protocol allowed CIA development in C57BL/6 mice [16]. In this case use of CFA in both primary and booster injections with collagen is a critical determinant for disease development. Each of these models has been used to show a critical requirement for LTB4 in the development and progression of arthritis.
Leukotriene B4 and its Receptors
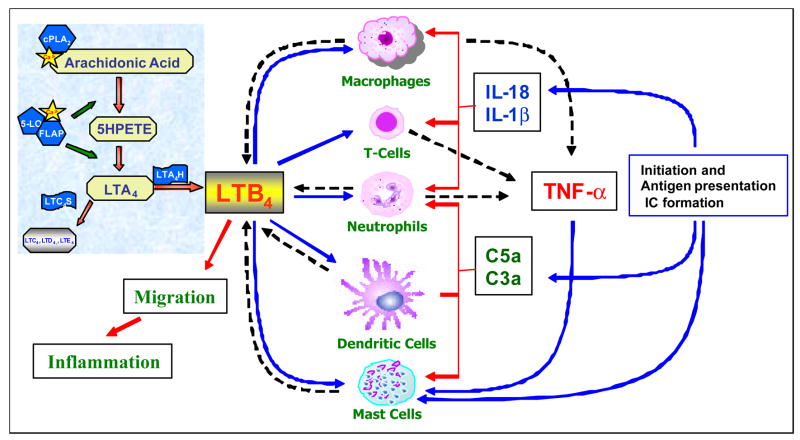
Leukotriene B4 is a potent mediator of inflammation derived from arachidonic acid by the sequential actions of 5-lipoxygenase (5-LO) and LTA4 hydrolase (Fig. 1) [21]. LTB4 acting through its receptors (BLT1) can cause chemotaxis, degranulation, adhesion and enhance the survival of neutrophils. Although BLT1 was long known to be a neutrophil chemoattractant receptor, recent studies identified BLT1 expression on macrophages [22], smooth muscle cells [23], endothelial cells [24], activated T-cells [25] and mast cells [26] considerably expanding the potential role of LTB4. Recent experiments from our laboratory demonstrated functional expression of BLT1 on both mature and immature dendritic cells and having a direct effect in the control of adaptive immune responses [27]. Knockout mice for BLT1 generated in several laboratories have been instrumental in defining a critical role for this receptor in diverse inflammatory diseases such as atherosclerosis [22] asthma, autoimmune uveitis and arthritis (see below). In contrast, the function and biological activities of BLT2 are completely unknown. BLT2 has been shown to be expressed widely in humans, with the spleen and peripheral blood leukocytes showing the highest expression [9]. However, murine BLT2 expression has been difficult to determine with several laboratories reporting varying results [26, 28, 29]. Although a BLT1/BLT2 double deficient mouse line was recently reported, complete understanding of the unique functions of BLT2 will not likely emerge until it can be targeted.
Figure 1. The role of LTB4 in the development of arthritis.
LTB4, a pro-inflammatory lipid mediator, is synthesized from the arachidonic acid pathway involving the biosynthetic enzymes 5-lipoxygenase (5-LO), Five-Lipoxygenase Activating Protein (FLAP) and LTA4 Hydrolase (LTA4H) via 5HPETE and LTA4 as intermediates. Arthritis might be initiated by infection mediated activation in the joints and/or from the formation of auto-antibodies leading to deposition of immune complexes (IC) in the synovium. The immune complexes activate mast cells and cause mast cell degranulation, which further leads to the influx of inflammatory cells through vasodilation and other mediators. Interplay of synovial cells and the incoming leukocytes leads to production of cytokines and activation of the complement cascade. Amplification loops between the cytokines and chemoattractants like LTB4 and other chemokines sets up perpetual joint inflammation. All of the innate immune cells such as dendritic cells, macrophages, neutrophils and mast cells are capable of producing LTB4 by the induction of complement or cytokines. Since all of these cells express LTB4 receptors they set up further amplification loops in the inflamed joints. The solid lines represent response to and broken lines represent production of indicated mediators.
Role of Leukotriene B4 Receptors in Arthritis
A role for LTB4 in rheumatoid arthritis was suggested by several observations over the past two decades. Neutrophils from RA patients undergoing methotrexate therapy displayed both acute and chronic suppression of LTB4 synthesis ex vivo [30].LTB4 is a potent chemoattractant of neutrophils and promotes the adhesion of neutrophils to vascular endothelium, which promotes arthritis development [31]. In a recent study, a significant increase in the mRNA levels of BLT1 and BLT2 was seen in the joint tissues and cells from RA patients relative to OA patients suggesting a role for these receptors in RA [32].
Development of targeted gene deletions in mice and of antagonists to the enzymes responsible for the production of LTB4 or to the receptors allowed for a better understanding of the requirement for LTB4 in arthritis (Table 1). In early studies the LTB4 receptor antagonists, CP-105,696 was shown to greatly reduce disease severity in an IL-1α accelerated CIA model [33]. Independent studies with two other LTB4 receptor antagonists also yielded similar results in IL-1α accelerated CIA model [34]. In an LPS accelerated CIA model, use of a FLAP inhibitor SA6541 reduced the severity of disease [35]. Studies in mice deficient in the LTB4 pathway quickly followed the antagonist experiments. FLAP knock-out mice were put through the IL-1α accelerated CIA model and were found to have a 73% reduction in disease severity, which was associated with a 23% decrease in disease incidence. FLAP heterozygous mice were shown to have a 37% decrease in disease severity and a similar decrease in disease incidence as the FLAP knock-out mice, suggesting that FLAP is a potential drug target [36]. Though this pathway looked like a good target for arthritis treatment, attempts to use the antagonists in clinical trials of arthritis apparently failed.
Table 1.
Role of LTB4 and its receptors in murine arthritis models
| Mouse Strain | Model of Arthritis | Experimental Results | References |
|---|---|---|---|
| DBA/1LacJ | IL-1 accelerated CIA | Complete protection against disease development by administration of BLT1 antagonist CP-105,696 | (35) |
| DBA/1 | IL-1 Accelerated CIA | A 38% reduction in disease severity by administration of BLT1 antagonist LY293111Na or CGS25019C | (36) |
| DBA/1 | LPS-accelerated CIA | Reduced severity of disease by administration of FLAP inhibitor SA6541 | (37) |
| DBA/1 | IL-18 accelerated CIA | 5-LO inhibitor MK-886 reduced the severity of disease | (41) |
| FLAP−/− DBA/1 | IL-1 Accelerated CIA | Reduced incidence and severity of disease in FLAP−/− mice relative to WT mice | (38) |
| C57BL/6 | K/BxN | Complete protection by preventive administration and reduced severity by therapeutic treatment with 5-LO inhibitor L-739,010 and with BLT1 antagonist CP-105,696 | (39) |
| BLT1−/− and BLT1/BLT2−/− C57BL/6 | CIA | Complete protection from disease development in BLT1−/− and BLT1/BLT2−/− mice. Similar levels of auto antibody (anti-C II) production in receptor deficient animals | (31) |
| 5-LO−/− and LTA4H−/− C57BL/6 | K/BxN | Complete protection from disease development in 5-LO−/− and LTA4H−/− mice. Adoptive transfer of WT neutrophils is sufficient for disease development in 5LO−/− mice | (20) |
| BLT1−/− C57BL/6 | K/BxN | Complete protection from disease development in BLT1−/− mice. Adoptive transfer of WT neutrophils is sufficient for disease development in BLT1−/− mice | (39) |
Recent data has renewed the interest in the LTB4 pathway as a target in arthritis. Chen et al., have shown that neither 5-LO nor LTA4 hydrolase knock-out mice develop disease in the K/BxN model, while LTC4 synthase knock-out mice develop full disease. Prophylactic treatment with a 5-LO antagonist also completely blocked disease from occurring in wild-type mice. They went on to show in mast cell and neutrophil transfer experiments that neutrophils but not the mast cells are the primary source ofLTB4 in K/BxN arthritis [19]. Using the BLT1 knock-out animals, Luster’s group showed a critical role for BLT1 in K/BxN arthritis [37]. BLT1−/− mice do not show any signs of arthritis. Adoptive transfer of neutrophils from wild-type mice restored disease in these mice but most of the recruited neutrophils in the synovium are BLT1 negative suggesting that BLT1 is required only for initiation of joint inflammation but not maintenance. CP-105,696 was also shown to block disease occurrence in this model [37]. Our studies with BLT1 and BLT1/BLT2 deficient mice in a CIA model also implicated a critical role for BLT1 in arthritis. Both these mice showed similar levels of anti collagen antibody levels indicating normal immune response to collagen but complete absence of joint inflammation [29]. Although BLT1/BLT2 double deficient mice are protected from disease in this model, complete protection of BLT1−/− mice does not allow for determining the independent functions of BLT2 in arthritis. Generation of BLT2−/− mice is critical for identification of its role, if any, in arthritis.
Based on the current knowledge, potential mechanisms involved in arthritis development for LTB4 and its receptors are shown in Figure 1. Arthritis might be initiated with the formation of auto-antibodies and deposition of immune complexes (IC) by as yet undefined mechanisms (reviewed in [38]). While the sequence of events is not clearly established studies have implicated macrophages, neutrophils, mast cells, dendritic cells and T-cells in different models of arthritis. All of these cells have recently been shown to express LTB4 receptors and many cells also produce LTB4. Amplification loops between key cytokines involved in arthritis such as TNFα, IL-1β and IL-18 to LTB4 production have been reported. In addition, studies have also shown that C5a mediated neutrophil recruitment requires BLT1 in a mouse model of peritonitis [39]. However, a cause and effect relationship between LTB4 responses of these cells in development of arthritis remains to be established.
Critical Questions about Leukotriene B4 Receptors in Rheumatoid Arthritis
Several important questions remain unanswered regarding the role of LTB4 receptors in RA. Pharmaceutical companies studied the involvement of LTB4 in RA for nearly 25 years, yet no clear candidate drugs emerged from these studies. The discovery of BLT2 with distinct antagonist specificity and tissue distribution may provide a clue to the ineffectiveness of some of the earlier antagonists [40]. It is not surprising that these antagonists did not work well on BLT2 since they were all selected and refined using assays based on neutrophil activation by BLT1. What is the role of BLT2 in inflammatory diseases? It could have a complementary and/or compensatory role during the development of human RA. Thus, blocking one of these receptors might be ineffective for RA treatment. A specific role for BLT2 in art hritis and other inflammatory diseases remains to be established.
Acknowledgments
Research in our laboratory is supported by the NIH grant AI52381 and by the James Graham Brown Cancer Center.
List of abbreviations
- LTB4
leukotriene B4
- CIA
collagen-induced arthritis
- TNF
tumour necrosis factor
- RA
rheumatoid arthritis
- COX-2
cyclooxygenase-2
- GPCR
G-protein coupled receptor
- CFA
complete Freund’s adjuvant
- IFA
incomplete Freund’s adjuvant
- 5-LO
5-lipoxygenase
- LTA4H
leukotriene A4 hydrolase
- LTA4
leukotriene A4
- LTC4
leukotriene C4
- FLAP
Five-Lipoxygenase Acivating Protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weyand CM, Goronzy JJ. Pathogenesis of rheumatoid arthritis. Med Clin North Am. 1997;81:29–5. doi: 10.1016/s0025-7125(05)70504-6. [DOI] [PubMed] [Google Scholar]
- 2.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167:1601–608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 3.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 4.Moller B, Villiger PM. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin Immunopathol. 2006;27:391–408. doi: 10.1007/s00281-006-0012-9. [DOI] [PubMed] [Google Scholar]
- 5.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, Antoni C, Leeb B, Elliott MJ, Woody JN, Schaible TF, Feldmann M. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–1563. doi: 10.1002/1529-0131(199809)41:9<1552::AID-ART5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 6.Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med. 2000;343:1520–1528. doi: 10.1056/NEJM200011233432103. 1522 p following 1528. [DOI] [PubMed] [Google Scholar]
- 7.Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Norgard B, Friis S, Sorensen HT. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med. 2005;165:978–984. doi: 10.1001/archinte.165.9.978. [DOI] [PubMed] [Google Scholar]
- 8.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- 9.Yokomizo T, Kato K, Terawaki K, Izumi T, Shimizu T. A second leukotriene B(4) receptor, BLT2. A new therapeutic target in inflammation and immunological disorders. J Exp Med. 2000;192:421–432. doi: 10.1084/jem.192.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuart JM, Townes AS, Kang AH. Nature and specificity of the immune response to collagen in type II collagen-induced arthritis in mice. J Clin Invest. 1982;69:673–683. doi: 10.1172/JCI110495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch AE, Kunkel SL, Strieter RM. Cytokines in rheumatoid arthritis. J Investig Med. 1995;43:28–38. [PubMed] [Google Scholar]
- 12.Bakker AC, Joosten LA, Arntz OJ, Helsen MM, Bendele AM, van de Loo FA, van den Berg WB. Prevention of murine collagen-induced arthritis in the knee and ipsilateral paw by local expression of human interleukin-1 receptor antagonist protein in the knee. Arthritis Rheum. 1997;40:893–900. doi: 10.1002/art.1780400517. [DOI] [PubMed] [Google Scholar]
- 13.van de Loo FA, Kuiper S, van Enckevort FH, Arntz OJ, van den Berg WB. Interleukin-6 reduces cartilage destruction during experimental arthritis. A study in interleukin-6-deficient mice. Am J Pathol. 1997;151:177–191. [PMC free article] [PubMed] [Google Scholar]
- 14.Lubberts E, Joosten LA, Van Den Bersselaar L, Helsen MM, Bakker AC, Xing Z, Richards CD, Van Den Berg WB. Intra-articular IL-10 gene transfer regulates the expression of collagen-induced arthritis (CIA) in the knee and ipsilateral paw. Clin Exp Immunol. 2000;120:375–383. doi: 10.1046/j.1365-2249.2000.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung BP, I, McInnes B, Esfandiari E, Wei XQ, Liew FY. Combined effects of IL-12 and IL-18 on the induction of collagen-induced arthritis. J Immunol. 2000;164:6495–6502. doi: 10.4049/jimmunol.164.12.6495. [DOI] [PubMed] [Google Scholar]
- 16.Campbell IK, Hamilton JA, Wicks IP. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur J Immunol. 2000;30:1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, Marchal P, Duchatelle V, Degott C, van Regenmortel M, Benoist C, Mathis D. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto I, Maccioni M, Lee DM, Maurice M, Simmons B, Brenner M, Mathis D, Benoist C. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat Immunol. 2002;3:360–365. doi: 10.1038/ni772. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binstadt BA, Patel PR, Alencar H, Nigrovic PA, Lee DM, Mahmood U, Weissleder R, Mathis D, Benoist C. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nat Immunol. 2006;7:284–292. doi: 10.1038/ni1306. [DOI] [PubMed] [Google Scholar]
- 21.Serhan CN, Haeggstrom JZ, Leslie CC. Lipid mediator networks in cell signaling: update and impact of cytokines. Faseb J. 1996;10:1147–1158. doi: 10.1096/fasebj.10.10.8751717. [DOI] [PubMed] [Google Scholar]
- 22.Subbarao K, V, Jala R, Mathis S, Suttles J, Zacharias W, Ahamed J, Ali H, Tseng MT, Haribabu B. Role of Leukotriene B4 Receptors in the Development of Atherosclerosis: Potential Mechanisms. Arterioscler Thromb Vasc Biol. 2003 doi: 10.1161/01.ATV.0000110503.16605.15. [DOI] [PubMed] [Google Scholar]
- 23.Back M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U S A. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu H, Johansson AS, Sjostrom M, Wan M, Schroder O, Palmblad J, Haeggstrom JZ. Differential induction of BLT receptor expression on human endothelial cells by lipopolysaccharide, cytokines, and leukotriene B4. Proc Natl Acad Sci U S A. 2006;103:6913–6918. doi: 10.1073/pnas.0602208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 26.Lundeen KA, Sun B, Karlsson L, Fourie AM. Leukotriene B4 Receptors BLT1 and BLT2: Expression and Function in Human and Murine Mast Cells. J Immunol. 2006;177:3439–3447. doi: 10.4049/jimmunol.177.5.3439. [DOI] [PubMed] [Google Scholar]
- 27.Del Prete A, Shao WH, Mitola S, Santoro G, Sozzani S, Haribabu B. Regulation of Dendritic Cell Migration and Adaptive Immune Response by Leukotriene B4 Receptors: A Role for LTB4 in Up-regulation of CCR7 Expression and Function. Blood. 2006 doi: 10.1182/blood-2006-02-003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iizuka Y, Yokomizo T, Terawaki K, Komine M, Tamaki K, Shimizu T. Characterization of a mouse second leukotriene B4 receptor, mBLT2: BLT2-dependent ERK activation and cell migration of primary mouse keratinocytes. J Biol Chem. 2005;280:24816–24823. doi: 10.1074/jbc.M413257200. [DOI] [PubMed] [Google Scholar]
- 29.Shao WH, Del Prete A, Bock CB, Haribabu B. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: a critical role for BLT1 in collagen-induced arthritis in mice. J Immunol. 2006;176:6254–6261. doi: 10.4049/jimmunol.176.10.6254. [DOI] [PubMed] [Google Scholar]
- 30.Sperling RI, Benincaso AI, Anderson RJ, Coblyn JS, Austen KF, Weinblatt ME. Acute and chronic suppression of leukotriene B4 synthesis ex vivo in neutrophils from patients with rheumatoid arthritis beginning treatment with methotrexate. Arthritis Rheum. 1992;35:376–384. doi: 10.1002/art.1780350403. [DOI] [PubMed] [Google Scholar]
- 31.Gimbrone MA, Jr, Brock AF, Schafer AI. Leukotriene B4 stimulates polymorphonuclear leukocyte adhesion to cultured vascular endothelial cells. J Clin Invest. 1984;74:1552–1555. doi: 10.1172/JCI111570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto A, Endo H, Hayashi I, Murakami Y, Kitasato H, Kono S, Matsui T, Tanaka S, Nishimura A, Urabe K, Itoman M, Kondo H. Differential expression of leukotriene B4 receptor subtypes (BLT1 and BLT2) in human synovial tissues and synovial fluid leukocytes of patients with rheumatoid arthritis. J Rheumatol. 2003;30:1712–1718. [PubMed] [Google Scholar]
- 33.Griffiths RJ, Pettipher ER, Koch K, Farrell CA, Breslow R, Conklyn MJ, Smith MA, Hackman BC, Wimberly DJ, Milici AJ, et al. Leukotriene B4 plays a critical role in the progression of collagen-induced arthritis. Proc Natl Acad Sci U S A. 1995;92:517–521. doi: 10.1073/pnas.92.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwabara K, Yasui K, Jyoyama H, Maruyama T, Fleisch JH, Hori Y. Effects of the second-generation leukotriene B(4) receptor antagonist, LY293111Na, on leukocyte infiltration and collagen-induced arthritis in mice. Eur J Pharmacol. 2000;402:275–285. doi: 10.1016/s0014-2999(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 35.Tsuji F, Oki K, Fujisawa K, Okahara A, Horiuchi M, Mita S. Involvement of leukotriene B4 in arthritis models. Life Sci. 1999;64:PL51–56. doi: 10.1016/s0024-3205(98)00556-6. [DOI] [PubMed] [Google Scholar]
- 36.Griffiths RJ, Smith MA, Roach ML, Stock JL, Stam EJ, Milici AJ, Scampoli DN, Eskra JD, Byrum RS, Koller BH, McNeish JD. Collagen-induced arthritis is reduced in 5-lipoxygenase-activating protein-deficient mice. J Exp Med. 1997;185:1123–1129. doi: 10.1084/jem.185.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim ND, Chou RC, Seung E, Tager AM, Luster AD. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med. 2006;203:829–835. doi: 10.1084/jem.20052349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muller-Ladner U, Pap T, Gay RE, Neidhart M, Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005;1:102–110. doi: 10.1038/ncprheum0047. [DOI] [PubMed] [Google Scholar]
- 39.Allendorf DJ, Yan J, Ross GD, Hansen RD, Baran JT, Subbarao K, Wang L, Haribabu B. C5a -mediated leukotriene B4-amplified neutrophil chemotaxis is essential in tumor immunotherapy facilitated by anti-tumor monoclonal antibody and beta-glucan. J Immunol. 2005;174:7050–7056. doi: 10.4049/jimmunol.174.11.7050. [DOI] [PubMed] [Google Scholar]
- 40.Yokomizo T, Kato K, Hagiya H, Izumi T, Shimizu T. Hydroxyeicosanoids bind to and activate the low affinity leukotriene B4 receptor, BLT2. J Biol Chem. 2001;276:12454–12459. doi: 10.1074/jbc.M011361200. [DOI] [PubMed] [Google Scholar]