Abstract
Inflammatory processes are increasingly recognized as important participants in the pathophysiology of hypertension and cardiovascular disease. Angiotensin II may be to a large degree responsible for triggering vascular inflammation by inducing oxidative stress, resulting in up-regulation of inflammatory mediators. Inflammatory markers such as C-reactive protein are increased in the blood of patients with hypertension and predict the development of cardiovascular disease. Moreover, C-reactive protein may be a pro-inflammatory molecule under certain circumstances. C-reactive protein and high blood pressure in combination have additional predictive value for cardiovascular outcomes, as they contribute as independent determinants of cardiovascular risk. Therapeutic intervention aimed to reduce vascular inflammation in hypertensive patients has been proposed. Recent lines of evidence suggest that lifestyle modification and pharmacological approaches may reduce blood pressure and inflammation in patients with hypertension. Antagonism of the renin-angiotensin system with the selective angiotensin receptor blockers may improve cardiovascular outcome beyond blood pressure control, by reducing vascular inflammation and remodeling.
Keywords: vascular inflammation, hypertension, ARBs
Introduction
Low grade inflammation localized in vascular tissue is increasingly recognized as an important contributor to the pathophysiology of hypertension (De Ciuceis et al 2005), to the initiation and progression of atherosclerosis and the development of cardiovascular disease (CVD) (Savoia and Schiffrin 2006, 2007). Patients with CVD present increased expression and plasma concentrations of inflammatory markers and mediators (Blake and Ridker 2001; Sesso et al 2003). Among them, C-reactive protein (CRP) has been demonstrated as an independent risk factor for the development of hypertension (Blake et al 2003; Thorand et al 2003), and has been associated with increased risk of diabetes (Barzilay et al 2004) and CVD. Numerous epidemiological studies have shown that plasma levels of high-sensitivity CRP (hsCRP) are a powerful predictor of ischemic cardiovascular events in patients with stable or unstable angina. They appears to correlate with softer plaques that are more prone to rupture, and may even predict cardiovascular events among apparently healthy subjects (Ridker et al 1997, 1998). Furthermore, hsCRP levels have been shown to correlate with systolic blood pressure (BP), pulse pressure, and incident hypertension (Chae et al 2001). Thus CRP and high BP in combination have additional predictive value for cardiovascular outcomes, as they contribute as independent determinants of cardiovascular risk.
The activation of the renin-angiotensin system (RAS) is primarily involved in the pathophysiology of hypertension and the development of CVD (Schiffrin and Touyz 2004). In addition to its effect on BP, angiotensin II is also a proinflammatory mediator (Kranzhofer et al 1999; Pastore et al 1999). Antagonism of the RAS may improve cardiovascular outcomes beyond BP control, by reducing vascular inflammation and remodeling.
In this review we discuss the role of CRP as marker and/or mediator of low-grade inflammation in the vasculature of hypertensive patients and the potential advantages of antihypertensive medication that may attenuate the inflammatory mechanisms associated with hypertension.
RAS, inflammation, and hypertension
The activated components of the RAS play a key role in the pathophysiology of hypertension (Schiffrin and Touyz 2004). Angiotensin II, one of the final products of the RAS, induces vascular remodeling and injury by several mechanisms. These include vasoconstriction, cell growth, generation of reactive oxygen species (ROS), and inflammation. Angiotensin II can induce inflammation within the vascular wall (Figure 1) and consequently trigger deposition of extracellular matrix, and hypertrophy and/or hyperplasia of vascular smooth muscle cells (VSMCs) (Touyz and Schiffrin 2000) by modulating cytokine release (Schieffer et al 2000) and pro-inflammatory transcription factors such as NF-κB (Hernandez-Presa et al 1997), which in turn regulate adhesion molecule (VCAM-1 and ICAM-1) expression (Pueyo et al 2000).
Figure 1.
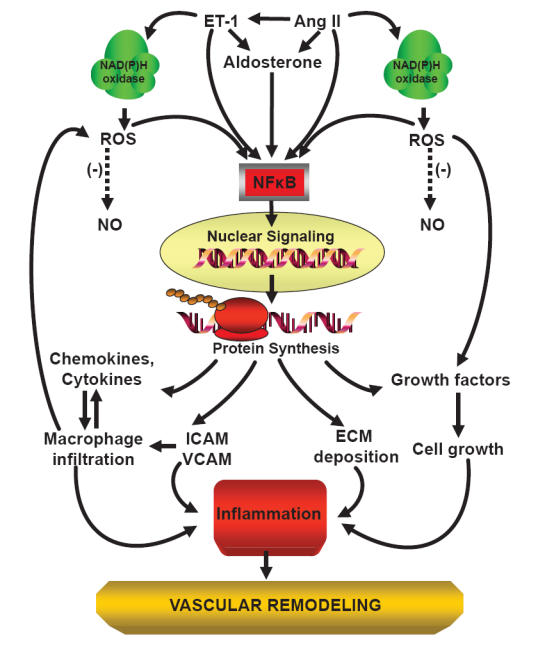
Scheme of angiotensin II-induced inflammation and vascular damage.
Abbreviations: Ang II, angiotensin II; EC, endothelial cell; ECM, extracellular matrix; ET-1, endothelin-1; ICAM, intercellular adhesion molecule; NAD(P)H oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NO, nitric oxide; NF-κB, nuclear factor kappa B; ROS, reactive oxygen species; VCAM, vascular cell adhesion molecule; (-), inhibition or reduction.
Angiotensin II, aldosterone, as well as endothelin-1, can modulate basal superoxide production by activation of reduced nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase and expression of its subunits (Lassegue and Clepmpus 2003). NAD(P)H oxidase is the major source of ROS in the vascular wall, and is expressed in endothelial cells, VSMCs, fibroblasts, and monocytes/macrophages (Griendling et al 2000; Touyz et al 2002) (Figure 1). ROS act as signaling molecules, but also in part by impairing endothelium-dependent vascular relaxation by reducing the bioavailability of nitric oxide (NO) (Rajagopalan et al 1996; Jung et al 2003). Vascular contractile responses are accordingly enhanced, associated with structural changes in the wall of small arteries and increased peripheral resistance (Touyz et al 2003). Increased ROS production induced by angiotensin II is involved in the mechanisms leading to vascular remodeling, through VSMC proliferation and hypertrophy and collagen deposition (Touyz and Schiffrin 2001). ROS promote vascular inflammation (Touyz and Schiffrin 2004) and exert effects on the development and progression of atherosclerosis (Kalinina et al 2002). Angiotensin II-induced ROS production increases ICAM-1 expression, macrophage infiltration, macrophage/monocyte chemoattractant protein-1 (MCP-1) production, and vascular hypertrophy independently of BP elevation (Liu et al 2003). Macrophages which infiltrate the adventitia or the media of blood vessels may generate oxidative stress by NAD(P)H oxidase, also in response to elevated BP (Schiffrin and Touyz 2003a). Mice deficient in monocytes/macrophages present less oxidative stress and less induction of inflammatory molecule upregulation by angiotensin II in the vasculature, and develop less endothelial dysfunction and vascular remodeling (De Ciuceis et al 2005). This suggests a central role for macrophages and pro-inflammatory mediators in angiotensin II-induced vascular injury.
Angiotensin II is also involved in atherosclerotic lesion progression and plaque instability by stimulating the activation of MMPs (matrix metalloproteinases), which can digest the fibrous cap and thereby participate in the triggering of plaque rupture (Galis et al 1994). In addition, angiotensin II stimulates the production of plasminogen activator inhibitor-I (PAI-1) (Vaughan et al 1995), which may contribute not only to the prothrombotic state but also to plaque rupture in atherosclerotic lesions.
C-reactive protein as biomarker and mediator of inflammation
CRP is a pentameric protein produced mainly by hepatocytes under the control of interleukin-6 as part of the non-specific acute-phase response to tissue damage, infection, inflammation and malignant neoplasia (Pepys and Hirschfield 2003). Recent findings have documented that CRP is also produced by vascular smooth muscle cells and endothelial cells, and in human atheroma (Yasojima et al 2001; Venugopal et al 2005). Increased levels of serum CRP, particularly sensed by high sensitivity assay, are generally regarded as a humoral sign of inflammation, and may identify subjects at moderate or high risk of cardiovascular disease. hsCRP has been related to insulin resistance, systolic BP, pulse pressure and hypertension (Chae et al 2001; Engstrom et al 2002). hsCRP correlates with plasma levels of von Willebrand factor (vWF), tissue plasminogen activator, and cellular fibronectin, which are markers of endothelial dysfunction (Yudkin et al 1999). Numerous epidemiological studies have shown that plasma hsCRP level is a powerful predictor of ischemic cardiovascular events in patients with stable or unstable angina, appears to be positively associated with softer plaques that are more prone to rupture (Liuzzo et al 1994), may even predict cardiovascular events among apparently healthy subjects (Ridker et al 1997, 1998), and may be useful in targeting medium-risk patients who could benefit from aggressive cardiovascular preventative therapy.
Vascular inflammation of large arteries exerts its effects in part by impairing artery elasticity and increasing vascular stiffness, in the presence or absence of hypertension. A correlation between CRP and measures of arterial wave reflection and stiffness in asymptomatic subjects was recently demonstrated (Kullo et al 2005). CRP levels were also associated with the future development of hypertension independently of baseline BP (Sesso et al 2003; Chrysohoou et al 2004), although this issue has been recently challenged (Niskanen et al 2004). In the Women’s Health Study which included over 15,000 women, after adjustment for the effects of other cardiovascular risk factors, high levels of CRP appeared to be predictive of cardiovascular events either in the absence or presence of high BP. In the latter case the risk was three-fold higher to that in the low CRP/low BP group (Blake et al 2003). However, after adjustment for confounding factors, elevated CRP levels appear not to lead to elevated BP (Smith et al 2005). Thus, both CRP and BP seem to be independent determinants of cardiovascular risk, and, in combination, each parameter may have additional predictive value (Chae et al 2001; Engstrom et al 2002; Blake et al 2003).
CRP may be more than an inflammatory biomarker of increased cardiovascular risk, as deposits of CRP have been demonstrated by immunohistochemical staining in atherosclerotic plaques (Figure 2), where it co-localizes with the terminal complement complex and appears to be involved in foam cell formation (Torzewski et al 2000). CRP promotes monocyte chemotaxis (Zwaka 2001) and facilitates low-density lipoprotein uptake by macrophages in vitro (Zwaka et al 2001). In endothelial cells, CRP facilitated the release of PAI-1 (Devaraj et al 2003) and endothelin-1 (Verma et al 2002), and increased the expression of cell adhesion molecules (Pasceri et al 2000), reduced NO bioavailability (Venugopal et al 2002), and NO-mediated dilation in the vasculature. In particular, CRP inhibited endothelium-dependent NO-mediated dilatation of coronary arterioles by inducing the generation of superoxide by NAD(P)H oxidase via p38 kinase activation (Qamirani et al 2005). On the other hand, it has been reported that CRP per se does not activate endothelial cells. The latter has been attributed to contamination of CRP preparations with azide and lipopolysaccharide, which could be responsible for the cell activation (Taylor et al 2005). In VSMCs, CRP increased angiotensin type 1 receptor number and ROS formation (Wang et al 2003), as well as activation of the stress-activated protein kinases p38 and c-jun N-terminal kinase (JNK) (Hattori et al 2003). It was recently reported that CRP caused a sustained increase in systolic BP in mice, related to an augmented pressor response to angiotensin II that is associated with a NO-sensitive downregulation of vascular angiotensin type 2 receptor expression (Vongpatanasin et al 2007).
Figure 2.
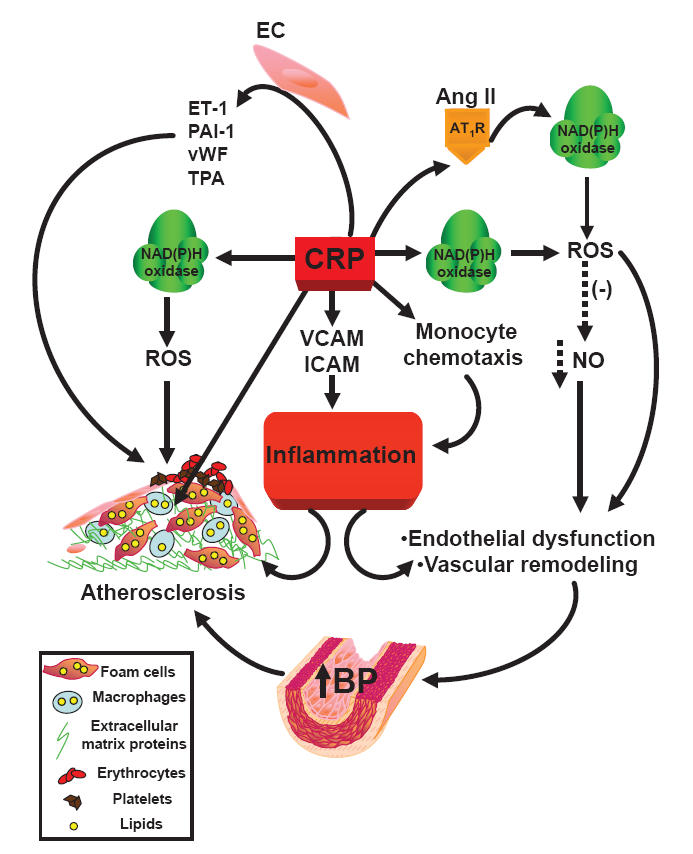
Scheme of C-reactive protein-induced inflammation.
Abbreviations: Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; BP, blood pressure; CRP, C-reactive protein; ET-1, endothelin-1; ICAM, intercellular adhesion molecule; NAD(P)H oxidase, nicotinamide adenine dinucleotide phosphate oxidase; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; ROS, reactive oxygen species; TPA, tissue plasminogen activator; VCAM, vascular cell adhesion molecule; vWF, von Willebrand factor; (-), reduction.
Along with the evidence that CRP is a marker and a modulator of inflammation, it has also been shown that CRP may possess anti-inflammatory actions. CRP inhibited neutrophil activation and adhesion (Heuertz and Webster 1997; Zouki et al 1997) and blocked platelet aggregation in vitro (Fiedel and Gewurz 1976; Vigo 1985; Miyazawa et al 1988). Accordingly, it has been proposed that distinct isoforms of CRP are formed during inflammation. CRP could dissociate into individual subunits that undergo conformational changes. The resulting monomeric CRP isoforms, express several neoepitopes and display properties distinct from those of native CRP (Potempa et al 1987; Zouki et al 2001; Khreiss et al 2002). Monomeric CRP is less soluble than pentameric CRP, it tends to aggregate, and induces a pro-inflammatory phenotype in human endothelial cells (Khreiss et al 2004a). Monomeric CRP antigens were detected in inflammed tissues and in the wall of normal human blood vessels, and displayed potent pro-thrombotic activity under low levels of shear. In contrast, native CRP inhibited platetelet activation and prevented neutrophil–endothelial-cell and neutrophil–platelet adhesion. Hence monomeric CRP rather than native CRP may be involved in the precipitation of acute coronary syndrome (Khreiss et al 2004b).
Thus, CRP may be a pro-inflammatory molecule under certain circumstances, as well as a marker to consider in medium-risk cardiovascular patients.
Reducing vascular inflammation via antagonism of the RAS
Arterial BP control is critical to reduce the burden of cardiovascular morbidity and mortality. Indeed, arterial hypertension contributes to increase the latter in combination with other cardiovascular risk factors (such as obesity, diabetes and dyslipidemia). JNC-VII and WHO-ISH and other national and international guidelines have suggested different non-pharmacological and pharmacological approaches to reduce BP in hypertensive patients (Chobanian et al 2003; Whitworth and Chalmers 2004). Since hypertension is a pro-inflammatory condition, non-pharmacological (weight loss, exercise and Mediterranean-style diet) and pharmacological therapeutic intervention to control BP have also been proposed to reduce vascular inflammation in patients with hypertension, in order to achieve a reduction of cardiovascular events and improved outcomes in randomized clinical trials.
Several therapeutic approaches have been shown to reduce CRP in patients at high cardiovascular risk. Exercise training, together with weight loss, reduced hsCRP levels significantly, although not in proportion to weight reduction (Obisesan et al 2004). CRP decrease was observed in the middle weight-reduction quartile, suggesting that there may be an optimal link between exercise and weight loss with respect to the inflammatory status (Obisesan et al 2004). Patients consuming a Mediterranean-style diet had a significant reduction in serum concentrations of hsCRP, IL-6, IL-7, and IL-18, as well as in insulin resistance (Esposito et al 2004).
In patients with high LDL-cholesterol levels, those with low hsCRP have better clinical outcomes than those with higher levels (Nissen et al 2005; Ridker et al 2005). Fenofibrate lowered hsCRP levels in patients with hypertriglyceridemia or combined hyperlipidemia (Koh et al 2005a). Statin-induced reduction of atherosclerosis progression was significantly related to greater reduction of hsCRP levels. Simvastatin or atorvastatin induced a reduction of CRP in coronary patients with hyperlipidemia (Strandberg et al 1999). In patients with diabetes and hypercholesterolemia, simvastatin combined with the angiotensin converting enzyme (ACE) inhibitor ramipril significantly reduced CRP levels more than monotherapy (Koh et al 2005b).
In hypertensive patients in the presence or absence of diabetes, BP control with ACE inhibitors, angiotensin receptor blockers (ARBs), and calcium channel blockers was associated with a reduction in the circulating levels of inflammatory mediators (Gasic et al 1999; Sardo et al 2004; Agabiti-Rosei et al 2005a, b). Several lines of evidence suggest that the improvement in systemic and vascular inflammatory status observed in patients treated with antihypertensive medications might involve BP-independent actions, which have been attributed in part to direct inhibition of pro-inflammatory effects of angiotensin II (Koh et al 2003; Schiffrin and Touyz 2003b). However, not all studies have demonstrated anti-inflammatory effects of agents that interrupt RAS. There is evidence that drugs that selectively block angiotensin II receptors or ACE may have superior anti-inflammatory properties than other antihypertensive agents. In subjects with uncomplicated hypertension, ACE inhibitors but not ARBs decreased plasma levels of adhesion molecules (VCAM-1, ICAM-1, E-selectin) (Jilma et al 2002). In older patients, ARBs reduced circulating levels of VCAM-1 but not of E-selectin (Rajagopalan et al 2002). In diabetic nephropathy, ARBs decreased plasma adhesion molecules (Andersen et al 2000). In type 2 diabetic patients, ARBs diminished VCAM-1, whereas ACE inhibitors reduced ICAM-1 (Guba et al 2000). We recently reported that antihypertensive treatment with an ARB ameliorated inflammatory processes and markedly reduced circulating pro-inflammatory mediators in diabetic hypertensive patients, independently by BP or glycemic control (Touyz et al 2007). Furthermore, CRP-induced up-regulation of the angiotensin type 1 receptor was attenuated by losartan (Wang et al 2003). In another study in hypertensive patients, although MCP-1 and PAI-1 were variably reduced, losartan, irbesartan, or candesartan did not significantly reduce serum CRP (Koh et al 2004). Also, whereas three small randomized clinical trials in patients with hypertension and cardiovascular disease showed a significant CRP-lowering effect by an ARB (Yasunari et al 2004; Fliser et al 2004; Schieffer et al 2004), in another study in hypertensive and diabetic patients no lowering of CRP could be detected (Agabiti-Rosei et al 2005a).
Recently, the Val-MARC (Valsartan-Managing blood pressure Aggressively and evaluating Reductions in hsCRP) study, addressed the issue of whether BP reduction per se lowers CRP levels, or whether selective antagonism of angiotensin type 1 receptors may have independent effects to reduce CRP levels (Ridker et al 2006). The study included 1668 patients with stage 2 hypertension randomly allocated to either the ARB valsartan alone (160–320 mg/day) or to valsartan/hydrochlorothiazide (HCTZ, 160–320 mg/12.5 mg/day) for a period of 6 weeks. At the end of the treatment, valsartan alone slightly but significantly reduced hsCRP levels, effect that was maintained over an extended follow-up period. In contrast the combination therapy did not reduce CRP levels. The difference between valsartan and valsartan/HCTZ was present in all subgroups evaluated despite the fact that BP reduction was greater in the combined therapy group. Minimal evidence of correlation between change in BP and change in hsCRP was observed. Interestingly, CRP reduction associated with valsartan treatment was observed not only in the total cohort but also among the subjects taking statins, a class of agents that has been shown to significantly reduce hsCRP levels (Strandberg et al 1999; Albert et al 2001; Koh et al 2005b; Ridker et al 2005). Thus, these data support the hypothesis that ARBs may have anti-inflammatory effects independently of the degree of BP reduction. However, the implication of the very small change in CRP found with valsartan vs valsartan plus HCTZ remains unclear, as does the therapeutic importance of this difference, since in the clinic, most patients who receive an ARB also receive a thiazide diuretic. Nevertheless, these findings have recently been confirmed by a study performed in a large multiethnic cohort of patients receiving pharmacologic treatment for hypertension in which the use of ACE inhibitors and ARBs as monotherapy was associated with lower CRP levels (Palmas et al 2007).
Observational studies in patients with cardiovascular disease have also suggested that ARBs and ACE inhibitors lower serum CRP. In patients with ischemic heart disease undergoing heart catheterization, lower serum levels of CRP were observed when the patients were treated with an ARB (Takeda et al 2003). After coronary artery bypass surgery, patients who had received ACE inhibitors had lower levels of IL-6, the main modulator of CRP release by the liver (Brull et al 2002). Moreover, ACE inhibitor use was associated with lower CRP levels in patients with heart failure (Joynt et al 2004). In the Val-Heft study, patients with congestive heart failure randomized to treatment with the ARB valsartan had lower hsCRP levels, whereas placebo-treated subjects did not (Arnand et al 2005).
Conclusion
Hypertension may be considered a disease associated with low-grade inflammation that contributes to cardiovascular disease. Non pharmacological and pharmacological approaches to control high BP may decrease vascular inflammation independently of BP reduction in patients with hypertension, resulting in reduced cardiovascular events in randomized clinical trials. Among other antihypertensive agents, ARBs have shown more potent anti-inflammatory properties unrelated to BP-lowering effect of this class of drugs, but more probably the result of a direct antagonism of the pro-inflammatory effects induced by angiotensin II. Thus, although reducing BP is the primary goal in order to decrease cardiovascular events in hypertensive patients, reduction of low-grade inflammation in hypertension may be an interesting and important target in order to reduce the cardiovascular morbidity and mortality associated with hypertension.
Acknowledgments
The work of the authors was supported by grants 13570 and 37917 of the Canadian Institutes of Health Research to ELS. ELS was supported in part by a Canada Research Chair on Hypertension and Vascular Research.
References
- Agabiti Rosei E, Morelli P, Rizzoni D. Effects of nifedipine GITS 20 mg or enalapril 20 mg on blood pressure and inflammatory markers in patients with mild-moderate hypertension. Blood Press. 2005b;1:14–22. doi: 10.1080/08037050510034257. [DOI] [PubMed] [Google Scholar]
- Agabiti Rosei E, Rizzoni D, Muiesan ML, et al. CENTRO (CandEsartaN on aTherosclerotic Risk factOrs) study investigators. Effects on candesartan cilexetil and enalapril on inflammatory markers of atherosclerosis in hypertensive patients with non-insulin dependent diabetes mellitus. J Hypertens. 2005a;23:435–44. doi: 10.1097/00004872-200502000-00027. [DOI] [PubMed] [Google Scholar]
- Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin Inflammation/CRP Evaluation (PRINCE), a randomized trial and cohort study. J Am Med Assoc. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- Andersen S, Schalkwijk CG, Stehouwer CD, Parving HH. Angiotensin II blockade is associated with decreased leukocyte adhesion molecule levels in diabetic nephropathy. Diabetes Care. 2000;23:1031–2. doi: 10.2337/diacare.23.7.1031. [DOI] [PubMed] [Google Scholar]
- Arnand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure prognostic value and the effect of valsartan. Circulation. 2005;112:1428–34. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Peterson D, Cushman M, et al. The relationship of cardiovascular risk factors to microalbuminuria in older adults with or without diabetes mellitus or hypertension: the cardiovascular health study. Am J Kidney Dis. 2004;44:25–34. doi: 10.1053/j.ajkd.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Blake JG, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89:763–71. doi: 10.1161/hh2101.099270. [DOI] [PubMed] [Google Scholar]
- Blake GJ, Rifai N, Buring JE, Ridker PM. Blood pressure, C-reactive protein, and risk of future cardiovascular events. Circulation. 2003;108:2993–9. doi: 10.1161/01.CIR.0000104566.10178.AF. [DOI] [PubMed] [Google Scholar]
- Brull DJ, Sanders J, Rumley A, et al. Impact of angiotensin converting enzyme inhibition on post-coronary artery bypass interleukin 6 release. Heart. 2002;87:252–5. doi: 10.1136/heart.87.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, et al. National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: The JNC 7 Report. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, Pitsavos C, Panagiotakos DB, et al. Association between prehypertension status and inflammatory markers related to atherosclerotic disease: the ATTICA Study. Am J Hypertens. 2004;17:568–73. doi: 10.1016/j.amjhyper.2004.03.675. [DOI] [PubMed] [Google Scholar]
- De Ciuceis C, Amiri F, Brassard P, et al. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol. 2005;25:2106–13. doi: 10.1161/01.ATV.0000181743.28028.57. [DOI] [PubMed] [Google Scholar]
- Devaraj S, Xu DJ, Jialal I. C-Reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implication for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- Engstrom G, Janzon L, Berglund G, et al. Blood pressure increase and incidence of hypertension in relation to inflammation-sensitive plasma protein. Arterioscler Thromb Vasc Biol. 2002;22:2054–8. doi: 10.1161/01.atv.0000041842.43905.f3. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. J Am Med Assoc. 2004;292:1440–6. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Fiedel BA, Gewurz H. Effects of C-reactive protein on platelet function, II: inhibition by CRP of platelet reactivities stimulated by poly-L-lysine, ADP, epinephrine, and collagen. J Immunol. 1976;117:1073–8. [PubMed] [Google Scholar]
- Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110:1103–7. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, et al. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2494–503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasic S, Wagner OF, Fasching P, et al. Fosinopril decreases levels of soluble vascular cell adhesion molecule-1 in borderline hypertensive type II diabetic patients with microalbuminuria. Am J Hypertens. 1999;12:217–22. doi: 10.1016/s0895-7061(98)00229-5. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–83. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- Guba M, Steinbauer M, Buchner M, et al. Differential effect of short term ace- and AT1 –receptor inhibition on postischemic injury and leukocyte adherence in vivo and in vitro. Shock. 2000;13:190–6. doi: 10.1097/00024382-200003000-00004. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc Res. 2003;58:186–95. doi: 10.1016/s0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- Hernandez-Presa M, Bustos C, Ortego M, et al. Angiotensin convertine enzyme inhibition prevents arterial nuclear factor kappa B activation monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–41. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- Heuertz RM, Webster RO. Role of C-reactive protein in acute lung injury. Mol Med Today. 1997;3:539–45. doi: 10.1016/S1357-4310(97)01145-3. [DOI] [PubMed] [Google Scholar]
- Jilma B, Li-Saw-Hee FL, Wagner OF, et al. Effects of enalapril and losartan on circulating adhesion molecules and monocyte chemotactic protein-1. Clin Sci (Lond) 2002;103:131–6. doi: 10.1042/cs1030131. [DOI] [PubMed] [Google Scholar]
- Joynt KE, Gattis WA, Hasselblad V, et al. Effect of angiotensin converting enzyme inhibitors, beta blockers statins, and aspirin on C-reactive protein levels in outpatients with heart failure. Am J Cardiol. 2004;93:783–5. doi: 10.1016/j.amjcard.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Jung O, Marklund SL, Geiger H, et al. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–9. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- Kalinina N, Agrotis A, Tararak E, et al. Cytochrome b558-dependent NAD(P)H oxidase-phox units in smooth muscle and macrophages of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2002;22:2037–43. doi: 10.1161/01.atv.0000040222.02255.0f. [DOI] [PubMed] [Google Scholar]
- Khreiss T, Jozsef L, Hossain S, et al. Loss of pentameric symmetry of C-reactive protein is associated with delayed apoptosis of human neutrophils. J Biol Chem. 2002;277:40775–81. doi: 10.1074/jbc.M205378200. [DOI] [PubMed] [Google Scholar]
- Khreiss T, Jozsef L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004a;109:2016–22. doi: 10.1161/01.CIR.0000125527.41598.68. [DOI] [PubMed] [Google Scholar]
- Khreiss T, Jozsef L, Potempa LA, Filep JG. Opposing effects of C-reactive protein isoforms on shear-induced neutrophil-platelet adhesion and neutrophil aggregation in whole blood. Circulation. 2004b;110:2713–20. doi: 10.1161/01.CIR.0000146846.00816.DD. [DOI] [PubMed] [Google Scholar]
- Kranzhofer R, Schmidt J, Pfeiffer CA, et al. Angiotensin induces inflammatory activation of human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1623–1629. doi: 10.1161/01.atv.19.7.1623. [DOI] [PubMed] [Google Scholar]
- Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–910. doi: 10.1016/s0735-1097(03)00846-5. [DOI] [PubMed] [Google Scholar]
- Koh KK, Han SH, Chung WJ, et al. Comparison of effect of losartan, irbesartan, and candesartan, on flow-mediated brachial artery dilation and on inflammatory and thrombolytic markers in patients with systemic hypertension. Am J Cardiol. 2004;93:1432–35. doi: 10.1016/j.amjcard.2004.02.050. [DOI] [PubMed] [Google Scholar]
- Koh KK, Han SH, Quon MJ, Ahn JY, Shin EK. Beneficial effect of fenofibrate to improve endothelial dysfunction and raise adiponectin levels in patients with primary hypertriglyceridemia. Diabetes Care. 2005a;28:1419–24. doi: 10.2337/diacare.28.6.1419. [DOI] [PubMed] [Google Scholar]
- Koh KK, Han SH, Quon MJ, et al. Vascular and metabolic effect of combined therapy with ramipril and simvastatin in patients with type 2 diabetes. Hypertension. 2005b;45:1088–93. doi: 10.1161/01.HYP.0000166722.91714.ba. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Seward JB, Bailey KR, et al. C-reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens. 2005;18:1123–9. doi: 10.1016/j.amjhyper.2005.03.730. [DOI] [PubMed] [Google Scholar]
- Lassegue B, Clepmpus RE. NAD(P)H oxidase: specific features, expression and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R97. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang F, Yang X-P, et al. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–82. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- Liuzzo G, BIasucci LM, Gallimore JR, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med. 1994;331:417–24. doi: 10.1056/NEJM199408183310701. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Kiyono S, Inoue K. Modulation of stimulus-dependent human platelet activation by C-reactive protein modified with active oxygen species. J Immunol. 1988;141:570–4. [PubMed] [Google Scholar]
- Niskanen L, Laaksonen DE, Nyyssonen K, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–65. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, et al. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- Obisesan TO, Leeuwemburgh C, Phillips T, et al. C-Reactive protein genotypes affect baseline, but not exercise training-induced changes, in C-reactive protein levels. Arterioscler Thromb Vasc Biol. 2004;24:1874–9. doi: 10.1161/01.ATV.0000140060.13203.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmas W, Ma S, Psaty B, et al. Antihypertensive medications and C-reactive protein in the multi-ethnic study of atherosclerosis. Am J Hypertens. 2007;20:233–41. doi: 10.1016/j.amjhyper.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-Reactive protein on human endothelial cells. Circulation. 2000;102:2165–8. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- Pastore L, Tessitore A, Martinottti S, et al. Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increase soluble ICAM-1 release in vivo. Circulation. 1999;100:1646–52. doi: 10.1161/01.cir.100.15.1646. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potempa LA, Siegel NJ, Fiedel BA, et al. Expression, detection and assay of neoantigen (neo-CRP) associated with a free C-reactive protein subunit. Mol Immunol. 1987;24:531–41. doi: 10.1016/0161-5890(87)90028-9. [DOI] [PubMed] [Google Scholar]
- Pueyo ME, Gonzalez W, Nicoletti A, et al. Angiotensin II stimulates endothelial vascular cell adhesion molecule-1 via nuclear factor-kappaB activation induced by intracellular oxidative stress. Arterioscler Thromb Vasc Biol. 2000;20:645–51. doi: 10.1161/01.atv.20.3.645. [DOI] [PubMed] [Google Scholar]
- Qamirani E, Ren Y, Kuo L, Hein W. C-reactive protein inhibits endothelin-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook R, Mehta RH, et al. Effect of losartan in aging related endothelial impairment. Am J Cardiol. 2002;89:562–66. doi: 10.1016/s0002-9149(01)02297-4. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Kurz S, Munzel T, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98:731–3. doi: 10.1161/01.cir.98.8.731. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, et al. Inflammation, aspirin and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–6. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Rifai N, Glynn RJ for the Val-MARC Investigators. Valsartan, blood pressure reduction, and C-reactive protein. Primary report of the Val-MARC trial. Hypertension. 2006;48:73–79. doi: 10.1161/01.HYP.0000226046.58883.32. [DOI] [PubMed] [Google Scholar]
- Sardo MA, Castaldo M, Cinquegrani M, et al. Effects of AT1 receptor antagonist losartan on sICAM-1 and TNF-alpha levels in uncomplicated hypertensive –patients. Angiology. 2004;55:195–203. doi: 10.1177/000331970405500212. [DOI] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;2:152–8. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–84. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- Schieffer B, Bunter C, Witte J, et al. Comparative effects of AT1-antagonism and angiotensin-converting enzyme inhibition on markers of inflammation and platelet aggregation in patients with coronary artery disease. J Am Coll Cardiol. 2004;44:362–8. doi: 10.1016/j.jacc.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Schieffer B, Luchtefeld M, Braun S, et al. Role of NAD(P)H oxidase in angiotensin II-induced JAK/STAT signaling and cytokine induction. Circ Res. 2000;87:1195–201. doi: 10.1161/01.res.87.12.1195. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. Inflammation and vascular hypertrophy induced by angiotensin II: role of NADPH oxidase-derived reactive oxygen species independently of blood pressure elevation? Arterioscler Thromb Vasc Biol. 2003a;23:707–9. doi: 10.1161/01.ATV.0000069907.12357.7E. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. Multiple actions of angiotensin II in hypertension: benefits of AT1 receptor blockade. J Am Coll Cardiol. 2003b;42:911–13. doi: 10.1016/s0735-1097(03)00845-3. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL, Touyz RM. From bedside to bench to bedside: role of renin-angiotensin-aldosterone system in remodeling of resistance arteries in hypertension. Am J Physiol Heart Circ Physiol. 2004;287:H435–H–46. doi: 10.1152/ajpheart.00262.2004. [DOI] [PubMed] [Google Scholar]
- Sesso HD, Buring JE, Rifai N, et al. C-reactive protein and the risk of developing hypertension. J Am Med Assoc. 2003;290:2945–51. doi: 10.1001/jama.290.22.2945. [DOI] [PubMed] [Google Scholar]
- Smith GD, Lawlor DA, Harbord R, et al. Association of C-reactive protein with blood pressure and hypertension. Life course confounding and Mendelian randomization tests of causality. Arterioscler Thromb Vasc Biol. 2005;25:1051–6. doi: 10.1161/01.ATV.0000160351.95181.d0. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Vanhanen H, Tikkanen MJ. Effects of statins on C-reactive protein in patients with coronary artery disease. Lancet. 1999;353:118–19. doi: 10.1016/S0140-6736(05)76154-7. [DOI] [PubMed] [Google Scholar]
- Takeda T, Oshida S, Nishino M, et al. Relationship between effects of statins, aspirin and angiotensin II modulators on high-sensitive protein levels. Atherosclerosis. 2003;169:155–8. doi: 10.1016/s0021-9150(03)00158-8. [DOI] [PubMed] [Google Scholar]
- Taylor KE, Giddings JC, van den Berg CW. C-reactive protein-induced in vitro endothelial cell activation is an artifact caused by azide and lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2005;25:1225–30. doi: 10.1161/01.ATV.0000164623.41250.28. [DOI] [PubMed] [Google Scholar]
- Thorand B, Lowel H, Schneider A, et al. C-reactive protein as a predictor for incident diabetes mellitus among middle-aged men: results from the MONICA Augsburg cohort study, 1984–1998. Arch Intern Med. 2003;163:93–9. doi: 10.1001/archinte.163.1.93. [DOI] [PubMed] [Google Scholar]
- Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–9. doi: 10.1161/01.atv.20.9.2094. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, et al. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–13. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Savoia C, He Y, et al. Increased inflammatory biomarkers in hypertensive type2 diabetic patients: improvement after angiotensin II type 1 receptor blockade. J Am Soc Hypertens (JASH) 2007 doi: 10.1016/j.jash.2007.01.009. in press. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–72. [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hypertens. 2001;19:1245–54. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–52. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30:860–6. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- Vaughan DE, Lazos SA, Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. J Clin Invest. 1995;95:995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal SK, Devaraj S, Jialal I. Macrophage conditioned medium induces the expression of C-reactive protein in human aortic endothelial cells: potential for paracrine/autocrine effects. Am J Pathol. 2005;166:1265–71. doi: 10.1016/S0002-9440(10)62345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal SH, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effect of C-reactive protein. Circulation. 2002;105:1890–6. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- Vigo C. Effect of C-reactive protein on platelet-activating factor-induced platelet aggregation and membrane stabilization. J Biol Chem. 1985;260:3418–22. [PubMed] [Google Scholar]
- Vongpatanasin W, Thomas GD, Schwartz L, et al. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115:1020–8. doi: 10.1161/CIRCULATIONAHA.106.664854. [DOI] [PubMed] [Google Scholar]
- Wang CH, Li SH, Weisel RD, et al. C-reactive protein upregulates angiotensin type 1 receptors in vascular smooth muscle. Circulation. 2003;107:1783–90. doi: 10.1161/01.CIR.0000061916.95736.E5. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Chalmers J. World health organisation-international society of hypertension (WHO/ISH) hypertension guidelines. Clin Exp Hypertens. 2004;26:747–52. doi: 10.1081/ceh-200032152. [DOI] [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerothic plaques. Am J Pathol. 2001;158:1039–51. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunari K, Maeda K, Watanabe T, et al. Comparative effects of valsartan versus amlodipine on left ventricular mass and reactive oxygen species formation by monocytes in hypertensive patients with left ventricular hypertrophy. J Am Coll Cardiol. 2004;43:2116–23. doi: 10.1016/j.jacc.2003.12.051. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Stehouwer CDA, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role of cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–8. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zouki C, Beauchamp M, Baron C, et al. Prevention of in vitro neutrophil adhesion to endothelial cells through shedding of L-selectin by C-reactive protein and peptides derived from C-reactive protein. J Clin Invest. 1997;100:522–9. doi: 10.1172/JCI119561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouki C, Haas B, Chan JSD, et al. Loss of pentameric symmetry of C-reactive protein is associated with promotion of neutrophil-endothelial cell adhesion. J Immunol. 2001;167:5355–61. doi: 10.4049/jimmunol.167.9.5355. [DOI] [PubMed] [Google Scholar]
- Zwaka TP, Hombach V, Torzewski J. C-reactive protein-mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–7. doi: 10.1161/01.cir.103.9.1194. [DOI] [PubMed] [Google Scholar]


