Abstract
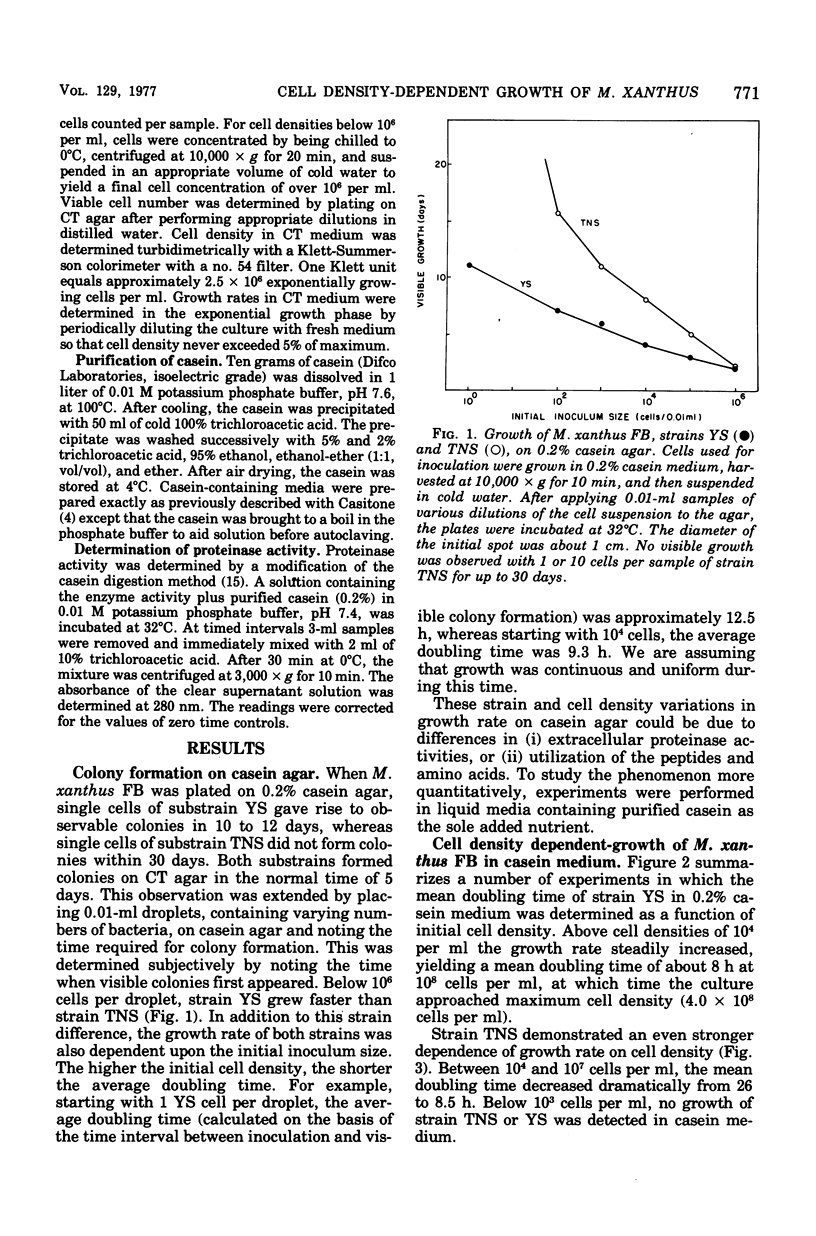
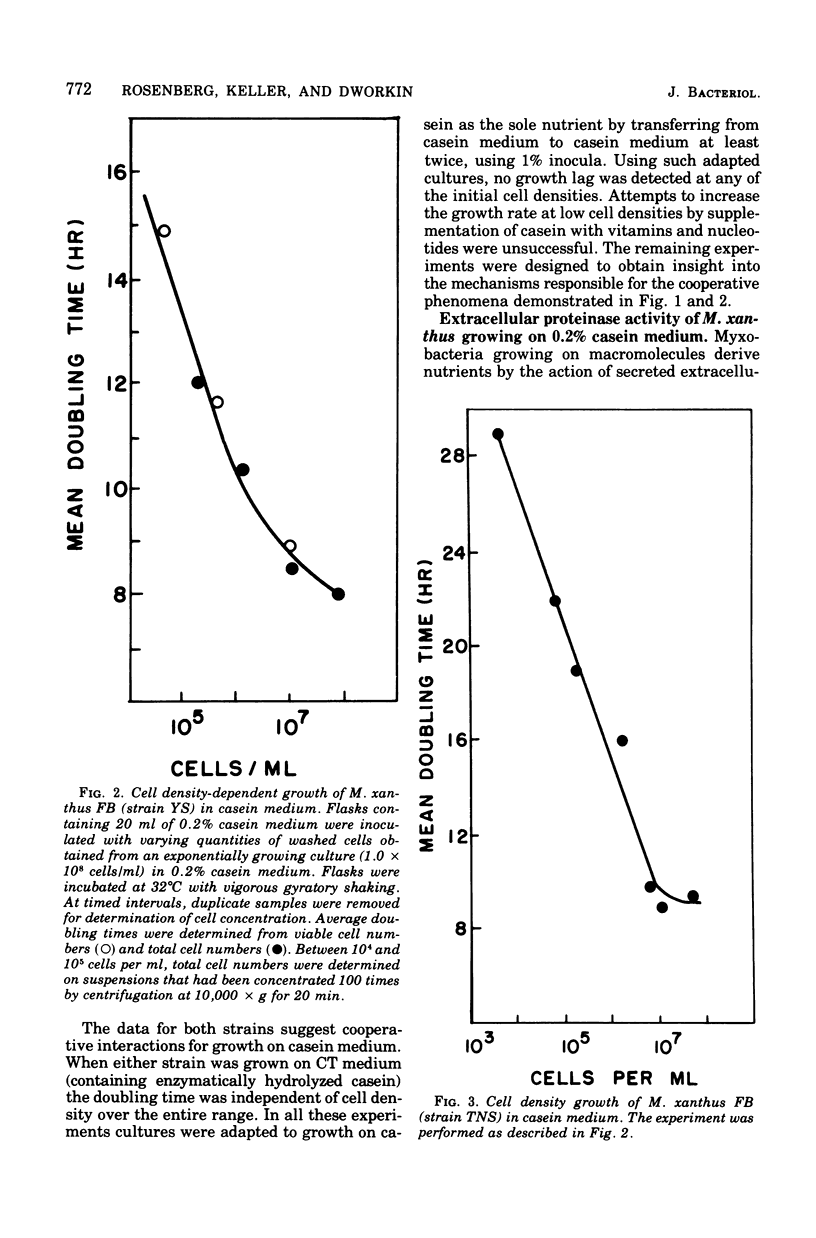
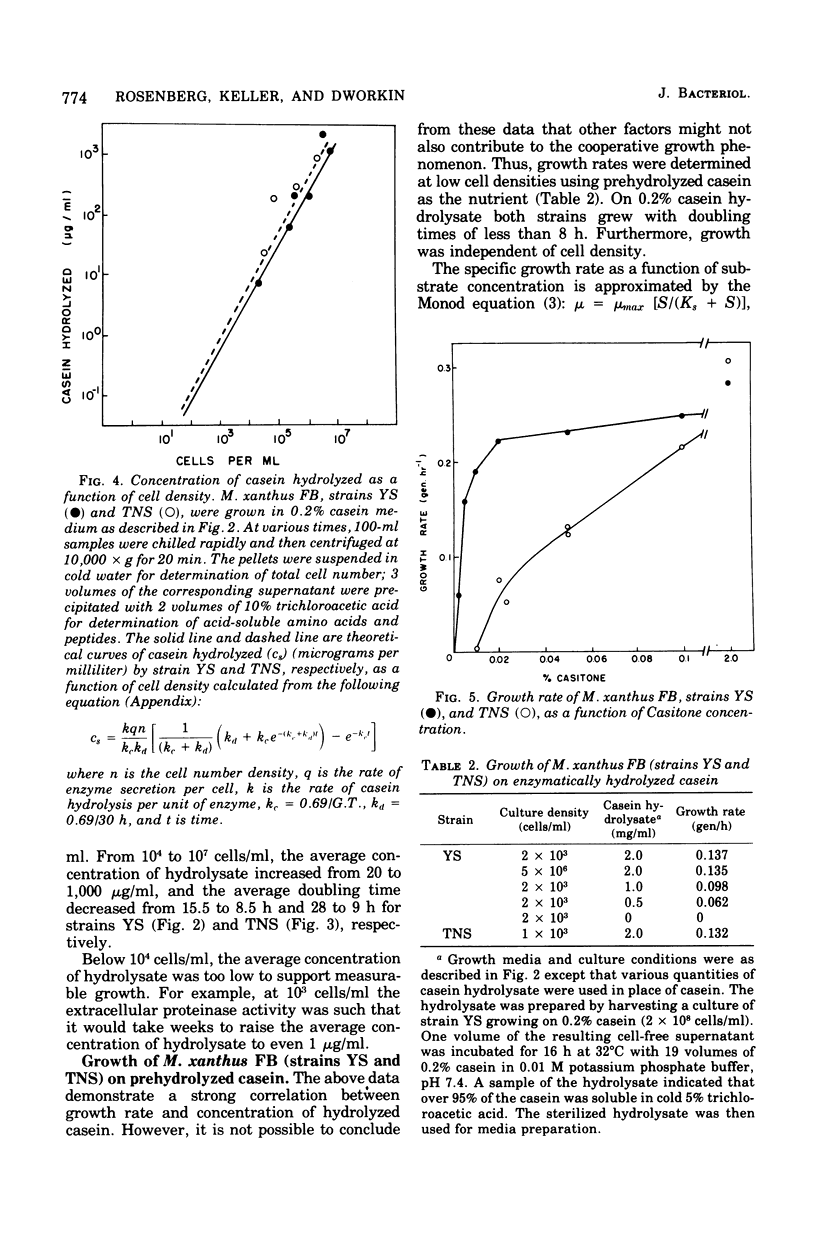
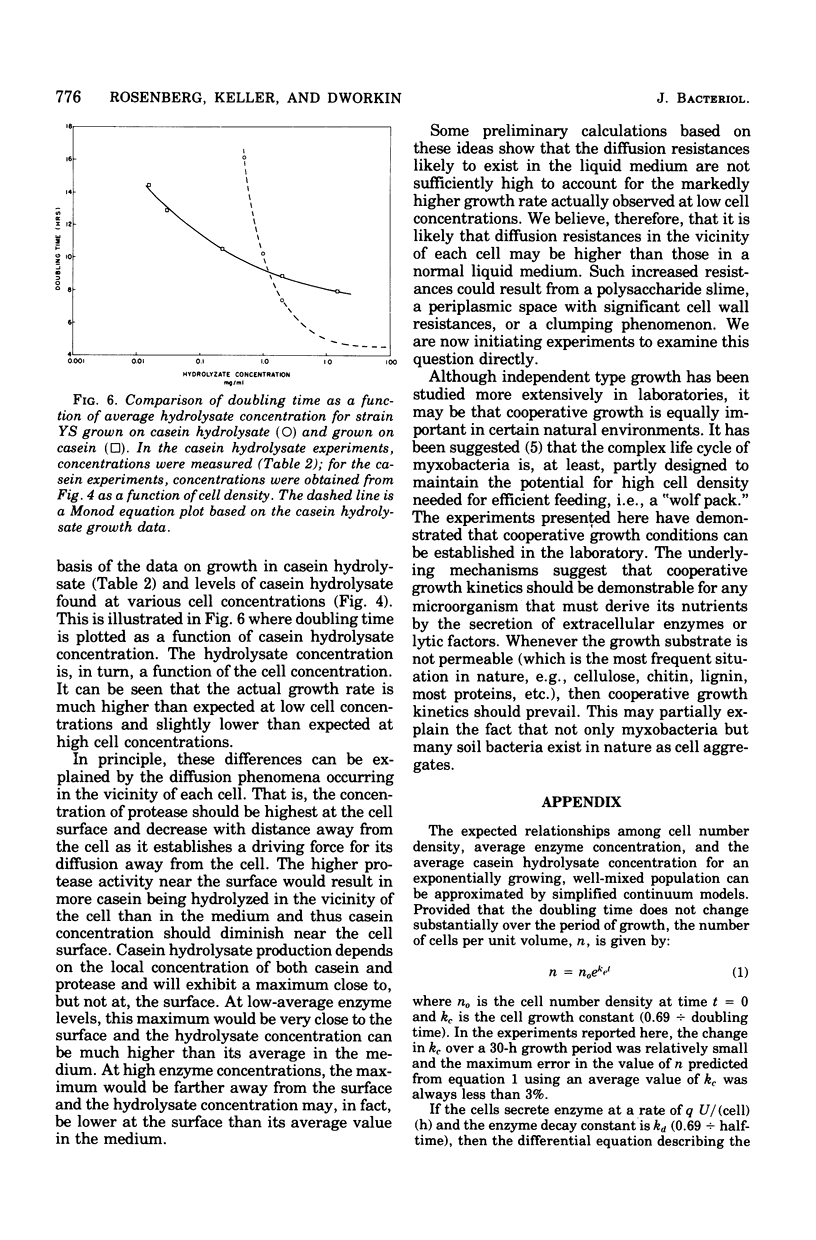
When Myxococcus xanthus FB was grown on 0.2% casein it exhibited a phenomenon we call cooperative growth. That is, above 104 cells per ml, both strains that were studied exhibited increasing growth rates as a function of increasing cell numbers. Between 104 and 107 cells per ml, the mean doubling times of strains YS and TNS decreased from 15.2 to 8 h and 26 to 8.5 h, respectively. The extracellular proteinase activity of the two strains was equivalent and directly proportional to cell number. Cooperative growth was correlated with increased concentration of hydrolyzed casein in the medium, suggesting cooperative hydrolysis of casein. At low cell densities neither strain was capable of measurable growth on casein in liquid media, and we have calculated that the average concentration of hydrolyzed casein in the medium was indeed too low to support growth. At low cell densities, growth on hydrolyzed casein (Casitone) was normal and independent of cell concentration. Demonstration of cooperative growth at higher cell densities supports the suggestion that the communal behavior of myxobacteria results in more efficient feeding.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNHAM B. F., NEILANDS J. B. Studies on the metabolic function of the ferrichrome compounds. J Biol Chem. 1961 Feb;236:554–559. [PubMed] [Google Scholar]
- Byers B. R., Powell M. V., Lankford C. E. Iron-chelating hydroxamic acid (schizokinen) active in initiation of cell division in Bacillus megaterium. J Bacteriol. 1967 Jan;93(1):286–294. doi: 10.1128/jb.93.1.286-294.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962 Aug;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. METABOLIC CONTROLS IN CULTURED MAMMALIAN CELLS. Science. 1965 Apr 2;148(3666):42–51. doi: 10.1126/science.148.3666.42. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., WOLFE R. S. LYSIS OF BACTERIAL CELL WALLS BY AN ENZYME ISOLATED FROM A MYXOBACTER. J Bacteriol. 1965 Aug;90:395–402. doi: 10.1128/jb.90.2.395-402.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIELD M. F., LICHSTEIN H. C. Growth stimulating effect of autoclaved glucose media and its relationship to the CO2 requirement of propionibacteria. J Bacteriol. 1958 Nov;76(5):485–490. doi: 10.1128/jb.76.5.485-490.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE D. C., COOK F. D. EXTRACELLULAR ENZYMES FROM STRAINS OF SORANGIUM. Can J Microbiol. 1965 Feb;11:109–118. doi: 10.1139/m65-014. [DOI] [PubMed] [Google Scholar]
- Hart B. A., Zahler S. A. Lytic enzyme produced by Myxococcus xanthus. J Bacteriol. 1966 Dec;92(6):1632–1637. doi: 10.1128/jb.92.6.1632-1637.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüttermann A. Studies on a bacteriolytic enzyme of Archangium violaceum (Myxobacteriales). II. Partial purification and properties of the enzyme. Arch Mikrobiol. 1969;67(4):306–317. doi: 10.1007/BF00412578. [DOI] [PubMed] [Google Scholar]
- KNOX R., COLLARD P. The effect of temperature on the sensitivity of Bacillus cereus to penicillin. J Gen Microbiol. 1952 May;6(3-4):369–373. doi: 10.1099/00221287-6-3-4-369. [DOI] [PubMed] [Google Scholar]
- LANKFORD C. E., RAMSEY H. H. Stimulation of growth initiation by heat degradation products of glucose. J Bacteriol. 1956 Oct;72(4):511–518. doi: 10.1128/jb.72.4.511-518.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lankford C. E., Walker J. R., Reeves J. B., Nabbut N. H., Byers B. R., Jones R. J. Inoculum-dependent division lag of Bacillus cultures and its relation to an endogenous factor(s) ("schizokinen"). J Bacteriol. 1966 Mar;91(3):1070–1079. doi: 10.1128/jb.91.3.1070-1079.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo S., Dworkin M. Bacteriolytic enzymes produced by Myxococcus xanthus. J Bacteriol. 1972 Apr;110(1):236–245. doi: 10.1128/jb.110.1.236-245.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker D. R. Lytic enzymes of Sorangium sp. Isolation and enzymatic properties of the alpha- and beta-lytic proteases. Can J Biochem. 1965 Dec;43(12):1935–1954. doi: 10.1139/o65-217. [DOI] [PubMed] [Google Scholar]
- Wireman J. W., Dworkin M. Morphogenesis and developmental interactions in myxobacteria. Science. 1975 Aug 15;189(4202):516–523. doi: 10.1126/science.806967. [DOI] [PubMed] [Google Scholar]



