Abstract
Normal aging is associated with both locomotor and orolingual motor deficits. Preclinical studies of motor function in normal aging, however, have focused primarily on locomotor activity. The purpose of this study was to measure age-related changes in orolingual motor function and compare these changes between two rat strains commonly used in aging studies: Fischer 344 (F344) and Fischer 344/Brown Norway hybrid (F344/BN) rats. Rats (6-, 12-, 18- and 24-months of age) were trained to lick water from an isometric force-sensing operandum so that the number of licks per session, licking rhythm (licks/second) and lick force could be measured. In both strains, the number of licks per session was greatest in the oldest group, while this measure was greater for F344/BN rats at all ages. Peak tongue force increased with age in F344/BN rats, did not change with age in the F344 rats, and was greater for the F344/BN rats at all ages. Both strains exhibited an age-related slowing of licking rhythm beginning with the 18-month-old group. These findings suggest that despite lifespan differences between these two rat strains, diminished tongue motility emerges at the same age.
Keywords: aging, oromotor, bradykinesia, movement, licking, operant, tongue, animal models
1. Introduction
Normal aging is accompanied by decreases in both quantity and quality of motor activity in humans and in animals [5,19]. In addition to bradykinesia (slowed movements) and gait disturbances, diminished orolingual motor function is also associated with human aging. These alterations, which include dysarthria, dysphagia and masticatory deficits, contribute to increased mortality and morbidity in the elderly [3,11,20]. Like locomotor deficits, orolingual motor deficits also accompany Parkinson’s disease (PD)[1,17], suggesting that altered basal ganglia function may play a role in their occurrence. In addition, age-related neuromuscular changes (e.g., motor neuronal innervation from the hypoglossal nucleus, changes in muscle fiber type) may also affect orolingual motor function [23].
Because of their functional relevance, orolingual deficits have been studied extensively in elderly human volunteers (e.g., [11]). Very few studies, however, have examined age-related changes in orolingual motor function using animal models. We recently addressed this issue by comparing orolingual motor function in young (6 mos) versus aged (24 mos) Fischer 344 (F344) rats [31]. Rats were trained to lick water from a disc that was attached to an isometric force transducer. While aged rats exhibited a greater number of licks per session (perhaps due to decreased tolerance to water restriction [27,34]), tongue motility as quantified by licks-per-second was significantly diminished in this group compared to the young group. This finding is consistent with studies reporting diminished tongue motility in elderly volunteers who performed an analogous repetitive tongue movement task [16]. We also reported a significant relationship between tongue motility and the ratio of dopamine (DA) metabolites to DA (thought to reflect DA turnover) in the substantia nigra of the aged rats. This finding, along with the fact that orolingual motor function is altered in rats with 6-hydroxydopamine depletions of the nigrostriatal pathway [26], supports the basal ganglia hypothesis.
The purpose of the current study was to determine the age of onset of rhythm slowing, and whether this function differs between F344 rats and another strain commonly used in aging research, the F344/Brown Norway (F344/BN) hybrid. While F344/BN rats exhibit a longer lifespan than F344 rats (50% mortality at 146 weeks of age versus 103 weeks, respectively; [28]), both strains have been reported to exhibit diminished locomotor function and changes in nigrostriatal dopamine function beginning at 18 months of age [14,36]. What is not known is whether orolingual rhythm slowing likewise emerges in this middle-aged group in these strains, or whether it is a characteristic of senescence.
2. Materials and methods
2.1 Subjects
Male F344 and F344/BN rats were obtained from the National Institute on Aging colonies (Harlan Sprague-Dawley, Indianapolis, IN). Group numbers were as follows: F344 (6 mos, n=10; 12 mos, n=8; 18 mos, n=10; 24 mos, n=4; there was more age-related attrition than anticipated in the 24-month-old F344 rats); F344/BN (6 mos, n=11; 12 mos, n=11; 18 mos, n=8; 24 mos, n=10). Procedures were approved by the University of Kansas Medical Center IACUC and adhered to the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Procedure
The apparatus has been previously described [26,31]. Briefly, data were recorded in a modified rodent operant chamber with a front panel containing a 6-cm square hole at floor level. Affixed to the square hole was a 6-cm cubic transparent enclosure that, on its lower horizontal surface, contained a 12-mm-diameter hole through which the rat’s tongue extended down to reach the operandum. The operandum was an 18 mm diameter aluminum disc rigidly attached to the shaft of a Model 31 load cell (Sensotec, Columbus, OH) and was centered 2 mm beneath the hole in the plastic enclosure. A computer-controlled peristaltic pump (Series E; Manostat Corp., New York, NY), fitted with a solid-state relay (Digikey, Thief River Falls, MN), and controlled by a LabMaster computer interface (LabMaster, Solon, OH), delivered water to the center of the lick disc through a 0.5-mm-diameter hole. The force transducer was capable of resolving force measurements to 0.2-g equivalent weights. A PC recorded the transducer's force-time output sampled at 100 samples/s. Following gradual water restriction to 10 minutes access per day, rats were exposed to the water-licking task during 6 minute sessions until they licked reliably (i.e., >100 licks). Rats continued to receive 10 min water access per day throughout the study. The force requirement was 1 g to register a response and 12 licks were required to produce 0.05 ml of water. Each session started with a free 0.05 ml delivery of water. Upon stabilization of baseline (~2 weeks), data for the licking task were then collected over a period of 2 days. The mean values for each variable for these two days were analyzed.
2.3. Data analysis
The effects of age and strain on orolingual motor function were assessed in terms of three dependent variables: 1) number of licks per session, 2) the rhythm of licking in licks/second (Hz), and 3) the mean peak lick force. The number of licks was a count of the number of tongue contacts that equaled or exceeded 1 g. The lick rhythm was determined as follows: computation of the power spectra was performed by MatLab's Signal Processing Toolbox (The Math Works, Inc, Natick, MA). For this analysis, each 6-min session was divided into 35 series of 1024 samples from the lick-force transducer. With the Hanning data window selected, MatLab produced 35 corresponding power spectra. The power spectra were truncated to 25 Hz (based on prior work indicating little of behavioral interest beyond 25 Hz) and averaged together to yield a single power spectrum. A peak-find program written in Free Pascal was used to identify the peak in the averaged power spectrum, and the frequency at this peak was taken as the lick rhythm for a particular session. This method resolved lick rhythm to the nearest 0.1 Hz. This method was used because, unlike lick rate, the peak in the power spectrum is not influenced by pauses in licking behavior. The spectral analysis method is therefore more analogous to measures of within-burst inter-lick intervals (e.g. [7]). The peak lick force was the mean of the peak forces exerted during a session. Body weights were also analyzed as a function of age and strain. For between-strain comparisons, data for each measure were analyzed using a two-way analysis of variance (ANOVA) with age and strain as the between-subjects variables using SYSTAT (SYSTAT Software, Inc., San Jose, CA). The level of significance was p<0.05.
3. Results
Representative force-time waveforms illustrating licking bouts for one rat in each age group and strain are presented in Figure 1. Note the substantially greater tongue forces in the F344/BN strain. The number of licks per session increased with age, F(3,68)=6.239, p<0.001, but was greater in the F344/BN rats at each age, F(1,68)=25.292, p<0.0001 (Fig. 2A). Both strains exhibited slowing of licking rhythm beginning at 18 months of age, F(3,64)=18.331, p<0.0001 (Fig. 2B). While the aging effect was more pronounced in the F344 rats, the age X strain interaction did not reach significance (p=0.07). Peak force of licks was significantly greater in the F344/BN rats than in the F344 rats at all ages, F(1,62)=86.745, p<0.0001 (Fig.2C). Although tongue force increased with age in the F344/BN rats but remained constant with age in the F344 rats, this interaction was not significant (p=0.07). Body weights were greater for the F344/BN rats, F(1,67)=137.697, p<0.0001, but increased with age in both strains, F(3,67)=46.120, p<0.0001 (Fig. 2D). In the F344 rats, body weights were maximum at 12 months, while F344/BN rats exhibited increases at each age, leading to a significant age X strain interaction, F(3,67)=15.234, p<0.0001.
Figure 1.
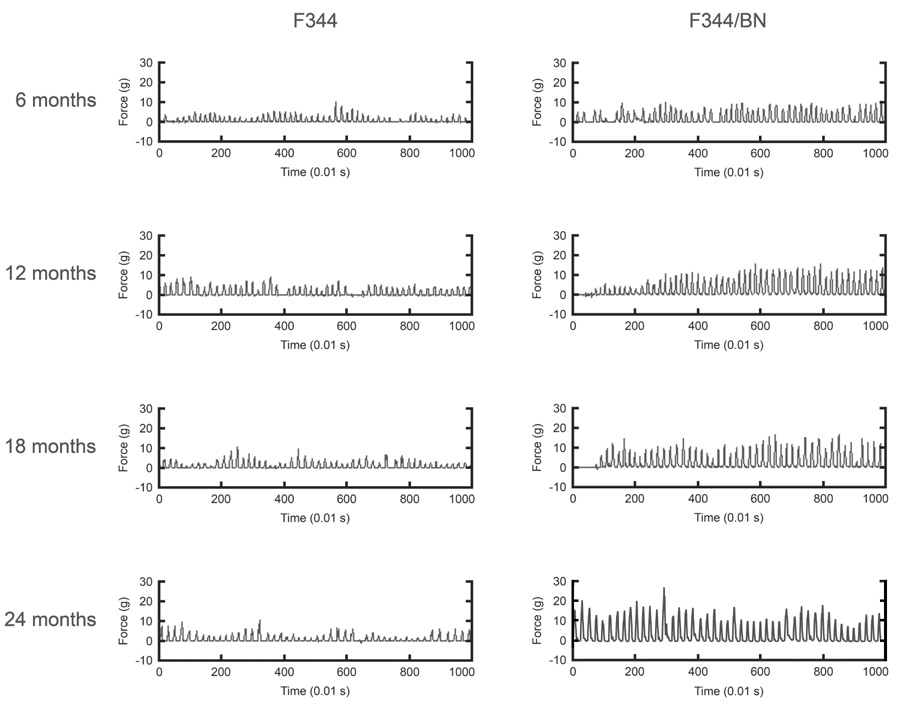
Raw force-time waveforms depicting 10 s samples of licking. Waveforms are from representative sessions for 6- month (top row), 12-month (second row), 18-month (third row) and 24-month (bottom row) F344 (left column) and F344/BN (right column) rats.
Figure 2.
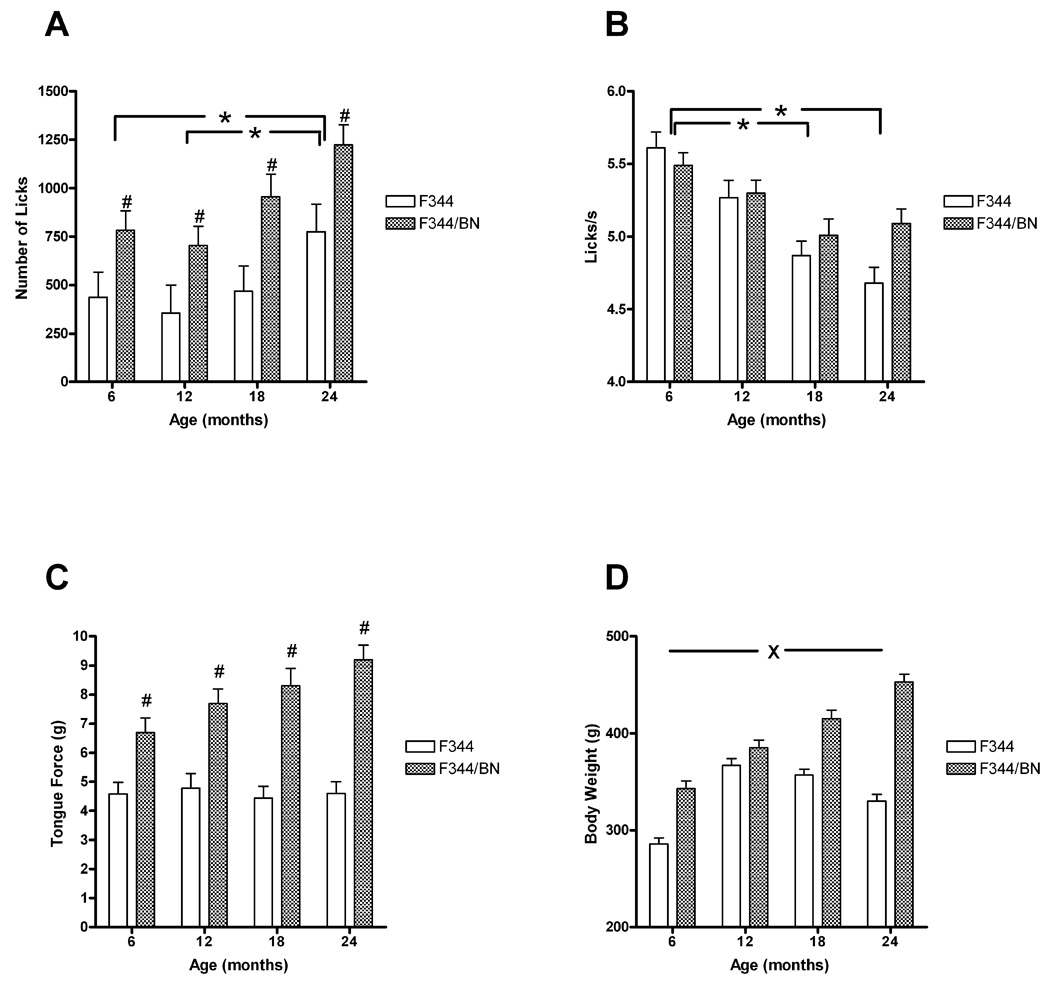
Orolingual motor measures and body weights as a function of age and strain. (A) Rats exhibited an age-related increase in the number of licks/session (#p<0.05). At each age, the number of licks/session was greater for F344/BN rats (*p<0.05). (B) Licking rhythm was significantly slower in both strains beginning with the 18-month group (#p<0.05). (C) Peak tongue force was significantly greater for F344/BN rats than for F344 rats at all ages (*p<0.05). Neither the main effect for age nor the age X strain interaction reached significance. (D) Both F344 and F344/BN rats exhibited age-related increases in body weights. After reaching a maximum at 12 months, body weights decreased with age in F344 rats (they remained higher than the 6-month group at each age however). F344/BN rats exhibited progressive weight increases across the four age groups. Note: #denotes significant difference from 6-months group, *denotes significant strain difference at this age, Xdenotes significant strain by age interaction.
4. Discussion
We report here that despite substantial strain-related differences in longevity and body weights, tongue motility decreases at approximately the same age in F344 and F344/BN rats. While older rats emitted a greater number of licks per session, their licking rhythm was significantly slower beginning at 18 months of age in both strains. Peak tongue forces did not change with age in the F344 rats, but increased with age in the F344/BN rats. Tongue force was greater in F344/BN rats than in F344 rats at all ages.
Despite a difference in lifespan between the F344 and F344/BN strains [28], previous results [14,29,36] combined with those reported here, suggest that the onset of at least some forms of age-related motor decline are similar for these two strains. The fact that tongue motility diminished with age while tongue force did not is consistent with recent findings reporting slower tongue muscle contractions but stability in tongue muscle forces and fatigability in aged (30–33 months) F344/BN rats [23]. It is also consistent with findings reporting no change in hypoglossal motor neuronal number with age (up to 30 months) in mice [32]. The fact that these findings stand in apparent contrast to studies reporting decreased tongue force output in elderly humans [6,21], probably reflects task differences between these studies. For example, studies in humans typically incorporate a measure of maximum force output, while our current and previous [31] studies only involved a minimum force criterion. Also, while the number of motor neurons in the hypoglossal nucleus may not decline with age, there are age-related changes in the tongue musculature (e.g., [18]).
The strain-related differences in tongue force (even at 12 months when body weights were similar) are striking. Recent studies have reported that F344/BN rats exhibit age-related signs of sarcopenia (i.e., decreases in skeletal muscle mass and contractile properties) while F344 rats do not [24]. It is possible that while factors relating to production of tongue force did not change with age in the F344 rats (either between 6–12 months when body weights increased, or between 12 and 24 months when they declined), changes in tongue force production mirrored age-related body weight increases in the F344/BN rats. While it is tempting to speculate that age-related sarcopenia would be reflected in tongue forces in even older F344/BN rats, the minimum force criterion (1g) adopted for this study may not be sufficient to test this hypothesis. Clearly, a more direct test involving increasing force requirements for reinforcement is warranted to clarify the extent to which these animals model tongue force deficits observed in elderly humans, and how isometric tongue force output is affected by neuromuscular changes in aging.
The slowing of licking rhythm with age likely reflects a multi-system process. In addition to an age-related slowing of tongue muscle contractions [23], multiple neural mechanisms probably play a role in this phenomenon. We recently reported that licking rhythm in aged rats is correlated with measures of dopamine and dopamine turnover in the substantia nigra [31]. Interestingly, despite lifespan differences, the age of onset of rhythm slowing is similar for these two strains and corresponds with the age of onset of changes in nigrostriatal function [14,36]. High speed licking behavior in the rat is governed by central pattern generators in the hypoglossal nucleus, the cerebellum, and the medial bulbar reticular formation, and age-related functional alterations have been reported for each of these regions [4,12,15,37]. Because motor-related information in the thalamus and the reticular formation is influenced by inputs from the substantia nigra and the cerebellum [35], altered dopamine transmission may further disrupt the processing of this information. We recently found that locomotor-activating doses [30] of the mixed dopamine/norepinephrine uptake inhibitor nomifensine did not speed licking rhythm in 24-month-old F344 rats [31]. It may be that drugs such as amphetamine that produce DA release in the substantia nigra [13,33], and not merely blockade of catecholamine uptake, are required to normalize licking rhythm in aged rats. Such an effect would be consistent with our previous finding that licking rhythm and nigral DA turnover were significantly correlated in aged F344 rats [31]. The fact that these changes emerge at 18 months in both strains suggest that changes in the nigrostriatal pathway (and possibly the other neural systems that affect high speed licking) precede other functional changes that occur with age, especially in F344/BN rats.
The significant increase in the number of licks with age was consistent with our previous study comparing young versus aged F344 rats in this task [31], but appears to be at odds with findings regarding diminished operant and general motor activity in aged rats [9,14,29,36]. However, the licking behavior examined under these conditions could be considered more consummatory than instrumental. As mentioned previously, aged rats are more sensitive to water restriction and exhibit altered fluid regulatory mechanisms compared to younger rats [27,34]. Because of this, and because the motor demands of licking are less than those involved in operant lever-pressing tasks, the number of licks emitted during these 6 min sessions should not be considered analogous to other motor measurements. An additional consideration is that the results from our water-based reinforcement may not be analogous to previous studies that have utilized other taste stimuli, such as varying sucrose concentrations or sweetened condensed milk. In a series of articles testing a range of interventions known to affect motivation, one group reported that while the within-burst interlick intervals (analogous to our measure of licking rhythm) were resistant to these manipulations, more macro-level patterns of consumption (e.g., overall licking rate, inter-burst intervals) were affected [7,8,25]. These findings, along with our previous [31] and current findings showing increased number of licks (more analogous to licking rate) but slowed licking rhythm in aged rats, support the relative independence of motor versus motivational influences on difference parameters measured in these studies.
Overall, these results show that like locomotor activity, orolingual motility is diminished in both F344 and F344/BN rats beginning at 18 months. The fact that aged rats exhibited a greater number of licks per session but slower licking rhythm suggests a relative independence of motor versus motivational influences on these measures. Conversely, tongue force production appears to remain intact with age in F344 rats, and even increase in aging F344/BN rats. It is interesting that despite substantial differences in lifespan between these two strains, the onset of changes in motor function that reflect changes in basal ganglia function is similar for F344 and F344/BN rats. Future studies are clearly warranted to delineate the roles that age-related changes in basal ganglia and brainstem nuclei, as well as functional neuromuscular changes play in the age-related orolingual motor deficits.
Acknowledgements
This work was supported by NIH grants AG023549, AG026491 and by a Lied Endowed Basic Science Research grant. The authors wish to thank Dr. Stephen Fowler for his assistance with MATLAB programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbs JH, Hartman DE, Vishwanat B. Orofacial motor control impairment in Parkinson’s disease. Neurology. 1987;37:394–398. doi: 10.1212/wnl.37.3.394. [DOI] [PubMed] [Google Scholar]
- 2.Aldes LD, Champman ME, Chronister RB, Haycock JW. Sources of noradrenergic afferents to the hypoglossal nucleus in the rat. Brain Res Bull. 1992;29:931–942. doi: 10.1016/0361-9230(92)90168-w. [DOI] [PubMed] [Google Scholar]
- 3.Baum BJ, Bodner L. Aging and oral motor function: evidence for altered performance among older persons. J Dent Res. 1983;62:2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- 4.Behan M, Brownfield MS. Age-related changes in serotonin in the hypoglossal nucleus of the rat: implications for sleep-disordered breathing. Neurosci Lett. 1999;267:133–136. doi: 10.1016/s0304-3940(99)00337-7. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 6.Crow HC, Ship JA. Tongue strength and endurance in different aged individuals. J Gerontol A Biol Sci Med Sci. 1996;51:M247–M250. doi: 10.1093/gerona/51a.5.m247. [DOI] [PubMed] [Google Scholar]
- 7.Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci. 1992;106:217–228. [PubMed] [Google Scholar]
- 8.Davis JD, Smith GP, Kung TM. Abdominal vagotomy alters the structure of the ingestive behavior of rats ingesting liquid diets. Behav Neurosci. 1994;108:767–769. [PubMed] [Google Scholar]
- 9.Emerich DF, Plone M, Francis J, Frydel BR, Winn SR, Lindner MD. Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain Res. 1996;736:99–110. [PubMed] [Google Scholar]
- 10.Fowler SC, Wang G. Chronic haloperidol produces a time- and dose-related slowing of lick rhythm in rats: implications for rodent models of tardive dyskinesia and neuroleptic-induced parkinsonism. Psychopharmacology. 1998;137:50–60. doi: 10.1007/s002130050592. [DOI] [PubMed] [Google Scholar]
- 11.Fucile S, Wright PM, Chan I, Yee S, Langlais M-E, Gisel EG. Functional oral-motor skills: do they change with age? Dysphagia. 1998;13:195–201. doi: 10.1007/PL00009571. [DOI] [PubMed] [Google Scholar]
- 12.Gould TJ, Bowenkamp KE, Larson G, Zahniser NR, Bickford PC. Effects of diatary restriction on motor learning and cerebellar noradrenergic dysfunction in aged F344 rats. Brain Res. 1995;684:150–158. doi: 10.1016/0006-8993(95)00407-h. [DOI] [PubMed] [Google Scholar]
- 13.Gulley JM, Kosobud AE, Rebec GV. Behavior-related modulation of substantia nigra pars reticulata neurons in rats performing a conditioned reinforcement task. Neuroscience. 2002;111:337–349. doi: 10.1016/s0306-4522(02)00018-0. [DOI] [PubMed] [Google Scholar]
- 14.Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in Fischer 344 rats. Brain Res. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 15.Hilber P, Caston J. Motor skills and motor learning in Lurcher mutant mice during aging. Neuroscience. 2001;102:615–623. doi: 10.1016/s0306-4522(00)00509-1. [DOI] [PubMed] [Google Scholar]
- 16.Hirai T, Tanaka O, Koshino H, Yajima T. Ultrasound observations of tongue motor behavior. J Prosthetic Dent. 1991;54:840–844. doi: 10.1016/s0022-3913(05)80024-1. [DOI] [PubMed] [Google Scholar]
- 17.Ho AK, Bradshaw JL, Cunnington R, Phillips JG, Iansek R. Sequence heterogeneity in Parkinsonian speech. Brain Lang. 1998;64:122–145. doi: 10.1006/brln.1998.1959. [DOI] [PubMed] [Google Scholar]
- 18.Hodges SH, Anderson AL, Connor NP. Remodeling of neuromuscular junctions in aged rat genioglossus muscle. Ann Otol Rhinol Laryngol. 2004;113:175–179. doi: 10.1177/000348940411300301. [DOI] [PubMed] [Google Scholar]
- 19.Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med Sci Sports Exerc. 2000;32:1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Lieu PK, Chong MS, Seshadri R. The impact of swallowing disorders in the elderly. Ann Acad Med Singapore. 2001;30:148–154. [PubMed] [Google Scholar]
- 21.McAuliffe MJ, Ward EC, Murdoch BE, Farrell AM. A nonspeech investigation of tongue function in Parkinson's disease. J Gerontol A Biol Sci Med Sci. 2005;60:667–674. doi: 10.1093/gerona/60.5.667. [DOI] [PubMed] [Google Scholar]
- 22.McComas AJ. Oro-facial muscles: internal structure, function and ageing. Gerodontology. 1998;15:3–14. doi: 10.1111/j.1741-2358.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- 23.Ota F, Connor NP, Konopacki R. Alterations in contractile properties of tongue muscles in old rats. Ann Otol Rhinol Lanyngol. 2005;114:799–803. doi: 10.1177/000348940511401010. [DOI] [PubMed] [Google Scholar]
- 24.Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005;6:335–343. doi: 10.1007/s10522-005-4808-0. [DOI] [PubMed] [Google Scholar]
- 25.Schneider LH, Davis JD, Watson CA, Smith GP. Similar effect of raclopride and reduced sucrose concentration on the microstructure of sucrose sham feeding. Eur J Pharmacol. 1990;186:61–70. doi: 10.1016/0014-2999(90)94060-b. [DOI] [PubMed] [Google Scholar]
- 26.Skitek EB, Fowler SC, Tessell RE. Effects of unilateral striatal dopamine depletion on tongue force and rhythm during licking in rats. Behav Neurosci. 1999;113:567–573. doi: 10.1037//0735-7044.113.3.567. [DOI] [PubMed] [Google Scholar]
- 27.Sladek CD, McNeill TH, Gregg CM, Blair ML, Baggs RB. Vasopressin and renin response to dehydration in aged rats. Neurobiol Aging. 1981;2:293–301. doi: 10.1016/0197-4580(81)90038-5. [DOI] [PubMed] [Google Scholar]
- 28.Sprott RL. Diet and calorie restriction. Exp Gerontol. 1997;32:205–214. doi: 10.1016/s0531-5565(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Stanford JA, Osterhaus GL, Vorontsova E, Fowler SC. Measuring forelimb force control and movement in Fischer 344/Brown Norway rats: effects of age and lorazepam. Behav Pharmacol. 2006;17:725–730. doi: 10.1097/FBP.0b013e32801155e8. [DOI] [PubMed] [Google Scholar]
- 30.Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered locomotion in the absence of decreased locomotor activity: exacerbation by nomifensine. Neurosci Lett. 2002;333:195–198. doi: 10.1016/s0304-3940(02)01105-9. [DOI] [PubMed] [Google Scholar]
- 31.Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered orolingual motor function: Relationships with nigrostriatal neurochemical measures. Neurobiol Aging. 2003;24:259–266. doi: 10.1016/s0197-4580(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 32.Sturrock RR. Stability of motor neuron and interneuron number in the hypoglossal nucleus of the ageing mouse brain. Anat Anz Jena. 1991;173:113–116. [PubMed] [Google Scholar]
- 33.Sulzer D, Maidment NT, Rayport S. Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem. 1993;60:527–535. doi: 10.1111/j.1471-4159.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 34.Swenson KL, Sands JM, Jacobs JD, Sladek CD. Effect of aging on vasopressin and aquaporin responses to dehydration in Fischer 344-Brown-Norway F1 rats. Am J Physiol. 1997;273:R35–R40. doi: 10.1152/ajpregu.1997.273.1.R35. [DOI] [PubMed] [Google Scholar]
- 35.Thach WT. Fundamentals of motor systems. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. San Diego: Academic Press; 1999. pp. 855–861. [Google Scholar]
- 36.Yurek DM, Hipkins SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 37.Zhang JH, Sampogna S, Morales FR, Chase MH. Age-related alterations in immunoreactivity of the midsized neurofilament subunit in the brainstem reticular formation of the cat. Brain Res. 1997;769:196–200. doi: 10.1016/s0006-8993(97)00853-6. [DOI] [PubMed] [Google Scholar]


