Abstract
Objective
To compare persistence with valsartan and enalapril in daily practice.
Methods
The PHARMO Record Linkage System includes various data registries including drug dispensing and hospitalizations for ≥2 million subjects in the Netherlands. Patients newly treated with valsartan or enalapril in the period of 1999–2002 were selected. Persistence was calculated by summing up the number of days of continuous treatment. Patients who remained on therapy with valsartan or enalapril for 12 or 24 months were defined as persistent at 1 or 2 years, respectively.
Results
3364 patients received valsartan and 9103 patients received enalapril. About 62% of patients treated with valsartan and 55% of patients treated with enalapril remained on therapy at 12 months after the initial dispensing, while 48% of patients treated with valsartan and 43% of patients treated with enalapril were persistent at 24 months. Patients treated with valsartan were about 20% more likely to stay on treatment than patients treated with enalapril (1 year RRadj: 1.23, 95% CI: 1.16–1.32; 2 years RRadj: 1.16, 95% CI: 1.11–1.23).
Conclusions
Real-life persistence is higher with valsartan than with enalapril. The results of this and other studies on persistence in daily practice should be taken into account when deciding upon drug treatment for hypertension.
Keywords: persistence, antihypertensive, valsartan, enalapril, angiotensin-converting enzyme inhibitors, angiotensin-2-receptor blockers
Introduction
Hypertension is a major risk factor for vascular disease and effective treatment with antihypertensive drugs has been shown to reduce both cardiovascular and cerebrovascular events (Gueyffier et al 1997). However, at least three quarters of antihypertensive patients fail to achieve optimal blood pressure control despite of treatment and according to the findings of the World Health Organization, poor adherence to treatment is an important cause of uncontrolled blood pressure (WHO 2003). Several methods have been developed to measure adherence using drug dispensing information from large pharmacy databases, including measurements of refill compliance and persistence (Steiner and Prochazka 1997; Catalan and LeLorier 2000; Dezii 2001; Halpern et al 2006). Between 50% and 70% of patients with treated hypertension adhere to antihypertensive medication, irrespective of the method used. Several observational studies have demonstrated that the choice of the initial antihypertensive drug class has an important impact on persistence with the treatment regimen (Caro et al 1999b; Hasford et al 2002; Marentette et al 2002; Morgan and Yan 2004; Erkens et al 2005; Koylan et al 2005; Mazzaglia et al 2005). Most studies showed highest persistence rates with angiotensin-2-receptor blockers (ARBs), followed by angiotensin converting enzyme inhibitors (ACE inhibitors) (Marentette et al 2002; Bourgault et al 2005; Erkens et al 2005; Koylan et al 2005; Gerth 2002). Lowest persistence rates were observed for diuretics (Caro et al 1999b; Hasford et al 2002; Bourgault et al 2005; Chou et al 2005). Important reasons for non-adherence with antihypertensive medication include patient demographic factors (such as age and gender), low confidence in the physician, perceived or experienced adverse effects of drugs, notion of hypertension as an intermittent condition, and complex dosing regimens (Svensson et al 2000; Mancia et al 2003; Ross et al 2004; Breekveldt-Postma and Herings 2005).
Although several studies have been published on persistence and compliance with ARBs compared to other groups of antihypertensive drugs, very little is known about the differences that exist between individual drugs. Wogen et al reported higher compliance and persistence rates with valsartan (ARB) than with lisinopril (ACE inhibitor) and amlodipine (calcium-channel blocker, CCB) after 1 year in patients that were new on the specific antihypertensive drug (Wogen et al 2003). In the Netherlands, use of ARBs and ACE inhibitors are recommended for high-risk hypertensive patients with similar compelling indications, such as diabetes mellitus, chronic kidney disease, heart failure, and post-myocardial infarction. These recommendations are in line with international guidelines (ESH 2003).
The objective of this study was to compare persistence with the ARB valsartan and the ACE inhibitor enalapril in daily practice during the first two years of treatment in hypertensive patients that were new on the index drug (valsartan or enalapril). Enalapril was selected as a comparator for valsartan, because it is the most frequently dispensed ACE inhibitor in the Netherlands.
Methods
Setting
Data were obtained from the PHARMO medical record linkage system (PHARMO RLS) in the Netherlands. The PHARMO RLS includes the demographic details and complete medication history of more than 2 million patients, further linked to hospital admission records as well as several other health registries, including pathology and clinical laboratory findings (Herings 1993). Computerized drug dispensing histories contain Anatomical Therapeutic Chemical (ATC) codes, the date of medication dispensing, prescriber information, prescribed dose of the drug, dispensed quantity, cost and duration of use. Hospital admission register comprises all hospital admissions in the Netherlands, including detailed information on the primary and secondary discharge diagnoses, diagnostic, surgical and treatment procedures, type and frequency of consultations with medical specialists and dates of hospital admission and discharge. All diagnoses are coded according to the International Classification of Diseases, 9th edition (ICD-9-CM).
Study cohort
The study population included all patients with at least one dispensing of valsartan (ATC code: C09CA03) or enalapril (ATC code: C09AA02) in the PHARMO community pharmacy database between 1 January 1999 and 31 December 2002. Only products with one active substance were included in the selection, patients starting a fixed combination therapy including valsartan or enalapril were excluded. The date of the first dispensing was defined as the index date and the drug prescribed was defined as the index drug. Patients were eligible for inclusion if they had a history of registration in PHARMO RLS of at least 12 months before the index date and a follow-up of at least 24 months. Patients did not use the index drug for at least 1 year before the index date and had at least 1 additional dispensing of the index drug within 60 days after the end date of the initial prescription to exclude patients not using the index drug for the treatment of chronic hypertension. For the same reason, patients who used nitrates (ATC code: C01DA) or alpha-blockers (ATC code: C02CA) during the 12-month period prior to the index date or during the first treatment episode were excluded. The patients included in the final study cohort were new users of the index drug, but not necessarily naïve to antihypertensive drug treatment.
Persistence
Medication persistence with the index drug was studied for 12 and 24 months, respectively (Halpern et al 2006). For each patient, the duration of use of each dispensing of the index drug was calculated by dividing the number of units dispensed by the prescribed number of units to be used per day as recorded in the pharmacies. All dispensings were subsequently converted into treatment episodes. A treatment episode was defined as a period of time in which a continuous specific pharmacotherapeutic treatment took place and was measured as the time span between the starting date of the first dispensing until the expiry date of the final dispensing including the permissible gap. In case of an interruption of 60 days or less between 2 dispensings, the treatment episode was considered uninterrupted. Patients were considered to discontinue treatment if the gap between the end of the dispensed supply of one dispensing and the start of the next dispensing was more than 60 days. Patients may have had multiple treatment episodes in their follow-up period, but only the first treatment episode was used to calculate persistence with the index drug. For patients continuously treated during the 12 months or 24 months of follow-up, the duration of treatment was censored at one or two years, respectively (Catalan and LeLorier 2000). In addition to the persistence with the index drug only, persistence was calculated based on the use of any antihypertensive drug during the first year after the index date. Persistence ended at discontinuation of all antihypertensive drugs after start of the index drug.
Possible confounders
Other factors that may influence persistence were determined including age, gender, first prescriber and year of start at the index date. Use of other antihypertensives (ATC-codes: C02, C03, C07, C08, C09A, C09B, C09C, C09D) was assessed in the period of 1 year prior to the index date and during the first treatment episode, whereas hospitalization for hypertension (ICD-9-CM: 401–405) was determined in the 1-year period prior to the index date. Cardiovascular co-morbidity (yes/no) was assessed in the 1-year period prior and during the first treatment episode based on drug use and hospital admission. Specific drugs that were determined included antithrombotics (ATC-code: B01), lipid lowering drugs (ATC-code: C10), antidiabetics (ATC-code: A10) and other cardiovascular drugs (ATC-codes: C01, C04 and C05). Cardiovascular hospitalizations included heart failure (ICD-9-CM: 428), ischemic heart diseases (ICD-9-CM: 410–414), cerebrovascular diseases (ICD-9-CM: 430–438), other cardiovascular events (ICD-9-CM: 390–459 excluding the before mentioned hospitalizations), diabetes (ICD-9-CM: 250), renal failure (ICD-9-CM: 584–586), and other renal events (ICD-9-CM: 580–589 excluding the before-mentioned hospitalizations). Hospitalization for any non-cardiovascular co-morbidity (yes/no) was assessed in the 1-year period prior to the index date.
Statistical analysis
Logistic regression analysis was performed to compare baseline characteristics between users of valsartan and users of enalapril. Survival functions describing persistence with valsartan and enalapril over time were computed using Kaplan Meier survival analyses. Sensitivity analyses were performed using a 30-day gap and 90-day gap, respectively. Crude and multivariate analyses to identify the effect of antihypertensive drug on persistence were conducted using Cox’s proportional hazard analyses. Factors univariately associated with persistence were included as potential confounders in the adjusted analyses. Statistical significance was defined at an alpha level of 0.05. All data were analyzed using SAS programs organized within SAS Enterprise Guide version 2.0 (SAS Institute Inc., Cary, NC, USA) and conducted under UNIX using SAS version 8.2.
Results
A total of 3364 patients treated with valsartan and 9103 patients treated with enalapril were included in the study cohort of which 8077 (65%) patients had been treated with other antihypertensive drugs during the 12-month period prior to and preceding the index date.
The significant differences in patient characteristics between the two treatment groups are described below. These characteristics were included as possible confounders in the multivariate analyses when studying persistence with valsartan and enalapril treatment. Among patients starting valsartan treatment there were less patients in the oldest (≥80 years of age) and youngest age category (<40 years of age) than among patients starting enalapril treatment (Table 1). The proportion of females was higher among patients taking valsartan compared to enalapril. The proportion of patients who had been treated with an ACE inhibitor, ARB, or CCB blocker during the one-year period prior to the index date was higher among patients starting valsartan than among patients starting enalapril, whereas the proportion of patients who had used a diuretic during the preceding year was higher for patients starting enalapril than for patients starting valsartan. A history of a lipid-lowering drug use was more common within valsartan than enalapril users whereas the use of antidiabetic agents was more common for patients starting enalapril. A previous hospitalization for any renal event was more common among enalapril than valsartan users.
Table 1.
General characteristics of patients starting valsartan or enalapril therapy
| Valsartan | Enalapril | |||
|---|---|---|---|---|
| Characteristic | N | % | N | % |
| Total | 3364 | 100.0 | 9103 | 100.0 |
| Gender | ||||
| Female (Ref.) | 1939 | 57.6 | 4951 | 54.4 |
| Male | 1425 | 42.4a | 4152 | 45.6 |
| Age (years) | ||||
| 0–39 | 180 | 5.4a | 609 | 6.7 |
| 40–64 (Ref.) | 1832 | 54.5 | 4695 | 51.6 |
| 65–79 | 1174 | 34.9 | 3124 | 34.3 |
| ≥80 | 178 | 5.3a | 675 | 7.4 |
| Mean ± sd | 60.5 ± 12.6 | 60.4 ± 13.9 | ||
| Prescriber | ||||
| GP (Ref.) | 2613 | 77.7 | 6955 | 76.4 |
| Specialist | 751 | 22.3 | 2148 | 23.6 |
| Previous use of antihypertensive drugs | ||||
| Any AHT | 2249 | 66.9a | 5828 | 64.0 |
| Diuretic | 862 | 25.6a | 3126 | 34.3 |
| Beta-blocker | 1253 | 37.2 | 3529 | 38.8 |
| ACE | 781 | 23.2a | 740 | 8.1 |
| ARB | 284 | 8.4a | 227 | 2.5 |
| CCB | 483 | 14.4a | 1011 | 11.1 |
| Miscellaneous | 49 | 1.5a | 89 | 1.0 |
| Previous use of other cardiovascular drugs | ||||
| Any CV drug | 1277 | 38.0a | 3775 | 41.5 |
| Antithrombotic | 682 | 20.3 | 1720 | 18.9 |
| Lipid-lowering drug | 557 | 16.6a | 1153 | 12.7 |
| Antidiabetic agent | 332 | 9.9a | 1829 | 20.1 |
| Other | 273 | 8.1a | 599 | 6.6 |
| Previous hospitalizations | ||||
| CV event | 162 | 4.8 | 437 | 4.8 |
| Diabetes | 4 | 0.1 | 28 | 0.3 |
| Renal event | 6 | 0.2a | 50 | 0.5 |
| Other | 378 | 11.2 | 1037 | 11.4 |
Statistically significant for valsartan vs enalapril p < 0.05.
Abbreviations: ACE, angiotensin converting enzyme inhibitor; AHT, antihypertensive; ARB, angiotensin-2-receptor blocker; CCB, calcium-channel blocker; CV, cardiovascular; GP, general practitioner; Ref, reference group.
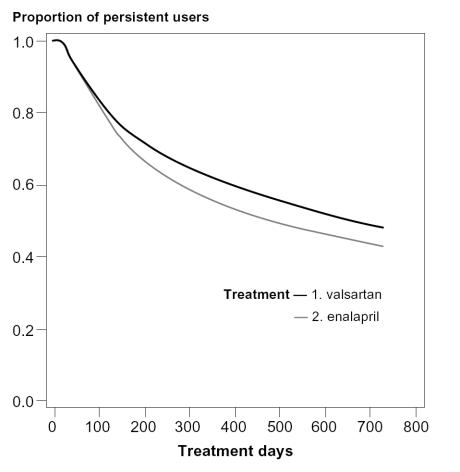
61.6% of patients starting valsartan and 55.0% of patients starting enalapril remained on therapy with the index drug at one year after the initial dispensing. Sensitivity analyses for 1-year persistence showed that about 50% of patients starting valsartan and 45% of patients starting enalapril remained on therapy when a 30-day gap was used and about 65% of patients starting valsartan and 60% of patients starting enalapril remained on therapy when a 90 day gap was used. At two years after start, 47.8% of patients starting valsartan and 43.0% of patients starting enalapril were still using the index drug. Patients starting valsartan were 20% (1.2 times) more likely to persist with the initial therapy than patients starting enalapril at 1 and 2 years, after adjustment for differences in patient characteristics (RR1year: 1.23, 95% CI: 1.16–1.32; RR2 years: 1.16, 95% CI: 1.11–1.23) (Table 2 and Figure 1). Factors adjusted for and associated with increased persistence in the multivariate analyses were higher age, male gender, specialist as prescriber, concomitant use of other antihypertensive drugs, and previous use of concurrent cardiovascular medication as well as previous cardiovascular hospitalizations (data not shown). The lower persistence with enalapril was mainly due to higher rate of discontinuation of the drug during the first year of treatment in the enalapril group (45%) than in the valsartan group (38%) (Figure 1).
Table 2.
Medication persistence with valsartan and enalapril
| Persistence | N | At 1 year after the index date | At 2 years after the index date | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Persistent (%) | RR | 95% CI | RRb | 95% CI | Persistent (%) | RR | 95% CI | RRb | 95% CI | ||
| Enalapril | 9103 | 55.0 | 1.00 | Reference | 1.00 | Reference | 43.0 | 1.00 | Reference | 1.00 | Reference |
| Valsartan | 3364 | 61.6 | 1.22a | 1.15–1.30 | 1.23a | 1.16–1.32 | 47.8 | 1.16a | 1.10–1.22 | 1.16a | 1.11–1.23 |
Statistically significant p < 0.05.
Adjusted for gender, age, type of prescriber, current and previous use of antihypertensive drugs, previous cardiovascular co-medication, and previous cardiovascular hospitalizations.
Figure 1.
Medication persistence with valsartan and enalapril treatment in the first 2 years after the index date (adjusted curves based on the Cox proportional hazard analysis). Percentage of patients still using index drug at (a) 1 year after the index date: 61.6% for valsartan and 55.0% for enalapril and (b) 2 years after the index date: 47.8% for valsartan and 43.0% for enalapril (p < 0.05).
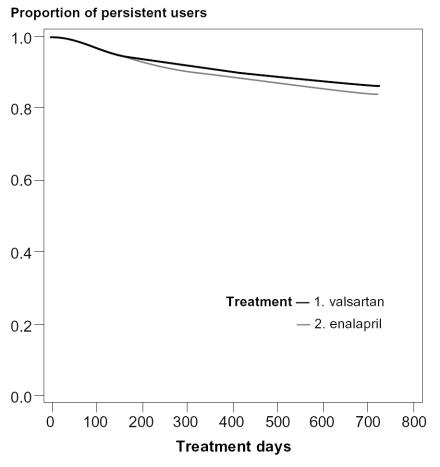
When persistence was assessed based on the use of the index drug or any other antihypertensive drug during the first year of follow-up, about 90% of the patients starting valsartan or enalapril were still on antihypertensive drug treatment after one year while 87% of patients starting valsartan and 84% of patients starting enalapril were on antihypertensive drug treatment after 2 years (Figure 2). No differences in overall persistence were observed between the treatment groups when using this definition (data not shown).
Figure 2.
Medication persistence assessed based on the use of the index drug or any other hypertensive drug during the first two years of follow-up (adjusted curves based on the Cox proportional hazard analysis).
Discussion
This study indicates that patients starting valsartan treatment are more persistent with their initial drug than patients starting enalapril treatment, after adjusting for differences in patient characteristics. Patients starting valsartan were 20% (1.2 times) more likely to persist with the initial therapy at one and two years after the start of the index drug than patients starting enalapril. Most patients (86%) were persistent with antihypertensive drug treatment after stopping the index drug but had switched either to another drug or to a combination of antihypertensive drugs.
The results of our study are in line with one other publication on persistence with individual antihypertensive drugs (Wogen et al 2003). The study by Wogen et al reported an increased persistence with valsartan compared to lisinopril (another representative of the ACE inhibitor class) in a usual- care setting of a managed care organization in the US. In their study, 63% of patients treated with valsartan and 50% of patients treated with lisinopril stayed on the initial therapy at one year. No information was available on 2-year persistence rates. Enhanced persistence with ARBs in general compared with ACE inhibitors was also found in several studies on persistence with antihypertensive drug classes (Gerth 2002; Marentette et al 2002; Wogen et al 2003; Bourgault et al 2005; Erkens et al 2005).
Lower persistence with enalapril was mainly due to a higher rate of discontinuation of this drug during the first year of treatment and may be related to the characteristic side-effects of ACE inhibitors, mainly cough (Dicpinigaitis 2006). However, the actual reasons for drug discontinuation remain to be investigated in primary research on patients’ behavior. Factors related to cardiovascular disease severity, such as previous use of concurrent cardiovascular drugs and concomitant use of antihypertensive drugs were associated with greater persistence, as previously observed by others (Caro et al 1999a; Breekveldt-Postma and Herings 2005; Erkens et al 2005; Perreault et al 2005). These data indicate that patients with more severe hypertension are more likely to take medications as directed because of the perceived need to treat their condition effectively.
The indications of use between ACE inhibitors and ARBs are not fully overlapping; both drugs are indicated for essential hypertension while specific indications for ACE inhibitors are chronic kidney disease and diabetes mellitus. ACE inhibitors are also used post-myocardial infarction and in patients with heart failure. ARBs are reserved for the latter patients in case they do not tolerate ACE inhibitors. These differences in indication of use were also observed when comparing baseline characteristics of valsartan and enalapril. The differences in indication of use do not explain the observed differences in persistence of use as the adjusted risk estimate was similar to the crude risk estimate. Antihypertensives should be used chronically for all indications. In addition, as ACE inhibitors are more often used in high-risk patients, it is assumed that these patients would be more persistent with their therapy than low-risk patients which would result in an underestimation of the difference in persistence between valsartan and enalapril when not fully adjusted for.
Although we included a number of known confounders in our analyses, there may be other confounding factors for which we did not have information available, such as patient perceptions and channeling of (higher propensity to prescribe) the study drugs to different groups of patients, which may have influenced the results. We did not have information on socio-economic factors; however, both drugs are fully reimbursed in The Netherlands. It is possible that valsartan is being prescribed to patients who have not been persistent with other antihypertensive drugs. Therefore, the differences in persistence may partly be explained by residual confounding. On the other hand, no statistically significant differences were observed in persistence rates based on the use of the index drug or any other antihypertensive drug between patients starting valsartan and patients starting enalapril. This strengthens the assumption that lower persistence with enalapril is related to differences between the individual drugs rather than differences in other factors associated with persistence between the two groups of patients. We cannot exclude that there may remain differences in persistence between valsartan and enalapril that relate to differences in indication of use, but we minimized possible differences in indication of use by excluding patients using nitrates previously or concomitantly with AHT treatment, by excluding patients with a single prescription of the index drug, and by concentrating on therapies that are representatives of drug classes recommended for the treatment of hypertension among patients with similar compelling indications.
In conclusion, patients treated with valsartan had higher persistence with their initial treatment compared to patients treated with enalapril. Lower real-life persistence may affect long-term health outcomes and lead to increased cost of illness. It is therefore important to take into account the results of this and other studies on persistence in daily practice when deciding upon drug treatment for hypertension.
Disclosures
Drs Gábor Vincze and Zeba M. Khan work at Novartis. Funding for this research was provided by Novartis Pharma AG, Switzerland.
References
- Bourgault C, Senecal M, Brisson M, et al. Persistence and discontinuation patterns of antihypertensive therapy among newly treated patients: a population-based study. J Hum Hypertens. 2005;19:607–13. doi: 10.1038/sj.jhh.1001873. [DOI] [PubMed] [Google Scholar]
- Breekveldt-Postma NS, Herings RM. Persistence with antihypertensives related to formulation: the case of nifedipine. Ann Pharmacother. 2005;39:237–42. doi: 10.1345/aph.1E163. [DOI] [PubMed] [Google Scholar]
- Caro JJ, Salas M, Speckman JL, et al. Persistence with treatment for hypertension in actual practice. CMAJ. 1999a;160:31–7. [PMC free article] [PubMed] [Google Scholar]
- Caro JJ, Speckman JL, Salas M, et al. Effect of initial drug choice on persistence with antihypertensive therapy: the importance of actual practice data. CMAJ. 1999b;160:41–6. [PMC free article] [PubMed] [Google Scholar]
- Catalan V, Lelorier J. Predictors of long-term persistence on statins in a subsidized clinical population. Value in Health. 2000;3:417–25. doi: 10.1046/j.1524-4733.2000.36006.x. [DOI] [PubMed] [Google Scholar]
- Chou CC, Lee MS, Ke CH, et al. Factors influencing the switch in the use of antihypertensive medications. Int J Clin Pract. 2005;59:85–91. doi: 10.1111/j.1742-1241.2005.00436.x. [DOI] [PubMed] [Google Scholar]
- Dezii CM. Persistence with drug therapy: a practical approach using administrative claims data. Manag Care. 2001;10:42–5. [PubMed] [Google Scholar]
- Dicpinigaitis PV. Angiotensin-converting enzyme inhibitor-induced cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:169S–73S. doi: 10.1378/chest.129.1_suppl.169S. [DOI] [PubMed] [Google Scholar]
- Erkens JA, Panneman MM, Klungel OH, et al. Differences in antihypertensive drug persistence associated with drug class and gender: a PHARMO study. Pharmacoepidemiol Drug Saf. 2005;14:795–803. doi: 10.1002/pds.1156. [DOI] [PubMed] [Google Scholar]
- ESH. European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–53. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- Gerth WC. Compliance and persistence with newer antihypertensive agents. Curr Hypertens Rep. 2002;4:424–33. doi: 10.1007/s11906-002-0021-6. [DOI] [PubMed] [Google Scholar]
- Gueyffier F, Boutitie F, Boissel JP, et al. Effect of antihypertensive drug treatment on cardiovascular outcomes in women and men. A meta-analysis of individual patient data from randomized, controlled trials. The INDANA Investigators. Ann Intern Med. 1997;126:761–7. doi: 10.7326/0003-4819-126-10-199705150-00002. [DOI] [PubMed] [Google Scholar]
- Halpern MT, Khan ZM, Schmier JK, et al. Recommendations for evaluating compliance and persistence with hypertension therapy using retrospective data. Hypertension. 2006;47:1039–48. doi: 10.1161/01.HYP.0000222373.59104.3d. [DOI] [PubMed] [Google Scholar]
- Hasford J, Mimran A, Simons WR. A population-based European cohort study of persistence in newly diagnosed hypertensive patients. J Hum Hypertens. 2002;16:569–75. doi: 10.1038/sj.jhh.1001451. [DOI] [PubMed] [Google Scholar]
- Herings R. Thesis. PHARMO: a record linkage system for postmarketing surveillance of prescription drugs in The Netherlands. Utrecht: Utrecht University; 1993. [Google Scholar]
- Koylan N, Acarturk E, Canberk A, et al. Effect of irbesartan monotherapy compared with ACE inhibitors and calcium-channel blockers on patient compliance in essential hypertension patients: a multicenter, open-labeled, three-armed study. Blood Press Suppl. 2005;1:23–31. [PubMed] [Google Scholar]
- Mancia G, Seravalle G, Grassi G. Tolerability and treatment compliance with angiotensin II receptor antagonists. Am J Hypertens. 2003;16:1066–73. doi: 10.1016/j.amjhyper.2003.07.012. [DOI] [PubMed] [Google Scholar]
- Marentette MA, Gerth WC, Billings DK, et al. Antihypertensive persistence and drug class. Can J Cardiol. 2002;18:649–56. [PubMed] [Google Scholar]
- Mazzaglia G, Mantovani LG, Sturkenboom MC, et al. Patterns of persistence with antihypertensive medications in newly diagnosed hypertensive patients in Italy: a retrospective cohort study in primary care. J Hypertens. 2005;23:2093–100. doi: 10.1097/01.hjh.0000186832.41125.8a. [DOI] [PubMed] [Google Scholar]
- Morgan SG, Yan L. Persistence with hypertension treatment among community-dwelling BC seniors. Can J Clin Pharmacol. 2004;11:e267–73. [PubMed] [Google Scholar]
- Perreault S, Lamarre D, Blais L, et al. Persistence with treatment in newly treated middle-aged patients with essential hypertension. Ann Pharmacother. 2005;39:1401–8. doi: 10.1345/aph.1E548. [DOI] [PubMed] [Google Scholar]
- Ross S, Walker A, Macleod MJ. Patient compliance in hypertension: role of illness perceptions and treatment beliefs. J Hum Hypertens. 2004;18:607–13. doi: 10.1038/sj.jhh.1001721. [DOI] [PubMed] [Google Scholar]
- Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–16. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- Svensson S, Kjellgren KI, Ahlner J, et al. Reasons for adherence with antihypertensive medication. Int J Cardiol. 2000;76:157–63. doi: 10.1016/s0167-5273(00)00374-0. [DOI] [PubMed] [Google Scholar]
- WHO. Adherence to long-term therapies: evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- Wogen J, Kreilick CA, Livornese RC, et al. Patient adherence with amlodipine, lisinopril, or valsartan therapy in a usual-care setting. J Manag Care Pharm. 2003;9:424–9. doi: 10.18553/jmcp.2003.9.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]