Abstract
Recent advances in our understanding of the immune response are allowing for the logical design of new approaches to cancer immunization. One area of interest is the development of new immune adjuvants. Immunostimulatory oligodeoxynucleotides containing the CpG motif (CpG ODN) can induce production of a wide variety of cytokines and activate B cells, monocytes, dendritic cells, and NK cells. Using the 38C13 B cell lymphoma model, we assessed whether CpG ODN can function as immune adjuvants in tumor antigen immunization. The idiotype served as the tumor antigen. Select CpG ODN were as effective as complete Freund’s adjuvant at inducing an antigen-specific antibody response but were associated with less toxicity. These CpG ODN induced a higher titer of antigen-specific IgG2a than did complete Freund’s adjuvant, suggesting an enhanced TH1 response. Mice immunized with CpG ODN as an adjuvant were protected from tumor challenge to a degree similar to that seen in mice immunized with complete Freund’s adjuvant. We conclude that CpG ODN are effective as immune adjuvants and are attractive as part of a tumor immunization strategy.
Bacterial DNA is capable of inducing activation of B cells, NK cells, and monocytes (1–5). In addition, bacterial DNA can induce production in vitro and in vivo of a variety of proinflammatory cytokines (6–8). In contrast, vertebrate DNA does not induce lymphocyte activation. Bacterial DNA contains a much higher frequency of unmethylated CpG dinucleotides than does vertebrate DNA due to (i) CpG suppression (the under representation of CpG in vertebrate genomes) and (ii) methylation of 80% of the CpG in vertebrates. It is possible that lymphocyte activation by the CpG motif in bacterial DNA represents an immune defense mechanism that can distinguish bacterial from host DNA (1). Select synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN) have immunologic effects similar to those seen with bacterial DNA. CpG ODN can stimulate monocytes, macrophages, and dendritic cells that then produce several cytokines, including the TH1 cytokine interleukin 12. This effect synergizes with CpG ODN to induce NK cell production of interferon γ (6). Both human and murine leukocytes respond to this novel pathway of immune activation, although individual CpG ODN differ somewhat in their ability to activate various immune cell populations and induce cytokine production in human and murine systems.
The molecular mechanisms responsible for CpG ODN-induced immune cell activation are still under investigation. We have recently reported that CpG ODN trigger the production of reactive oxygen species that activate NF-κB (9). This activation, in turn, leads to cellular activation. Irrespective of the mechanism involved, it is clear that select CpG ODN can have powerful immunologic effects and might be useful therapeutic agents in a number of circumstances, including cancer immunotherapy. For example, we have demonstrated that CpG ODN can enhance antibody-dependent cellular cytotoxicity and improve the in vivo efficacy of monoclonal antibody therapy in a syngeneic murine lymphoma model (10).
CpG ODN can induce activation of antigen-presenting cells and enhance production of cytokines known to participate in the development of an active immune response. They also enhance B cell activation, particularly when the B cell receptor is cross-linked (1). These effects are likely to promote antigen-specific responses. Indeed, Branda et al. (11) demonstrated that an anti-sense ODN, which in retrospect is noted to contain the CpG motif, enhances antibody response to antigen. We therefore used a well-established animal model to assess whether CpG ODN can function as an immune adjuvant in antitumor immunization.
METHODS
Tumor Model.
The 38C13 murine B cell lymphoma model has been used extensively in studies of antibody-based therapy and active immunization (12–15). The idiotype (Id) of the 38C13 surface IgM serves as a highly specific tumor-associated antigen (17, 18). 38C13 Id was obtained from the supernatant of a cell line that secretes 38C13 IgM as described (18), and purified by protein A affinity chromatography. Purified Id was conjugated to keyhole limpet hemocyanin (KLH) using gluteraldehyde and used as the immunogen.
Immunization.
Female C3H/HeN mice, obtained from Harlan-Sprague-Dawley, were housed in the University of Iowa Animal Care Unit and used at 6–9 weeks of age. Mice were immunized with 50 μg Id-KLH in a total volume of 200 μl PBS with the indicated antigen and injected subcutaneously except where indicated. Phosphorothioate ODN were produced in a GMP facility and purchased commercially (Oligos Etc., Wilsonville, OR). All CpG ODN were tested for endotoxin content (BioWhittaker), which was undetectable. All CpG ODN cytosines were unmethylated unless indicated. When CpG ODN was used as an adjuvant, both the antigen and CpG ODN were in aqueous phase. When CFA (Sigma) was used as an adjuvant, antigen and complete Freund’s adjuvant (CFA) were homogenized before injection.
ELISA Determination of Anti-Id Levels.
Serum was obtained by retroorbital puncture from mice following inhalation anesthesia with metophane. Microtiter plates were coated with 5 μg/ml 38C13 IgM or irrelevant IgM overnight. IgM-coated plates were blocked with 5% milk, and serial dilutions of serum were added. Plates were washed, and heavy chain-specific goat anti-mouse IgG, IgG1, or IgG2a (Southern Biotechnology Associates) added following by the colorimetric substrate p-nitrophenylphosphate. Serum from naive mice to which a known concentration of monoclonal anti-Id was added served as a standard. Plates were evaluated using a microplate reader and curves established for each sample. Test curves were compared with standard curves to determine the concentration of anti-Id. Values were considered valid only if the standard curves and the sample curves had the same shape.
In Vivo Survival Studies.
Two weeks after a single subcutaneous immunization, mice were inoculated i.p. with 1,000 38C13 cells. Mice that developed tumor displayed inguinal and abdominal masses, ascites, and cachexia. All mice that developed tumor died. Survival was determined, and significance with respect to time to death was assessed using Cox regression analysis.
RESULTS
CpG ODN 1758 Was Most Effective as an Adjuvant at Enhancing Production of Anti-Id Following Immunization with Id-KLH.
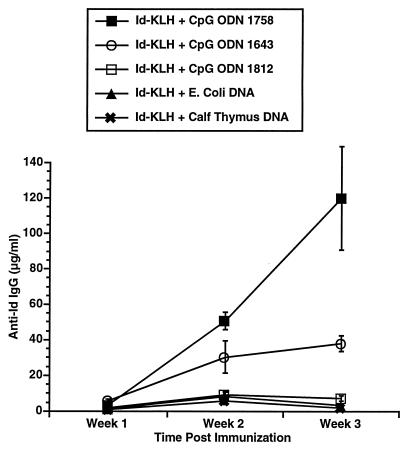
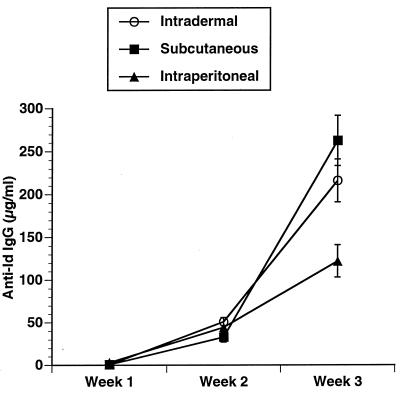
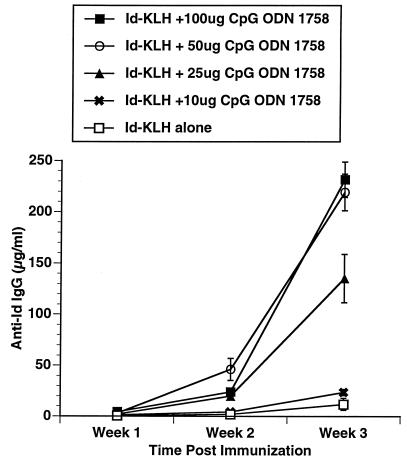
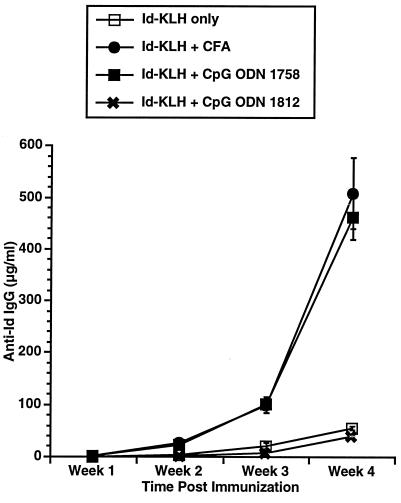
Three CpG ODN, Escherichia coli DNA, and calf thymus DNA were evaluated for their in vivo adjuvant effect. The CpG ODN were selected as representative based on our prior studies of their effects on B cells, cytokine secretion, and induction of NK activity. The sequences for these CpG ODN and their previously described in vitro effects are listed in Table 1. C3H mice were immunized with a single subcutaneous injection of 50 μg of Id-KLH in PBS mixed in aqueous solution with 50 μg of CpG ODN or DNA. Serum was obtained weekly and evaluated by ELISA for the presence of antigen-specific antibody (anti-Id IgG) and nonspecific anti-IgM (i.e., rheumatoid factor). As illustrated in Fig. 1, CpG ODN 1643 had a modest effect on enhancing anti-Id IgG levels. CpG ODN 1758 was most effective as an immune adjuvant at inducing production of anti-Id IgG. CpG ODN 1812, which is identical to 1758 in sequence but contains methylcytosines instead of unmethylated cytosines as part of the CpG dinucleotides, had little effect. No nonspecific anti-IgM was noted in any samples (data not shown), demonstrating the antibody response was directed against the idiotype of the 38C13 IgM. The development of an anti-Id response was somewhat higher after subcutaneous and intradermal injection of the antigen and adjuvant when compared with intraperitoneal administration (Fig. 2). There also was a dose-response effect with a maximum effect reached at a dose of 50 μg/mouse (Fig. 3).
Table 1.
Different CpG motifs
| ODN | Sequence | Predominant in vitro effects |
|---|---|---|
| 1758 | TCTCCCAGCGTGCGCCAT | Monocyte activation; enhanced NK activity (10) |
| 1812 | TCTCCCAGZGTGZGCCAT | Minimal immunostimulatory effect (10) |
| 1643 | GAGAACGCTCGACCTTCGAT | B cell mitogen (9) |
CpG dinucleotides are underlined; Z indicates 5-methylcytosine in the control ODN with methylated CpGs, 1812. All oligonucleotides were synthesized with phosphorothioate modified backbones to improve their nuclease resistance.
Figure 1.
Comparison of anti-Id titers following immunization using CpG ODN or DNA as an adjuvant. 38C13 Id IgM was conjugated to KLH using gluteraldehyde to produce Id-KLH. A total of 50 μg Id-KLH was mixed with adjuvant in aqueous solution and injected subcutaneously into naive 6- to 8-week-old female C3H mice in a volume of 0.2 ml. Each mouse received a single immunization. Blood was obtained weekly, and serum was evaluated for the presence of anti-Id IgG by ELISA. Normal mouse serum supplemented with a known concentration of monoclonal anti-Id was used as a standard. Three mice were included in each group.
Figure 2.
Route of immunization. Mice were immunized and anti-Id levels determined as in Fig. 1. Development of anti-Id was determined after subcutaneous, intradermal, or intraperitoneal immunization.
Figure 3.
Dose response to CpG ODN. Mice were immunized and anti-Id levels determined as in Fig. 1. All mice received 50 μg Id-KLH. The dose of CpG ODN 1758 was varied to assess dose response to adjuvant.
The Adjuvant Effect of CpG ODN Is Local, Not Systemic.
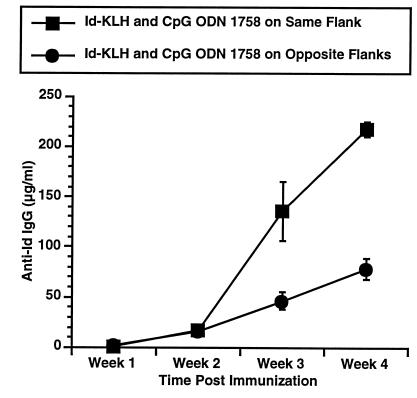
We next assessed whether the adjuvant effect of CpG ODN was local or systemic. One group of mice were immunized subcutaneously with Id-KLH on one flank and simultaneously injected with 50 μg CpG ODN 1758 on the contralateral flank, whereas a second group received a single injection of both agents (Fig. 4). Injection of CpG ODN and antigen on the same flank was required for maximal adjuvant effect. Thus, CpG ODN exerts much of its adjuvant effect locally. This finding is consistent with our prior observations that footpad injection with CpG ODN enhances NK activity of cells in the ipsilateral but not contralateral lymph node (19).
Figure 4.
Comparison of ipsilateral and contralateral injection. Mice were immunized and anti-Id levels determined as in Fig. 1. All mice received 50 μg Id-KLH and 50 μg CpG ODN 1758. Mice were injected with Id-KLH and CpG ODN 1758 mixed together (ipsilateral) or were injected on the right flank with Id-KLH and CpG ODN 1758 on the left flank (contralateral) to assess whether the adjuvant effect of CpG ODN is systemic or local.
CpG ODN Is as Effective as CFA After Primary and Secondary Immunization.
We next compared CpG ODN 1758 as an adjuvant to CFA. Mice were immunized with Id-KLH and CpG ODN 1758 or Id-KLH and CFA on day 0 and boosted with Id-KLH and CpG ODN or Id-KLH and incomplete Freund’s adjuvant on day 14. Serum was obtained weekly and anti-Id IgG levels determined. Mice immunized with CFA developed inflammatory masses at the sites of immunization, were less mobile, and demonstrated ruffled fur, whereas no such masses or other changes were noted in mice immunized with CpG ODN. No other toxicity was observed in either group. As illustrated in Fig. 5, CpG ODN 1758 and CFA were similar in their ability to induce anti-Id.
Figure 5.
Comparison of anti-Id titers following immunization with CpG ODN and CFA. Id-KLH was mixed with either CpG ODN 1758 in PBS or homogenized with CFA and injected subcutaneously in a volume of 0.2 ml. Mice were boosted with a second injection at 2 weeks with Id-KLH and CpG ODN 1758 or Id-KLH and incomplete Freund’s adjuvant.
CpG ODN Is More Effective Than CFA at Inducing Production of Antigen-Specific Antibody of the IgG2a Isotype.
Murine IgG2a is more effective than murine IgG1 at mediating antibody-dependent cellular cytotoxicity, and monoclonal IgG2a has been shown to be more effective as an antitumor agent in animal models of antibody therapy of cancer when compared with an antibody with the identical variable region of the IgG1 isotype (20). Enhanced production of IgG2a also suggests a TH1 response (21). We therefore evaluated the level of IgG2a and IgG1 anti-Id induced by immunization with CpG ODN 1758, and compared it to that induced by CFA. As shown in Table 2, CpG ODN 1758 induced enhanced production of IgG2a compared with that seen with CFA.
Table 2.
CpG ODN 1758 enhances production of antigen-specific IgG2a
| Anti-Id levels | CFA | CpG ODN 1758 | CpG ODN 1812 |
|---|---|---|---|
| IgG2a, μg/ml | 54 ± 9 | 121 ± 17 | 5 ± 1 |
| IgG1, μg/ml | 178 ± 20 | 127 ± 4 | 21 ± 2 |
| IgG2a/IgG1 | 0.30 | 0.95 | 0.23 |
Mice were immunized and serum obtained 3 weeks after a single immunization. Antigen-specific IgG1 and IgG2a was determined by ELISA as described. Data are presented as isotype specific anti-Id ± SEM.
Immunization Using CpG ODN as an Adjuvant Leads to Protection from Tumor Growth.
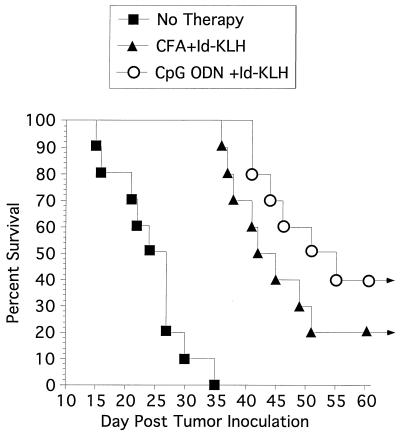
The goal of immunization with tumor antigen is to inhibit tumor growth. We therefore evaluated whether 38C13 tumor growth is inhibited in immunocompetent, syngeneic mice immunized with Id-KLH using CpG ODN 1758 as an immune adjuvant. Prior studies in the 38C13 model have involved multiple boosts with Id-KLH in CFA prior to tumor challenge (16). Because we sought to assess whether CpG ODN might be a more effective adjuvant that CFA, we challenged mice with tumor 2 weeks after a single immunization with Id-KLH using CpG ODN 1758 or complete Freund’s adjuvant. As illustrated in Fig. 6, all unimmunized mice developed tumor and died within 35 days, with a median survival of 25 days. Mice immunized with Id-KLH and CFA had a median survival of 42 days, and two animals remained disease free indefinitely whereas mice immunized with Id-KLH and CpG ODN 1758 had a median survival of 51 days with four mice remaining disease free. Use of CpG ODN 1758 or CFA as an adjuvant markedly improved survival when compared with control animals (P < 0.001). Although mice immunized with CpG ODN 1758 had longer survival than mice immunized with CFA, this difference did not reach statistical significance (P = 0.18). CpG ODN 1758 itself without the use of antigen had no detectable protective effect (data not shown).
Figure 6.
Tumor protection in C3H mice. Mice were immunized subcutaneously with a single dose of Id-KLH and CpG ODN 1758, Id-KLH, and CFA or were unimmunized. Two weeks later they were challenged with 1,000 38C13 cells intraperitoneally. Survival was followed for 100 days. All mice that were alive after 55 days remained tumor-free for the entire observation period. Ten mice were included in each group.
DISCUSSION
Recent advances in our understanding of tumor immunology and the immune response in general are allowing for the development of new, rational approaches to cancer immunotherapy. One promising approach is immunization with tumor-specific proteins or peptides. Recent studies suggest development of an immune response to a tumor specific antigen after immunization correlates with improved clinical outcome (22). CFA is the standard adjuvant in animal models, although the intensity of the local inflammation that results following injection with this adjuvant prevents its use clinically. Other adjuvants, such as QS21 (23), are currently being evaluated in clinical studies. Recombinant cytokines have also been explored as adjuvants with some success. However, immunization using an adjuvant that induces the orchestrated activation of various immune subsets and the production of multiple cytokines known to participate in the development of an active immune response is likely to be more effective and perhaps less toxic than immunization using single cytokine as an adjuvant.
Bacterial DNA has significant immunostimulatory effects on B cells, monocytes, and NK cells and can induce production of many of the cytokines that have been shown to be important in the development of antitumor immunity. The studies outlined above confirm that synthetic CpG ODN containing unmethylated CpG motifs can have similar effects. Although our approach is based on the hypothesis that the immune activation in response to bacterial DNA can be used to enhance the immune response to tumor, bacterial DNA itself had no detectable adjuvant effect in our studies. Because of its sensitivity to nucleases, bacterial DNA may not have survived long enough for an adjuvant effect to develop. Indeed, phosphodiester ODN have very short half-lives (24) and would not be expected to have much of an adjuvant effect unless administered repeatedly. The phosphorothioate backbone of the CpG ODN prolongs the half-life of ODN, and this may have contributed to its increased efficacy. It is also possible that bacterial DNA activates different subsets of cells than does CpG ODN, and that some negative feedback due to enhanced production of select cytokines, such as TH2 cytokines, limits the adjuvant effect of bacterial DNA. This contention is supported by the fact that CpG ODN 1643 is a more potent B cell mitogen than is CpG ODN 1758, yet had less of an effect as an immune adjuvant.
CpG ODN was as effective as CFA at enhancing production of anti-Id antibody following immunization, and was more effective at inducing production of IgG2a anti-Id. This effect was not seen with an ODN consisting of an identical sequence containing methylated CpG motifs, nor was it as extensive when the CpG ODN was administered on the opposite flank, indicating the adjuvant effect of CpG ODN is secondary to the CpG motif and is largely a local, not a systemic, effect.
A number of important questions remain to be answered. The molecular mechanisms responsible for CpG ODN-induced immunostimulation, and an explanation for why different CpG ODN have different effects, remain unclear and need to be elucidated. The enhanced production of IgG2a suggests a TH1 response to CpG ODN; however, changes in T cell function in response to CpG ODN or enhanced induction of cytotoxic T cells has yet to be explored rigorously. It will be important to determine whether immunization using CpG ODN as an adjuvant indeed enhances the cellular immune response and whether this plays a role in the observed tumor rejection. Synergy with other adjuvants also needs to be explored. Nevertheless, these studies demonstrate that adjuvant CpG ODN can orchestrate an immune response that leads to enhanced antigen-specific antibody formation. CpG ODN could therefore supply a unique approach to enhancing the efficacy of immunization including enhancing antitumor immunity.
Acknowledgments
These studies were supported by the Department of Veteran’s Affairs (G.J.W. and A.M.K.), the University of Iowa Cancer Center, and Training Grant HL07344 from the National Institutes of Health (J.E.W.). Services were provided by the University of Iowa Diabetes and Endocrinology Center, National Institutes of Health Grant DK25295.
ABBREVIATIONS
- CpG ODN
oligodeoxynucleotides containing the CpG motif ODN
- Id
idiotype
- KLH
keyhole limpet hemocyanin
- CFA
complete Freund’s adjuvant
References
- 1.Krieg A M, Yi A K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Klinman D M. Nature (London) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 2.Pisetsky D S, Reich C, Crowley S D, Halpern M D. Ann NY Acad Sci. 1995;772:152–163. doi: 10.1111/j.1749-6632.1995.tb44740.x. [DOI] [PubMed] [Google Scholar]
- 3.Stacey J J, Sweet M J, Hume D A. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 4.Yamamoto S, Yamamoto T, Shimada T, Kuramoto E, Yano O, Kataoka T, Tokunaga T. Microbiol Immunol. 1992;36:983–997. doi: 10.1111/j.1348-0421.1992.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 5.Messina J P, Gilkeson G S, Pisetsky D S. J Immunol. 1991;147:1759–1764. [PubMed] [Google Scholar]
- 6.Cowdery J S, Chace J H, Yi A K, Krieg A M. J Immunol. 1996;156:4570–4575. [PubMed] [Google Scholar]
- 7.Halpern M D, Kurlander R J, Pisetsky D S. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 8.Klinman D M, Yi A K, Beaucage S L, Conover J, Krieg A M. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi A-K, Klinman D M, Martin T L, Matson S, Krieg A M. J Immunol. 1996;157:5394–5402. [PubMed] [Google Scholar]
- 10.Wooldridge J E, Ballas Z, Krieg A M, Weiner G J. Blood. 1997;89:2994–2998. [PubMed] [Google Scholar]
- 11.Branda R F, Moore A L, Lafayette A R, Mathews L, Hong R, Zon G, Brown T, McCormack J J. J Lab Clin Med. 1996;128:329–338. doi: 10.1016/s0022-2143(96)90035-9. [DOI] [PubMed] [Google Scholar]
- 12.Campbell M J, Esserman L, Byars N E, Allison A C, Levy R. J Immunol. 1990;145:1029–1036. [PubMed] [Google Scholar]
- 13.Maloney D G, Kaminski M S, Burowski D, Haimovich J, Levy R. Hybridoma. 1986;4:191–209. doi: 10.1089/hyb.1985.4.191. [DOI] [PubMed] [Google Scholar]
- 14.Weiner G J, Kaminski M S. J Immunol. 1989;142:343–351. [PubMed] [Google Scholar]
- 15.Weiner G J, Kaminski M S. J Immunol. 1990;144:2436–2445. [PubMed] [Google Scholar]
- 16.Kaminski M S, Kitamura K, Maloney D G, Levy R. J Immunol. 1987;138:1289–1296. [PubMed] [Google Scholar]
- 17.Bergman Y, Haimovich J. Eur J Immunol. 1977;7:413–417. doi: 10.1002/eji.1830070702. [DOI] [PubMed] [Google Scholar]
- 18.Eshhar Z, Blatt Y, Bergman Y, Haimovich J. J Immunol. 1979;122:2430–2434. [PubMed] [Google Scholar]
- 19.Ballas Z K, Rasmussen W L, Krieg A M. J Immunol. 1996;157:1840–1845. [PubMed] [Google Scholar]
- 20.Kaminski M S, Kitamura K, Maloney D G, Campbell M J, Levy R. J Immunol. 1986;136:1123–1130. [PubMed] [Google Scholar]
- 21.Stevens T L, Bossie A, Sanders V M, Fernandez-Botran R, Coffman R L, Mosmann T R, Vitetta E S. Nature (London) 1988;334:255–158. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 22.Hsu F J, Caspar C B, Czerwinski D, Kwak L W, Liles T M, Syrengelas A, Taidilaskowski B, Levy R. Blood. 1997;89:3129–3135. [PubMed] [Google Scholar]
- 23.Livingston P O. Immunol Rev. 1995;145:147–166. doi: 10.1111/j.1600-065x.1995.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 24.Agrawal S, Temsamani J, Galbraith W, Tang J. Clin Pharmacokinet. 1995;28:7–16. doi: 10.2165/00003088-199528010-00002. [DOI] [PubMed] [Google Scholar]