Abstract
IL-4 receptor α chain (IL-4Rα)-deficient mice were generated by gene-targeting in BALB/c embryonic stem cells. Mutant mice showed a loss of IL-4 signal transduction and functional activity. The lack of IL-4Rα resulted in markedly diminished, but not absent, TH2 responses after infection with the helminthic parasite Nippostrongylus brasiliensis. CD4+, CD62L-high, and CD62L-low T cell populations from uninfected IL-4Rα−/− mice were isolated by cell sorting. Upon primary stimulation by T cell receptor cross-linkage, the CD62L-low, but not the CD62L-high, cells secreted considerable amounts of IL-4, which was strikingly enhanced upon 4-day culture with anti-CD3 in the presence or absence of IL-4. CD62L-low cells isolated from IL-4Rα−/−, β2-microglobulin−/− double homozygous mice produced less IL-4 than did either IL-4Rα−/− or wild-type mice. These results indicate that an IL-4-independent, β2-microglobulin-dependent pathway exists through which the CD62L-low CD4+ population has acquired IL-4-producing capacity in vivo, strongly suggesting that these cells are NK T cells.
IL-4 is a pleiotropic cytokine that binds to high-affinity receptors expressed on hematopoietic and nonhematopoietic cells (1). The IL-4 receptor is a heterodimeric molecule comprised of the 140-kDa high-affinity binding chain, IL-4 receptor α chain (IL-4Rα) (2, 3) and the γc subunit (4, 5). The γc chain is also shared with receptors for IL-2, IL-7, IL-9, and IL-15 (5–10). Both IL-4Rα and γc chains are involved in mediating IL-4 binding and signaling. The IL-4Rα chain also associates with the IL-13Rα chain to form a high-affinity IL-13 receptor (11).
Two types of T helper subsets have been defined based on the profile of cytokines they produce (12). TH1 clones secrete predominately interferon γ (IFN-γ) and little IL-4, whereas TH2 clones secrete IL-4, IL-5, IL-10, and IL-13 and little IFN-γ. The addition of exogenous IL-4 to in vitro cultures is required for the differentiation of naive CD4+ T cells into IL-4-secreting TH2 cells (13). Evidence suggesting an in vivo requirement for IL-4 in the induction of TH2 cells comes from studies of IL-4−/− and STAT6−/− mice. In the former, in vivo priming for IL-5 production in response to infection with Nippostrongylus brasiliensis is diminished whereas in the latter N. brasiliensis infection results in diminished IL-4 production upon in vitro stimulation (14, 15). The cell types responsible for producing the IL-4 needed for TH2 priming have not been conclusively identified. Possible candidates include CD4+, NK1.1+ T (NK T) cells (16), cells of the mast cell or basophil lineage (17–19), γδ T cells (20), and conventional T cells (21).
To more definitively investigate the role of IL-4 in TH2 development, we disrupted the IL-4Rα gene by homologous recombination in BALB/c-I embryonic stem (ES) cells (22). Because these mice lack sensitivity to IL-4, we could evaluate the relative importance of IL-4 in development of TH2 responses and could determine what cell populations retain the capacity to produce IL-4 even when they differentiate in the total absence of IL-4. Here we describe results from these studies in which we tested the relative ability of CD4+ T cells from wild-type and IL-4Rα-deficient mice to develop into IL-4 producers.
MATERIALS AND METHODS
Mice.
BALB/c β2 microglobulin (β2m)-deficient mice (23) were derived after 10 backcrosses to the BALB/c strain and were obtained from The Jackson Laboratory.
IL-4Rα Targeting.
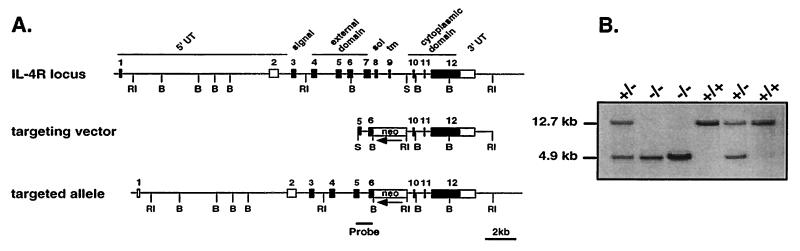
The IL-4Rα targeting vector (see Fig. 1A) was constructed from a 12.7-kb BALB/c genomic clone spanning IL-4Rα exons 4–12 (24). A 0.8-kb PCR product generated from sequences in exons 5 and 6 and incorporating a 5′ SmaI site in exon 5 was digested with SmaI/BamHI and ligated to a 1.7-kb BamHI/EcoRI fragment from plasmid PGKneotk (provided by Heiner Schrewe, Max-Planck-Institut für Immunbiologie, Freiburg, Germany) and a 5.5-kb EcoRI/SmaI fragment containing exons 10, 11, and 12 (Fig. 1A). Ten micrograms of PvuI-digested IL-4Rα target vector was electorporated into 1 × 107 BALB/c-I ES cells (22). Cells were maintained in DMEM containing 15% ES cell tested fetal bovine serum, 0.1 mM nonessential amino acids, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, and 1000 units/ml ESGRO recombinant leukemia inhibitory factor (all from GIBCO/BRL). Selection media containing 0.2 mg/ml G418 (Geneticin; GIBCO/BRL) was added 2 days after electorporation. G418-resistant ES cell colonies were isolated 7–8 days after addition of selection medium and screened for correct targeting by nested-primer PCR as described (25). First-round PCR primers sequences were as follows: IL-4Rp1, 5′-AACCTCATATGCATCCCGAG-3′ and neop3, 5′-CGGTATCGCCGCTCCCGATTCG-3′. Cycling conditions were 94°C for 2 min primary denaturation, then 94°C for 20 sec, 60°C for 20 sec, and 72°C for 30 sec for 20 cycles in a 50 μl reaction. Ten microliters of the first-round product was amplified with nested primers with the same cycling conditions for an additional 33 cycles. Second-round primer sequences were as follows: IL-4Rp2, 5′-AACTGTGGGCTGAGCACAGACA-3′ and neop4, 5′-GCGCATCGCCTTCTATCGCCTTC-3′. The final product was run through a 1.6% agarose gel containing ethidium bromide. A mimic control vector transfected and isolated under similar conditions was used as a positive control for the screenings. One targeted clone was injected into 3.5-day blastocysts from C57BL/6 mice and transferred into foster females. Chimeric males were bred to BALB/cJ females. Albino coat-color offspring from chimeric matings were screened for the mutated IL-4Rα allele by Southern blot and PCR analyses.
Figure 1.
Targeting of IL-4Rα. The IL-4Rα locus was disrupted in BALB/c ES cells. (A) The IL-4Rα gene, targeting construct, and locus after homologous recombination are shown. Exons 7–9 (4.5 kb) were replaced by a PGKneo gene orientated antiparallel to the IL-4Rα gene. The targeting vector contained 6.3-kb homology to the genomic sequence. (B) Southern blot analysis of EcoRI-digested DNA obtained from offspring of heterozygous matings. The 12.7-kb band indicates the presence of the wild-type allele. The 4.9-kb band reveals the disrupted IL-4Rα locus.
Southern Blot and PCR Analyses.
Genomic DNA from offspring of IL-4Rα heterozygous matings was prepared from tail biopsies (26) (see Fig. 1B). DNA (5 μg) was digested with EcoRI, run through a 0.7% agarose gel, and transferred to nylon membrane (Zeta-Probe; Bio-Rad) under denaturing conditions. The filter was hybridized with 25 ng of [α-32P]dCTP-labeled DNA probe (Ready-To-Go; Pharmacia) with QuikHyb solution (Stratagene) and exposed to film overnight (Kodak X-Omat AR). Genotyping was also performed by PCR using primer pairs specific for exon 7 and the neo gene in a single reaction. Primer sequences for exon 7 were as follows: CR1.1, 5′-AATGTGACCTACAAGGAACC-3′ and CR1.2, 5′-GGACTCCACTCACTCCAG-3′. Neo sequences were as follows: IMR013, 5′-CTTGGGTGGAGAGGCTATTC-3′ and IMR14, 5′-AGGTGAGATGACAGGAGATC-3′. PCR products reveal a 125-bp exon 7 band in IL-4Rα+/+ mice, a 280-bp neo band in IL-4Rα−/− mice, and 125-bp and 280-bp bands in IL-4Rα+/− mice.
Culture Medium.
Complete RPMI (cRPMI) consisted of RPMI 1640 medium (Biofluids, Rockville, MD) supplemented with 10% fetal bovine serum (GIBCO/BRL), 1 mM sodium pyruvate, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Cytokines and Antibodies.
Mouse IL-4 was obtained from a recombinant baculovirus prepared by Cynthia Watson (National Institute of Allergy and Infectious Diseases). Human IL-2 was a gift of Cetus. IFN-γ was purchased from Genzyme. Anti-IL-4 (11B11) (27) was prepared by Verax (Lebanon, NH). Goat anti-IL-12 was purchased from R & D Systems. Anti-IFN-γ (XMG 1.2), biotinylated mouse-anti-rat IgG2a, fluorescein inothiocyanate (FITC)-anti-CD45R, FITC-anti-CD62L, phycoerythrin (PE)–anti-major histocompatibility complex (MHC) I-Ab,d, PE–anti-CD4, and streptavidin–PE were purchased from PharMingen. Monoclonal rat ant-mouse IL-4Rα M1 (28) was purchased from Genzyme. Monoclonal rat IgG2a (PharMingen) was used as an isotype control for the IL-4Rα stainings. Rat anti-mouse Fcγ receptor antibody 2.4G2 was used as 1:500 diluted ascites. Anti-CD3 antibody (2C11) was prepared by Jane Hu-Li (National Institute of Allergy and Infectious Diseases).
Cytokine and Ig Measurements.
IL-4, IL-5 (Endogen), IL-2, and IL-10 (PharMingen) ELISAs were performed according to manufacturer’s directions. IFN-γ was measured with a two-site ELISA (29, 30). In some experiments, IL-4 activity was quantitated using the IL-4 indicator cell line CT.4S (31). IgG1 and IgG2a isotyping reagents were purchased from Southern Biotechnology Associates. IgE ELISA was performed as described (32). 96-well plates were coated with 2 μg/ml each of two monoclonal anti-IgE antibodies (02131D from PharMingen and AMI2501 from BioSource, Camarillo, CA). After blocking and overnight incubation with serum samples, plates were developed with horseradish peroxidase-conjugated goat anti-IgE antibodies (Southern Biotechnology Associates) followed by peroxidase substrate (Bio-Rad).
Nippostrongylus Infection.
Five hundred third-stage N. brasiliensis larvae were injected subcutaneously into IL-4R−/− and IL-4R+/− controls. Mice were killed 7 days after infection and CD4+ T cells were isolated from the mesenteric lymph node by passing though a CD4-enrichment column (Cedarlane Laboratories). CD4+ cells were further isolated to 100% purity by flow sorting. Cells were cultured at 1 × 106/ml in 24-well plates coated with anti-CD3 (10 μg/ml). Supernatants were removed at 24 hr and assayed for IL-4, IL-5, IL-10, and IFN-γ by ELISA. In a separate experiment, serum Ig levels were measured 13 days after infection.
In Vitro Stimulation of CD4+ Subsets.
CD4+ lymph node cells from wild-type and IL-4Rα−/− mice were isolated by passing through a CD4-enrichment column (Cedarlane Laboratories). Purified CD4+ cells were stained with PE–anti-CD4 and FITC–anti-CD62L. Cells were flow-sorted into CD4+/CD62L-high and CD4+/CD62L-low populations and plated at 1 × 106/ml in 96-well plates coated with anti-CD3 (10 μg/ml). Supernatants were removed at 24 hr and assayed for IL-4 and IFN-γ by ELISA.
Cell Priming Conditions.
CD4+ cells from mesenteric lymph nodes were isolated and sorted for CD4+/CD62L-high and -low populations as described. Sorted populations (1 × 106/ml) were cultured with T-depleted irradiated spleen cells (7 × 106/ml) from BALB/c IL-4-deficient mice (22) as antigen-presenting cells in 24-well plates in cRPMI containing 3 μg/ml soluble anti-CD3. Cells were cultured in the presence of IL-2 (10 units/ml), anti-IFN-γ (10 μg/ml), and anti-IL-12 (10 μg/ml). Some cultures also contained IL-4 (1,000 units/ml) or anti-IL-4 (20 μg/ml). After 4 days of culture, cells were harvested, washed twice, and plated at 1 × 106/ml in cRPMI with IL-2 on 96-well plates coated with anti-CD3 (10 μg/ml). Supernatants were harvested at 24 hr and assayed for IL-4 and IFN-γ by ELISA.
Anti-CD3 in Vivo.
Mice were injected i.v. with 1.33 μg anti-CD3 as described (16). After 90 min, spleens were removed, dispersed into single cells, and cultured at 5 × 106/ml in 24-well plates for 1 hr. Supernatants were assayed for IL-4 by CT.4S proliferation.
STAT6 Electrophoretic Mobility-Shift Assay.
Freshly isolated spleens cells (10 × 106) from IL-4Rα+/− and IL-4Rα−/− mice were stimulated with 10,000 units/ml IL-4 for 10 min at room temperature. Cell lysates were prepared as described (33). A double-stranded oligonucleotide, the STAT6 element, corresponding to an IFN-γ activation site-like element found in the mouse IL-4 promoter (gatcAAGACCTTCACAGGAACTTTAATCT, provided by Hua Huang, National Institute of Allergy and Infectious Diseases) was synthesized with a 5′-gatc overhang (denoted by lowercase letters) and labeled with [32P]dCTP using Klenow DNA polymerase by standard techniques. For supershift of STAT6 complexes, cell lysates were incubated with a 32P-labeled STAT6 element and then incubated with 1 μl anti-mouse STAT6 antibody (Santa Cruz Biotechnology) before electrophoresis.
RESULTS
The IL-4Rα locus was disrupted as shown in Fig. 1A. Homozygous IL-4Rα−/− mice showed no overt phenotypic abnormalities and had normal lymphocyte cell numbers in spleen and thymus. The expression of CD3, Vβ8, CD4, CD8, CD23, CD45, class II MHC, sIgM, and sIgD molecules were unaltered in IL-4Rα−/− mice (data not shown).
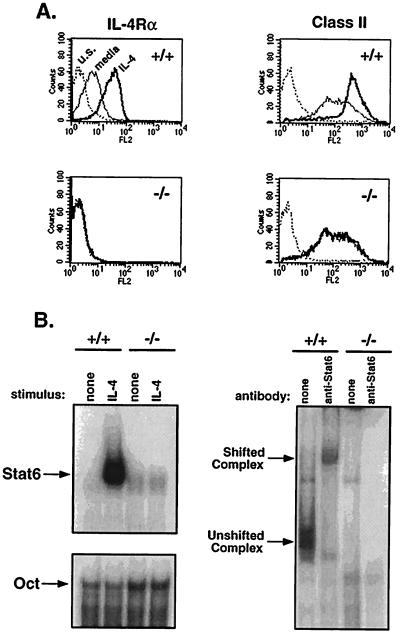
IL-4 ligand binding to its receptor up-regulates surface expression of class II MHC, CD23 molecules, and IL-4Rα itself (34–36). To confirm the absence of IL-4Rα expression in mutant mice, splenocytes from IL-4Rα−/− mice and littermate controls were cultured in the presence or absence of IL-4 for 40 hr. IL-4Rα and class II MHC levels were measured by flow cytometry. Expression levels of IL-4Rα and class II MHC were up-regulated on splenocytes from wild-type mice after culture with IL-4 (Fig. 2A). In contrast, IL-4Rα−/− mice lacked detectable IL-4Rα expression and such expression was not induced after the addition of IL-4. Class II MHC expression was also not up-regulated in mutant mice, corresponding with the loss of IL-4 functional activity.
Figure 2.
IL-4Rα is not expressed in targeted mice. (A) IL-4Rα expression and induction of IL-4Rα and class II MHC were measured by flow cytometry. Spleen cells from IL-4Rα−/− mice and wild-type littermates were cultured at 1 × 106/ml in cRPMI medium for 40 hr in medium alone or with IL-4 (1000 units/ml). For detection of IL-4Rα, splenocytes were stained with 20 μg/ml rat anti-mouse IL-4Rα (M1) and detected with biotinylated mouse anti-rat IgG2a in the presence of 10 μl normal mouse serum, followed by streptavidin-PE. Class II MHC Iab,d expression was gated on B220+ cells. Dotted line, unstained cells; thin line, medium alone; bold line, with IL-4. (B) STAT6 EMSA. Spleen cells were cultured with IL-4 (10,000 units) for 10 min. Cell lysates were incubated with a double-stranded STAT6-element probe and run on a acrylamide gel. Oct1 sequences were used to control for loading. A polyclonal anti-STAT6 antibody supershifted the complex (Right).
Interaction of IL-4 with IL-4Rα on the cell surface specifically triggers the activation of STAT6, which is a key step in the induction of many IL-4-inducible genes (15, 37, 38). IL-4Rα−/− cells did not activate STAT6 in response to IL-4 as revealed by the failure of extracts from these cells to form a complex with the IFN-γ activation site element derived from the IL-4 promoter, whereas extracts from IL-4-treated wild-type cells formed such a complex that could be supershifted with an anti-STAT6 antibody (Fig. 2B).
TH2 responses in IL-4Rα−/− Mice.
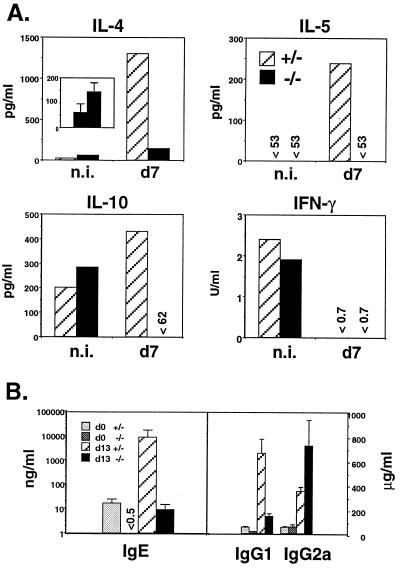
IL-4−/− mice have diminished TH2 responses and STAT6−/− mice diminished in vitro production of IL-4 in response to infection with N. brasiliensis, implicating a role for IL-4 the development of TH2 cells in vivo (14, 15). We asked whether TH2 responses in IL-4Rα−/− mice were also impaired. Mice were infected with N. brasiliensis. Purified CD4+ T cells were prepared 7 days later and stimulated with immobilized anti-CD3; supernatants were assayed for cytokine production. IL-4 production by CD4+ T cells from the IL-4Rα−/− mice was strikingly impaired and IL-5 and IL-10 were undetectable (Fig. 3A). Further evidence for the marked diminution in TH2 responses was reflected by the lower levels of total serum IgE and IgG1 in infected and uninfected IL-4Rα−/− mice, as compared with wild-type mice, and increased IgG2a levels in infected IL-4Rα−/− mice (Fig. 3B). Interestingly, IFN-γ production by CD4+ T cells from infected IL-4Rα−/− mice was not elevated, suggesting that IL-4 may play a limited role in regulating IFN-γ production from CD4+ T cells in this infection in BALB/c mice. It is interesting that serum IgG2a levels were increased in these mice. Because IFN-γ can induce switching to the γ2a CH chain, this increase might reflect an up-regulation in IFN-γ that was not detected by stimulation of CD4+ T cells from mesenteric lymph node cells.
Figure 3.
TH2 cytokines are reduced after N. brasiliensis infection. (A) Mesenteric lymph nodes were removed from noninfected controls (n.i.) and mice infected 7 days with N. brasiliensis. Purified CD4+ cells (106/ml) were stimulated for 24 hr with immobilized anti-CD3 and supernatant cytokine content measured by ELISA. (Inset) The graph compares IL-4 production in noninfected (Left) and infected (Right) IL-4Rα−/− mice. (B) Serum IgE, IgG1, and IgG2a were measured at days 0 and 13 after infection. Error bars represent standard deviations (n = 3).
Although serum IgE levels were reduced by 1,000-fold in the IL-4Rα−/− mice after infection, small amounts of IgE were detectable (9 ng/ml), suggesting that an IL-4Rα-independent pathway of IgE production exists in vivo. An IL-4-independent pathway for induction of IgE has also been observed in anti-IgD treated IL-4−/− mice (39) and STAT6−/− mice (38).
IL-4 Production from CD62L-Low CD4+ T Cells.
The diminished but still measurable production of IL-4 by CD4+ T cells from N. brasiliensis-infected IL-4Rα−/− mice implied that some CD4+ cells were capable of secreting IL-4 independently of IL-4 action in vivo. This led us to examine the capacity of lymph node CD4+ T cells to produce IL-4 in vitro in response to challenge with immobilized anti-CD3 and to develop into IL-4-producers as a result of in vitro stimulation with anti-CD3 and subsequent rechallenge. We chose to use mesenteric lymph nodes as a source of CD4+ T cells because these cells in other mouse strains have been shown to have relatively few NK T cells in comparison to T cell populations from spleen (16) or certain sites within the gut-associated lymphoid tissues (40). We did this because we were particularly interested in determining whether a source of IL-4 other than NK T cells might be found in the CD4 population of IL-4Rα−/− mice.
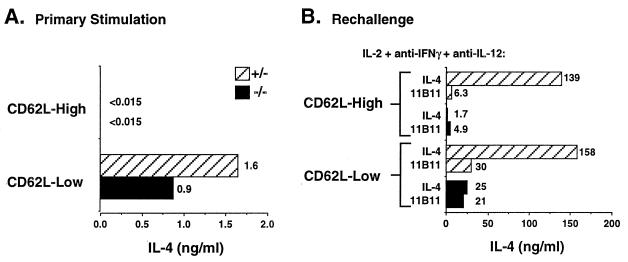
We used the expression of the peripheral lymph node homing receptor CD62L to discriminate resting and/or naive CD4+ T cells from other CD4 cell populations (41). Lymph node CD4+ cells were sorted into CD62L-low and CD62L-high populations and stimulated with immobilized anti-CD3 for 24 hr. Such stimulation of CD4+, CD62L-low cells resulted in similar levels of IL-4 production by wild-type and IL-4Rα−/− cells whereas the CD62L-high populations from both sets of mice produced no detectable IL-4 (Fig. 4A). IFN-γ was also undetectable in supernatants of the CD62L-high populations, but was produced in equivalent amounts from the CD62L-low cells from both wild-type and IL-4Rα−/− mice (11 units/ml each, data not shown).
Figure 4.
IL-4 is produced by CD62L-low CD4+ cells independent of IL-4 priming. (A) Purified CD4+ T cells were sorted for CD4+, CD62L-high and CD4+, CD62L-low populations. Cells were stimulated (106/ml) for 24 hr with immobilized anti-CD3 and supernatants measured by ELISA. (B) CD4+ T cells were purified and sorted as described and cultured with soluble anti-CD3 with T- depleted splenocytes from BALB/c IL-4−/− mice along with IL-2, anti-IFN-γ, anti-IL-12, and either IL-4 or anti-IL-4 (11B11). After 4 days, cells were washed and rechallenged (106/ml) for 24 hr with immobilized anti-CD3, and supernatants were assayed for IL-4 production.
Both CD62L-high and -low cells from wild-type and IL-4Rα−/− mice were stimulated for 4 days with soluble anti-CD3 and T-depleted spleen cells from IL-4-deficient BALB/c mice in the presence of IL-2, anti-IFN-γ, anti-IL-12, and either IL-4 or anti-IL-4 antibody (11B11). At the end of the culture period, cells were washed and restimulated, and supernatants were assayed for cytokine production (Fig. 4B). After in vitro “priming” of CD62L-low cells from wild-type mice in the absence of IL-4, an ≈20-fold increase in IL-4 production was observed. The degree of IL-4 production by these cells, however, was ≈5-fold less than that produced by the same cells that had been cultured with IL-4. CD62L-low cells from the IL-4Rα−/− mice cultured without IL-4 showed the same degree of increase in IL-4 production as did the CD62L-low cells from wild-type mice, but no further increase in IL-4-production was obtained by including IL-4 in the primary culture. Not surprisingly, in the cells that had been CD62L-high at the outset, IL-4 production by wild-type and mutant cells that had been cultured without IL-4 was modest. Addition of IL-4 to the priming culture led to vigorous production of IL-4 by cells from the wild-type but not IL-4Rα−/− cells.
IL-4 Production Is β2m-Dependent.
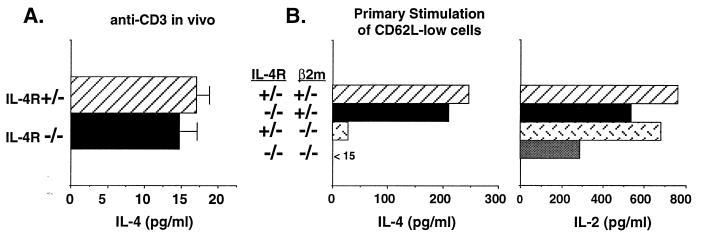
As already noted, a possible source of IL-4 among CD62L-low cells are NK T cells. These are a population of CD4+ T cells that express NK1.1 when derived from strains that possess the appropriate NKR-P1 allele, such as C57BL/6. In these strains, NK T cells have been shown to promtly produce IL-4 in response to injection of anti-CD3 (16). In vitro, they produce IL-4 and, upon in vitro “priming” with immobilized anti-CD3, increase their IL-4-producing capacity without requiring IL-4 (42). The NKR-P1 allele of BALB/c mice does not encode NK1.1 so these cells cannot be simply enumerated. However, they do appear to be present in these mice since injection of anti-CD3 causes the production of IL-4 by spleen cells within 90 min (Fig. 5A). Spleen cells from IL-4Rα−/− mice produce equivalent amounts of IL-4 in response to injection of anti-CD3 implying that NK T cells are present in the spleens of these mice and that IL-4 is not required for NK T cells to acquire IL-4-producing activity.
Figure 5.
CD4+, NK1.1+ T cells produce IL-4 in IL-4Rα−/− mice. (A) For measurement of IL-4 from CD4+, NK1.1+ T cells, mice were injected i.v. with anti-CD3. After 90 min spleens were removed and cells cultured for 1 hr. Supernatants were measured for IL-4 by bioassay. Error bars represent standard deviations from three individual mice. (B) CD4 T cells were purified from mesenteric lymph node cells from offspring of IL-4Rα+/− × β2m+/− matings. Cells were sorted for CD4+, CD62L-low populations and stimulated (106/ml) for 24 hr with immobilized anti-CD3 and supernatants measured for IL-4 and IL-2 by ELISA.
Because NK T cells from a variety of tissues express low levels of CD62L, these cells might be responsible for the anti-CD3-stimulated IL-4 production by CD62L-low mesenteric lymph node cells from IL-4Rα−/− mice. As noted above, we intentionally used mesenteric lymph nodes from IL-4Rα−/− mice to search for IL-4-producing cells because NK T cells have been reported to be present at relatively low levels in lymph nodes (16). Nonetheless, to test the possibility they might be responsible for the in vitro production of IL-4 that we observed, we took advantage of the finding that NK T cells are lacking in β2m−/− mice because the bulk of NK T cells are specific for and selected by CD1, a class Ib MHC molecule that depends upon β2m for expression (43). IL-4Rα+/− mice were crossed to BALB/c β2m+/− mice and CD4+ T cells from the various classes of offspring studied for IL-4 production. CD62L-low cells from wild-type (IL-4Rα+/−, β2m+/−) and IL-4Rα−/− (IL-4Rα−/−, β2m+/−) mice produced comparable amounts of IL-4 in response to immobilized anti-CD3 (Fig. 5B). In contrast, the same cell population derived from either β2m−/− (IL-4Rα+/−, β2m−/−) or double knockouts (IL-4Rα−/−, β2m−/−) produced little or no IL-4 in response to anti-CD3. As a control, each cell population was capable of producing IL-2 (Fig. 5B). Thus, the cells among the CD62L-low mesenteric lymph node population that can produce IL-4 in IL-4Rα−/− mice are β2m-dependent, strongly suggesting that they are NK T cells.
DISCUSSION
Here we have shown that CD4+ T cells from IL-4Rα mutant mice infected with N. brasiliensis display a strikingly diminished capacity to produce IL-4 in response to challenge with immobilized anti-CD3, in contrast to such T cells from heterozygous littermates in which a vigorous IL-4 response was observed. These results and those reported with STAT6-deficient mice provide strong evidence that TH2 responses in vivo, to this helminth, like induction of TH2 responses in vitro are critically dependent upon signaling through the IL-4 receptor. In other studies, IL-4 production in response to infection with Leishmania major was also markedly diminished in CD4+ T cells from IL-4Rα−/− mice (N.N.-T., unpublished results). These studies imply that IL-4 is a principal physiological regulator of the differentiation of naive T cells into IL-4-producing cells. Obviously, a much wider range of in vivo challenges will be needed to demonstrate whether there are any circumstances in which a vigorous TH2 response can be mounted by mice that lack the ability to receive IL-4-mediated signals.
These results emphasize the need to determine what cells produce the IL-4 that plays a central role in the development of TH2 cells, and how this production is regulated. Several cell types have been reported to be capable of producing IL-4 promptly upon stimulation. We have observed that two of these cell types, NK T cells and FcɛRI+ cells (data not shown), develop IL-4-producing capacity without any need for signals through the IL-4 receptor either at the time of stimulation or at any time in their development. Whether other cells, such as γ/δ T cells (20) or conventional, class II-restricted CD4+ T cells produce IL-4 promptly upon stimulation remains to be determined.
NK T cells are a cell population that have a characteristic set of markers (CD44-high, CD62L-low, CD45RB-low), intermediate levels of T cell receptor (TCR) expression and a skewed TCR repertoire (43). They are dependent upon CD1 for development and many are specific for CD1. Despite the absence of the NK1.1 allele in BALB/c mice, the presence of these cells can be inferred from two lines of evidence. First, injection of anti-CD3 into BALB/c mice causes prompt production of IL-4, a property of NK T cells in other mouse strains. Spleen cells of IL-4Rα−/− also produce IL-4 promptly upon i.v. injection of anti-CD3, indicating that this function does not depend upon signaling through the IL-4 receptor. Second, CD62L-low cells from BALB/c lymph nodes display the capacity to produce IL-4; this ability is not found in such cells from BALB/c β2m−/− mice, consistent with the importance of CD1, a β2m-associated molecule, in the development of NK T cells. Our results showing that CD62L-low cells from IL-4Rα−/− mice retain their capacity to produce IL-4 and that cells which are deficient in both IL-4R and β2m lack this capacity further argues that the ability of NK T cells to produce IL-4 is independent of IL-4R transmitted signals.
Nonetheless, the importance of NK T cells physiologically is still not certain (21, 44–47). In particular, it has been shown β2m−/− mice do display a TH2-type response to infection with N. brasiliensis, Schistosoma mansoni, and L. major (21, 44, 45). Our data, establishing the critical role for IL-4 in determining this phenotype in response to N. brasiliensis infection, coupled with the former observations indicates that another cell type must be sought to account for the production of the “early-inducing IL-4” in these infections.
It has been proposed that early-inducing IL-4 may actually be produced by conventional T cells as they develop into TH2 cells. That is, small amounts of IL-4 may be produced when these cells are initially stimulated; IL-6 may play a role in this IL-4 production (48). The resultant IL-4 then would act through the IL-4 receptor in a STAT6-dependent manner to commit the cells to become TH2 cells. Although it is difficult to rule this mechanism out, we would anticipate that stimulation of IL-4Rα−/−, β2m−/− cells should lead to the production of small amounts of IL-4. However, we failed to detect IL-4 in supernatants from CD62L-low CD4+ T cells after anti-CD3 stimulation of these cells. Similarly, CD62L-high cells of IL-Rα−/− mice failed to produce IL-4 at 24 hr after stimulation with immobilized anti-CD3 even when anti-CD28 was added (data not shown). Because the IL-4Rα−/− cells should have limited capacity to consume IL-4, these cultures would be expected to be a particularly favorable setting in which to detect such early IL-4 production. A more careful study of this issue will require both the addition of IL-6 and the use of more sensitive techniques to detect this postulated early burst of IL-4 production.
IL-4Rα-deficient mice provide a powerful tool for analyzing the physiological pathways through which priming for TH2 responses are induced and the role of IL-4 in a wide variety of infectious and other pathological conditions.
Acknowledgments
We thank Charles Lerner and the Microinjection Services at The Jackson Laboratory for ES cell injection, Christine Richardson for establishing the genotype screening, Jane Hu-Li for technical advice, Robert Seder and Mayumi Naramura for helpful discussions, Osami Kanagawa for providing the IL-4Rα genomic clone through Nicholas Wrighton, Derry Roopenian for deriving the BALB/c β2m−/− congenic strain, and the Flow Cytometry Service and the Animal Care Staff at the National Institute of Allergy and Infectious Diseases for excellent technical assistance. L.D.S. was supported by National Institutes of Health Grant CA20408. F.B. was supported by the German–Israeli Foundation.
ABBREVIATIONS
- IL
interleukin
- β2m
β2-microglobulin
- IFN-γ
interferon γ
- FITC
fluorescein isothiocyanate
- ES
embryonic stem
- PE
phycoerythrin
- cRPMI
complete RPMI
- MHC
major histocompatibility complex
References
- 1.Paul W E. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 2.Keegan A D, Nelms K, Wang L M, Pierce J H, Paul W E. Immunol Today. 1994;15:423–432. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 3.Beckmann M P, Cosman D, Fanslow W, Maliszewski C R, Lyman S D. In: Interleukins: Molecular Biology and Immunology. Kishimoto T, editor. Basel: Karger; 1992. pp. 107–134. [PubMed] [Google Scholar]
- 4.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K-I, Sugamura K. Science. 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 5.Russell S M, Keegan A D, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann M C, Miyajima A, Puri R K, Paul W E, Leonard W J. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 6.Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, Munakata H, Nakamura M, Sugamura K. Science. 1992;257:379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- 7.Noguchi M, Nakamura Y, Russell S M, Ziegler S F, Tsang M, Cao X, Leonard W J. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 8.Kondo M, Takeshita T, Higuchi M, Nakamura M, Sudo T, Nishikawa S I, Sugamura K. Science. 1994;263:1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- 9.Kimura Y, Takeshita T, Kondo M, Ishii N, Nakamura M, Van Snick J, Sugamura K. Int Immunol. 1995;7:115–120. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 10.Giri J G, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park L S, Cosman D, Anderson D. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilton D J, Zhang J-G, Metcalf D, Alexander W S, Nicola N A, Wilson T A. Proc Natl Acad Sci USA. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann T R, Cherwinski H, Bond M W, Giedlin M A, Coffman R L. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 13.Seder R A, Paul W E. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 14.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Köhler G. Nature (London) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S-I, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Nature (London) 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimoto T, Paul W E. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plaut M, Pierce J H, Watson C J, Hanley-Hyde J, Nordan R P, Paul W E. Nature (London) 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 18.Brown M A, Pierce J H, Watson C J, Falco J, Ihle J N, Paul W E. Cell. 1987;50:809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- 19.Seder R A, Paul W E, Ben-Sasson S Z, Le Gros G S, Kagey-Sobotka A, Finkelman F D, Pierce J H, Plaut M. Int Arch Allergy Appl Immunol. 1991;94:137–140. doi: 10.1159/000235345. [DOI] [PubMed] [Google Scholar]
- 20.Ferrick D A, Schrenzel M D, Mulvania T, Hsieh B, Ferlin W G, Lepper H. Nature (London) 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 21.Launois P, Ohteki R, Swihart K, MacDonald H R, Louis J A. Eur J Immunol. 1995;25:3298–3307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 22.Noben-Trauth N, Köhler G, Bürki K, Ledermann B. Transgenic Res. 1996;5:487–491. doi: 10.1007/BF01980214. [DOI] [PubMed] [Google Scholar]
- 23.Koller B H, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 24.Wrighton N, Campbell L A, Harada N, Miyajima A, Lee F. Growth Factors. 1992;6:103–118. doi: 10.3109/08977199209011014. [DOI] [PubMed] [Google Scholar]
- 25.Nitschke L, Kopf M, Lamers M C. BioTechniques. 1993;14:914–916. [PubMed] [Google Scholar]
- 26.Laird P W, Zijderveld A, Linders K, Rudnicki M A, Jaenisch R, Berns A. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohara J, Paul W E. Nature (London) 1985;315:333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- 28.Beckmann M P, Schooley K A, Gallis B, Vanden Bos T, Friend D, Alpert A R, Raunio R, Prickett K S, Baker P E, Park L S. J Immunol. 1990;144:4212–4217. [PubMed] [Google Scholar]
- 29.Curry R C, Kiener P A, Spitalny G L. J Immunol Methods. 1987;104:137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- 30.Mosmann T R, Fong T A. J Immunol Methods. 1989;116:151–158. doi: 10.1016/0022-1759(89)90198-1. [DOI] [PubMed] [Google Scholar]
- 31.Hu-Li J, Ohara J, Watson C, Tsang W, Paul W E. J Immunol. 1989;142:800–807. [PubMed] [Google Scholar]
- 32.Rizzo L V, DeKruyff R H, Umetsu D T. J Immunol. 1992;148:3733–3739. [PubMed] [Google Scholar]
- 33.Ryan J J, McReynolds L J, Keegan A, Wang L-H, Garfein E, Rothman P, Nelms K, Paul W E. Immunity. 1996;4:123–132. doi: 10.1016/s1074-7613(00)80677-9. [DOI] [PubMed] [Google Scholar]
- 34.Noelle R, Krammer P H, Ohara J, Uhr J W, Vitetta E S. Proc Natl Acad Sci USA. 1984;81:6149–6153. doi: 10.1073/pnas.81.19.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad D J, Waldschmidt T, Lee W T, Rao M, Keegan A D, Noelle R J, Lynch R G, Kehry M R. J Immunol. 1987;139:2290–2296. [PubMed] [Google Scholar]
- 36.Ohara J, Paul W E. Proc Natl Acad Sci USA. 1988;85:8221–8225. doi: 10.1073/pnas.85.21.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan M H, Schindler U, Smiley S T, Grusby M J. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 38.Shimoda K, van Deursen J, Sangster M Y, Sarawar S R, Carson R T, Tripp R A, Chu C, Quelle F W, Nosaka T, Vignali D A, Doherty P C, Grosveld G, Paul W E, Ihle J N. Nature (London) 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 39.Morawetz R A, Gabriele L, Rizzo L V, Noben-Trauth N, Kühn R, Rajewsky K, Müller W, Doherty T M, Finkelman F, Coffman R L, Morse H C., III J Exp Med. 1996;184:1651–1661. doi: 10.1084/jem.184.5.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hornqvist E, Enerbach L, Chen X J, Lycke N. Cell Immunol. 1993;148:71–90. doi: 10.1006/cimm.1993.1092. [DOI] [PubMed] [Google Scholar]
- 41.Gollob K J, Coffman R L. J Immunol. 1994;152:5180–5188. [PubMed] [Google Scholar]
- 42.Chen, H. & Paul, W. E. (1997) J. Immunol. in press.
- 43.Bendelac A, Rivera M N, Park S-H, Roark J H. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 44.von der Weid T, Beebe A M, Roopenian D C, Coffman R L. J Immunol. 1996;157:4421–4427. [PubMed] [Google Scholar]
- 45.Brown D R, Fowell D J, Corry D B, Wynn T A, Moskowitz N H, Cheever A W, Locksley R M, Reiner S L. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guery J C, Galbiati F, Smiroldo S, Adorini L. J Exp Med. 1996;183:485–497. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Rogers K H, Lewis D B. J Exp Med. 1996;184:1507–1512. doi: 10.1084/jem.184.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]