Abstract
Nebivolol is a third generation beta-blocker, which can be distinguished from other beta-blockers by its hemodynamic profile. It combines beta-adrenergic blocking activity with a vasodilating effect mediated by the endothelial L-arginine nitric oxide (NO) pathway. The effects of nebivolol have been compared with other beta-blockers and also with other classes of antihypertensive agents. In general, response rates to treatment are higher, and the frequency and severity of adverse events are either comparable or lower with nebivolol. Nebivolol is also effective in reducing cardiovascular morbidity and mortality in elderly patients with heart failure, regardless of the initial ejection fraction. Endothelium-derived NO is important in the regulation of large arterial stiffness, which in turn is a major risk factor for cardiovascular disease. Treatment with nebivolol increases the release of NO from the endothelium and improves endothelial function, leading to a reduction in arterial stiffness. Decreased arterial stiffness has beneficial hemodynamic effects including reductions in central aortic blood pressure. Unlike first generation beta-blockerrs, vasodilator beta-blockerrs such as nebivolol have favorable hemodynamic effects, which may translate into improved cardiovascular outcomes in patients with hypertension.
Keywords: nebivolol, hypertension, heart failure, beta-blocker, nitric oxide, arterial stiffness
Introduction
Hypertension is a major risk factor for cardiovascular disease, and aggressive reduction of blood pressure can significantly improve cardiovascular outcomes (Staessen et al 2003). However, there is still debate as to whether it is blood pressure reduction per se or the antihypertensive agent used that is most important in terms of improving cardiovascular outcome. The latest guidelines issued by the National Institute for Clinical Excellence (NICE) for England and Wales recommend an angiotensin-converting enzyme inhibitor (or an angiotensin receptor blocker if an ACE inhibitor is not tolerated) as first-line treatment for hypertension in patients less than 55 years old (NICE 2006). In patients over 55 years and in black patients of any age, the recommended first-line therapy is either a calcium channel blocker or a thiazide-type diuretic.
The NICE guidelines no longer recommend beta-blockers for the first or second line treatment of hypertension. This recommendation was prompted by two recent meta-analyses which showed that despite reducing blood pressure, beta blockade was not effective in reducing cardiovascular events when compared with either placebo or other antihypertensive agents (Carlberg et al 2004; Lindholm et al 2005). Beta-blockers have also recently been shown to increase the risk of type 2 diabetes, especially if treatment is in combination with a thiazide-type diuretic. However, atenolol was the beta-blocker used in most of these studies and, given the relative lack of clinical outcome data from trials of treating hypertension with beta-blockers other than atenolol, it is unclear whether this conclusion applies to all beta-blockers.
Isolated systolic hypertension is associated with increased large artery stiffness, a strong independent predictor of cardiovascular risk. Recently endothelium-derived nitric oxide (NO) has been shown to be involved in the regulation of large arterial stiffness, with a reduced bioavailability of NO production linked to increased arterial stiffness (Kinlay et al 2001; Wilkinson et al 2002; Schmitt et al 2005). Arterial stiffening associated with age and disease has therefore become a new and important therapeutic target in terms of blood pressure reduction and cardiovascular disease prevention. Drugs such as nebivolol that reduce blood pressure and improve endothelial function may be especially useful in this regard and should be considered as an alternative first-line treatment for hypertension and in elderly patients with chronic heart failure.
Nebivolol
Nebivolol is a third generation beta-blocker, which can be distinguished from other beta-blockers by its hemodynamic profile. The hemodynamic effects of nebivolol are due to its vasodilator properties including a reduction in systemic vascular resistance and an increase in cardiac output (Ritter 2001). It is the most beta-1-selective adrenoceptor antagonist currently in clinical use and has no alpha-1-blocking action (Van Bortel et al 1997). The enantiomers have different pharmacological properties. The d-isomer provides the beta-blocking component (Van Nueten and De Cree 1998) and both the d- and l-isomers have an endothelial NO-dependent vasodilating effect. Thus racemic nebivolol is needed for the drug to be most effective. Such characteristics are in contrast to those of carvedilol which also has vasodilatory and anti-inflammatory properties, but in this case due to its ability to block alpha1 receptors. The effects of carvedilol on NO bioactivity also remain unclear.
Nebivolol is rapidly absorbed after oral administration of a standard 5-mg dose and reaches peak plasma levels between 30 minutes to 2 hours after intake. It is extensively metabolized and excretion is mainly in the feces and urine. The pharmacokinetics of nebivolol are not affected by age. However, the recommended starting dose for patients over 65 years is 2.5 mg a day. This is in line with many other antihypertensive treatments where dosage is lowered for elderly patients.
Nebivolol (5 mg) is indicated for the treatment of essential hypertension and, in elderly patients ≥70 years, for the treatment of stable mild and moderate chronic heart failure in addition to standard therapies. A starting dose of 2.5 mg is suggested in patients over 65 years or in patients with renal insufficiency.
Nebivolol combines beta-adrenergic blocking activity with a vasodilating effect via increased NO availability, mediated by the endothelial L-arginine NO pathway, leading to a reduction in peripheral vascular resistance. Treatment with nebivolol also leads to improvements in left ventricular function in patients with heart failure (Uhlir et al 1991; Erdogan et al 2007; Lombardo et al 2006), and arterial compliance (Van Merode et al 1989). Left ventricular function is preserved and left ventricular mass is reduced in hypertensive patients with left ventricular hypertrophy (Stoleru et al 1993).
Hypertensive patients are at high risk of coronary artery disease and subsequent impaired cardiac function (Robertson and Ball 1994) and congestive heart failure. Endothelial dysfunction, characterized by decreased bioavailability of NO, also occurs early in various forms of cardiovascular disease. NO has powerful antiatherogenic effects and a decrease in NO production is associated with a number of cardiovascular risk factors including hypertension, diabetes mellitus and hypercholesterolemia. Endothelial dysfunction may therefore contribute to the pathogenesis of atherosclerosis in hypertension (Moncada 1994). Treatment with nebivolol may thus favorably impact on the vascular complications of hypertension either directly by reducing blood pressure or indirectly by increasing the bioavailability of NO. In healthy volunteers, nebivolol (5 mg) decreases systemic vascular resistance with no impairment of left ventricular function (Van de Water et al 1988). In addition, chronic treatment with nebivolol maintains left ventricular function in healthy volunteers and in patients with hypertension (De Cree et al 1991), acute myocardial infarction and congestive heart failure (Stoleru et al 1993).
Central aortic pressure is a strong predictor of cardiovascular morbidity and mortality but classic beta-blockers such as atenolol have little effect on reducing central aortic pulse pressure in hypertensive patients. Studies comparing atenolol with other antihypertensive agents show that although decreases in peripheral blood pressure are similar, treatment with atenolol results in significantly less reduction of central aortic pressure compared with either fosinopril (Chen et al 1995) or eprosartan (Dhakam et al 2006). Improvements in arterial stiffness and arterial compliance are also greater with calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers compared with atenolol (Resnick and Lester 2002).
The lack of efficacy of atenolol in reducing central pressure can have a direct effect on cardiovascular outcomes. In the CAFE study (Williams et al 2006) both brachial and aortic pressures were measured using pulse wave analysis in 2199 patients originally enrolled for the ASCOT trial. Patients treated with amlodipine/perindopril had greater reductions in central aortic systolic pressure and central aortic pulse pressure compared with patients given atenolol/bendrofluazide, even though reductions in brachial blood pressure were similar across the treatment groups. In addition, central aortic pulse pressure was an independent determinant of cardiovascular outcomes and may help to explain why, in the ASCOT trial, clinical outcomes were worse in patients treated with atenolol/bendrofluazide.
Atenolol may be less effective at reducing central aortic pressure because of its effects on reducing heart rate which may enhance the effect of wave reflections (Wilkinson et al 2006). The additional vasodilatory effects of nebivolol contribute to a lower reduction in heart rate, and the subsequent decrease in wave reflection together with improvements in arterial stiffness, and endothelial dysfunction may offset such deleterious hemodynamic effects and thus lower central pressure more than atenolol. This may translate clinically into greater reductions in cardiovascular morbidity and mortality than those seen with traditional beta-blockers (Kelly et al 1989; Pedersen and Cockcroft 2006), although this remains as yet unproven.
Clinical efficacy of nebivolol in hypertension
The efficacy of nebivolol monotherapy has been extensively studied in patients with mild to moderate hypertension. Early double-blind, placebo-controlled studies showed significant reductions of blood pressure with a daily dose of 5 mg nebivolol (Van Bortel and Van Baak 1992; Van Nueten et al 1997a). Nebivolol was equally effective in black patients, with a notable absence of typical side-effects usually associated with beta-blockade. Nebivolol did not impair Quality of life, measured with the Inventory of Subjective Health (ISH), and the frequency of adverse events was similar between nebivolol and placebo (Van Bortel et al 1993).
A recent follow-up study was conducted in order to establish whether the reported efficacy and safety of nebivolol can be generalized in a large nationwide study (Cleophas et al 2006). A total of 6356 patients with mild hypertension were treated with nebivolol for 6 weeks. No serious adverse events occurred during the study, and the occurrence of minor adverse events was very limited. Blood pressure was significantly reduced and the efficacy of nebivolol monotherapy and add-on therapy was similar. Nebivolol was also highly effective in patients with isolated systolic hypertension.
Cleophas et al (2001) have assessed the long-term efficacy of nebivolol monotherapy. The study found a greater reduction in blood pressure and a higher percentage of responders after 6 months of nebivolol treatment. Nebivolol was well tolerated and patients reported a better feeling of general well being compared with any previous monotherapies. Although current guidelines recommend the adjustment of antihypertensive drug therapy after 6–8 weeks of treatment (WHO-ISH 1999), such a strategy may not be appropriate for optimal nebivolol treatment.
In a 6-week observational study, nebivolol reduced both systolic and diastolic blood pressures and, unlike first generation beta-blockerrs, there were significant reductions in cholesterol, triglycerides, and blood sugar (Fallois and Faulhaber 2001). Results of studies comparing the efficacy and safety of nebivolol compared with other beta-blockers and other classes of antihypertensive agents generally find response rates to treatment are higher, and the frequency and severity of adverse events are either comparable or lower with nebivolol.
Nebivolol vs other beta-blockers
The antihypertensive effects of nebivolol are similar to those of the classic beta-blockers but the unique hemodynamic profile of nebivolol may contribute to its additional reported benefits. For example, in a double blind, randomized study in patients with untreated essential hypertension (Kamp et al 2003), both nebivolol (5 mg/day) and atenolol (100 mg/day) significantly reduced blood pressure to a similar extent. However, nebivolol also significantly reduced heart rate and peripheral resistance and increased stroke volume, leading to a small increase in cardiac output whereas cardiac output was significantly decreased and peripheral resistance increased with atenolol. The improvements in diastolic function with nebivolol highlight its potential use in the treatment of heart failure.
A separate double blind, randomized, parallel group trial compared patients treated for 4 weeks with nebivolol (5 mg/day), atenolol (50 mg/day), or placebo. Both nebivolol and atenolol significantly reduced blood pressure compared with placebo while nebivolol had no orthostatic effects and was better tolerated than atenolol (Van Nueten et al 1998b). Similar results were found when nebivolol was compared with metoprolol (Uhlir et al 1991).
The unique hemodynamic profile of nebivolol may also contribute to the maintenance of exercise capacity compared with other beta-blockers. In a double blind, placebo-controlled, cross-over study of exercise tolerance in healthy volunteers given nebivolol (5 mg) or atenolol (100 mg) for 2 weeks, exercise capacity was lower and fatigue higher with atenolol compared with nebivolol (Van Bortel and Van Baak 1992). Nebivolol also significantly decreased the total peripheral resistance during exercise compared with placebo.
Nebivolol vs other classes of antihypertensive agents
Nebivolol is an effective antihypertensive agent with a superior tolerability profile in comparison with other classes of antihypertensive agents. In a double-blind study comparing hypertensive patients treated with nebivolol (5 mg) or the angiotensin-converting enzyme inhibitor enalapril (10 mg) for 3 months, the decrease in blood pressure was significantly higher and response rates were higher with nebivolol (Van Nueten et al 1997b). The incidence of cough was also higher with enalapril.
Although nebivolol (5 mg) and the calcium antagonist nifedipine (20 mg) were equally effective in lowering blood pressure, nebivolol also significantly reduced heart rate (Van Nueten et al 1998a) and adverse events associated with nifedipine treatment caused a significantly higher number of patients to withdraw from the study compared with nebivolol-treated patients. Heart rate was also significantly reduced with nebivolol in a study comparing nebivolol (2.5–5 mg) with the calcium channel blocker amlodipine (5–10 mg) in elderly patients with mild to moderate hypertension (Mazza et al 2002). A high heart rate is linked to an increased risk of death in the elderly (Palatini et al 1999) and so an antihypertensive such as nebivolol that effectively lowers blood pressure and also lowers heart rate has dual benefits in this population.
Effects of nebivolol and the angiotensin receptor blocker losartan on quality of life and antihypertensive effects were compared in a double-blind, randomized, parallel group study (Van Bortel et al 2005). Patients with hypertension were treated for 12 weeks with 5 mg of nebivolol or 50 mg of losartan once daily. Quality of life parameters did not differ between the two treatments and although both drugs decreased systolic blood pressure similarly, the decrease in diastolic blood pressure was significantly greater with nebivolol.
Type 2 diabetes
Endothelial dysfunction, leading to decreased bioavailability of NO, is one of the major underlying mechanisms linking cardiovascular risk factors such as hypertension, diabetes mellitus, and dyslipidemia to overt cardiovascular disease (Mason 2006). In addition, tight control of blood pressure is more effective at reducing cardiovascular events than tight control of blood sugar in diabetic patients (Palatini et al 1999). Unlike some beta-blockerrs, nebivolol had no effect on insulin sensitivity and glucose tolerance (Fogari et al 1997) and may therefore have potential therapeutic benefits in patients with type 2 diabetes, especially as many diabetic patients develop hypertension during the course of their disease (Kannel et al 1991).
Arterial stiffness and cardiovascular risk
Arterial stiffness is a consequence of age (Kass 2002) and also of conditions such as diabetes and hypercholesterolemia, which cause premature vascular aging. Arterial stiffness is a major risk factor for cardiovascular disease and is an important predictor of mortality in hypertensive patients (Laurent et al 2001). Increased aortic stiffness is also an independent predictor of diastolic dysfunction in patients with hypertensive heart disease (Yambe et al 2004; Mottram et al 2005) (see also Figure 1), and may also limit exercise tolerance in patients with dilated cardiomyopathy (Bonapace et al 2003). Patients who have heart failure with a preserved ejection fraction present with ventricular-systolic and arterial stiffening beyond that expected with age and/or hypertension (Kawaguchi et al 2003). Ventricular-vascular stiffening may also be greater in elderly women compared with elderly men (Redfield et al 2005). Therefore, therapies aimed at reducing arterial stiffening may be of use in the treatment of diastolic dysfunction and heart failure.
Figure 1.
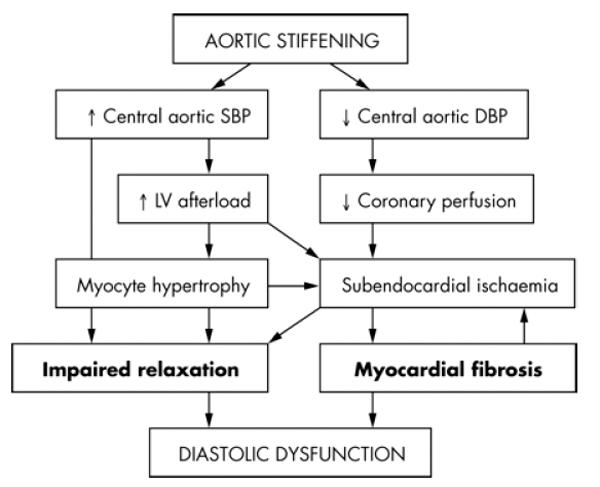
Pathophysiological pathways through which aortic stiffness may contribute to the development of diastolic dysfunction. DBP, diastolic blood pressure; SBP, systolic blood pressure. Reproduced with permission from Mottram PM, Haluska BA, Leano R et al 2005. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart, 91:1551–6. Copyright © 2005. BMJ Publishing Group Ltd.
Aortic pulse wave velocity (PWV) is a direct measure of arterial stiffness and may be a better predictor of future cardiovascular events compared with established risk factors such as age, hypertension, hypercholesterolemia, and diabetes. Studies using PWV find that increased arterial stiffness can predict cardiovascular risk in apparently healthy subjects (van Popele et al 2006), patients with hypertension (Boutouyrie et al 2002), diabetes (Cruickshank et al 2002), end-stage renal failure (Blacher et al 1999), and older individuals (Meaume et al 2001). Arterial stiffness is also a possible risk factor for diastolic heart failure in hypertensive patients.
Pulse pressure, determined by large artery compliance and the pattern of left ventricular ejection, is a surrogate measure of arterial stiffness. In older patients, pulse pressure is a more important determinant of cardiovascular prognosis than mean arterial pressure in normotensive (Benetos et al 1997), hypertensive (Benetos et al 1998) and post-myocardial infarction populations (Mitchell et al 1997). These findings are confirmed by recent re-analysis (Millar et al 1999) and meta-analysis (Blacher et al 2000) of blood pressure lowering studies. Patients with congestive heart failure have an elevated central pulsatile load, which may help to explain why an increased pulse pressure is linked to an increase in clinical events in such patients (Mitchell et al 2001). Therapies that help to reduce such abnormal loading of the heart may be of use in the treatment of congestive heart failure.
An increased PWV and amplitude of the pressure wave reflected back to the aorta can cause increases in central aortic pressure, leading to increased left ventricular workload. Central aortic pressure is therefore an independent predictor of cardiovascular morbidity and mortality. Indeed, patients with high aortic pressure have a worse cardiovascular prognosis than patients with more effective control of central aortic pressure (Williams et al 2006).
Increased arterial stiffness is linked to endothelial dysfunction and reduced bioavailability of NO (Wilkinson et al 2004). Endothelial dysfunction, which is found in patients with most cardiovascular risk factors, may explain why these conditions are also associated with increased arterial stiffness at an early stage before the development of manifest atheroma (Cockcroft et al 1997). Therefore, drugs such as nebivolol that can increase NO production may help to reduce large artery stiffness which in turn may lead to a reduction of cardiovascular risk.
Antihypertensive agents differ in their effects on large arterial stiffness and pulse wave reflection (Chen et al 1995; Van Bortel et al 1995; Breithaupt-Grogler et al 1996; Dreary et al 2002). However, some of the beneficial effects observed may simply be due to the passive effect of lowering mean arterial pressure. The classical first generation beta-blockers such as atenolol have been shown to actually increase pulse wave reflection and central pressure acutely (O’Rourke et al 1989). Nebivolol decreases arterial stiffness independently of any effect on blood pressure (McEniery et al 2004). Such vasodilating effects of nebivolol may help to reduce cardiovascular risk and improve outcomes, although such benefits need to be confirmed by the data from longer-term intervention trials.
Clinical efficacy of nebivolol in chronic heart failure
A large, randomized, double-blind, placebo-controlled study (SENIORS study), has assessed the effects of nebivolol on mortality and morbidity in elderly patients ≥70 years with a history of heart failure (Flather et al 2005). Patients were started on a dose of 1.25 mg nebivolol once daily and titrated to a target dose of 10 mg over a mean of seven weeks. Although nebivolol did not significantly reduce mortality, the composite risk of all cause mortality or cardiovascular hospital admission (time to first event) was significantly reduced by 15% with nebivolol compared with placebo (Figure 2). This risk reduction was lower than that seen in previous trials with other beta-blockers (Shibata et al 2001). However, a sub-analysis of data from patients most similar to patients of these earlier trials showed the risk reduction to increase to 27%. Such results indicate that nebivolol has comparable benefits to those of other beta-blockers studied in heart failure. The benefits of treatment appeared after 6 months and the risk reduction increased if treatment was continued. The benefits of beta-blockade were independent of the initial ejection fraction and were observed even in patients with mild left ventricular dysfunction or preserved ventricular function. The vasodilating effects specific to nebivolol may help to improve tolerability in elderly patients with heart failure and support the use of this particular beta-blocker to treat heart failure in an elderly population.
Figure 2.
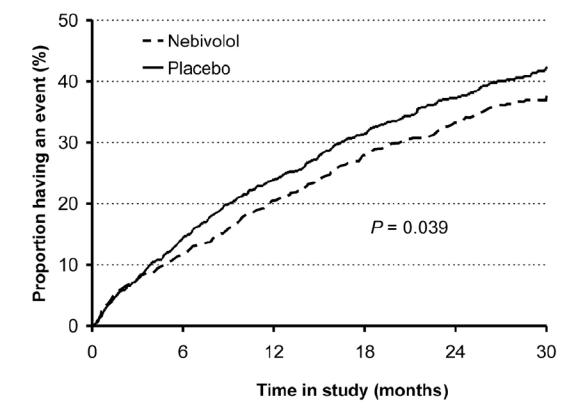
Time to first occurrence of events (all cause death or hospital admission for a cardiovascular reason – primary endpoint). Flather MD, Shibata MC, Coats AJ et al 2005. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J, 26:215–25. Copyright © 2005. Oxford University Press.
Safety and tolerability
Nebivolol is well-tolerated in patients with hypertension. In clinical trials, reported adverse events are mostly mild to moderate in nature with an incidence similar to that observed with placebo (Tzemos et al 2001). Meta-analysis of the incidence of adverse events in double-blind, placebo-controlled trials finds the occurrence of adverse events to be no different with nebivolol compared with placebo (Lacourciere and Arnott 1994; Van Nueten et al 1998a) and doses of up to 30 mg (6 times the recommended dose) have been well tolerated. Adverse events typical of classical beta-blockers are lower with nebivolol (Steiner et al 1990). Classical beta-blockers may also alter plasma lipids in a potentially adverse manner (Cruickshank and Prichard 1987) whereas patients treated for 3 months with up to 10 mg daily nebivolol show no changes in plasma total cholesterol, triglycerides, lipoproteins, and apolipoproteins (Lacourciere et al 1992; Van Nueten et al 1997b; Tzemos et al 2001). Quality of life, assessed using the Inventory of Subjective Health, is not impaired with nebivolol treatment (Van Bortel et al 1993) and in general is similar to that reported with both atenolol and losartan (Van Nueten et al 1998c; Van Bortel et al 2005).
In the SENIORS study in elderly patients with chronic heart failure, the tolerability of nebivolol was similar to placebo (Flather et al 2005). Drug-related adverse events were typical of those associated with beta-blockers and included hypotension, bradycardia and dizziness.
Endothelial function and the role of nitric oxide
The vascular endothelium modulates the tone and structure of the blood vessel smooth muscle by releasing various vasoactive and relaxing factors. One of the most important appears to be NO, a potent endogenous smooth muscle dilator, synthesized from the amino acid L-arginine, via the action of the constitutive enzyme nitric oxide synthase (Palmer et al 1988). NO regulates basal vascular tone and blood pressure and also has powerful antiatherogenic properties. Endothelial dysfunction, manifested by reduced arterial vasodilation, is linked to abnormalities of the L-arginine/NO pathway resulting in decreased bioavailability of NO. Such dysfunction can therefore predispose to atherogenesis and may represent a link between conditions associated with increased cardiovascular risk (including diabetes, hypercholesterolemia and hypertension) and the development of overt cardiovascular disease (Moncada 1994; Brunner et al 2005).
Nebivolol can improve endothelial function directly via an effect on the endothelial L-arginine/NO pathway. Nebivolol relaxes precontracted canine coronary artery strips only if the endothelium is intact (Stoleru et al 1993) and this vasorelaxant effect is antagonized by nitro-L-arginine, an inhibitor of NO production, implying that the effect is mediated via release of endothelium-derived NO (Gao et al 1991).
Similar findings have been reported in vivo in a human vascular bed (Cockcroft et al 1995). Infusion of nebivolol into the brachial artery of healthy volunteers resulted in vasodilation and an increase in forearm blood flow by an average of 90% whereas atenolol had no effect. The vasodilator effect was significantly reduced with co-infusion of L-NMMA and this inhibition was abolished by L-arginine, the substrate for NO production, suggesting that the L-arginine/nitric oxide pathway was involved. Similar effects of nebivolol have also been demonstrated in experiments of nebivolol infusion into superficial hand veins (Bowman et al 1994) and in patients with hypertension (Dawes et al 1999). Oral nebivolol, but not atenolol, can also improve both basal and stimulated NO release relative to placebo in patients with essential hypertension (Tzemos et al 2001).
The improvements in endothelial function, secondary to release of NO, seen with nebivolol are of particular importance in black patients. This patient group has a reduced bioavailability of NO and alterations in endothelial function which may contribute to the greater susceptibility to cardiovascular disease observed in black patients. Pre-treatment with nebivolol of endothelial cells from black patients can increase NO bioavailability to levels similar to those in endothelial cells from white patients thereby helping to reverse endothelial dysfunction (Mason et al 2005).
Conclusion
Nebivolol is a third generation beta-blocker and is an effective antihypertensive with a good tolerability profile. Adverse events are generally mild with an incidence similar to placebo and there is a low incidence of many of the side effects usually associated with the use of classical beta-blockers. Nebivolol is also effective in reducing morbidity and mortality in elderly patients with heart failure, regardless of the initial ejection fraction. In addition to its effectiveness as an antihypertensive, nebivolol has a unique hemodynamic profile. Treatment with nebivolol increases NO bioavailability, improves endothelial function, leading to a reduction in arterial stiffness which in turn can help lower central aortic pressure. Such effects of nebivolol have the potential to reduce cardiovascular events. Additional studies are needed to determine the long term clinical relevance of these findings in the treatment of hypertension and cardiovascular disease.
References
- Benetos A, Rudnichi A, Safar M, et al. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560–4. doi: 10.1161/01.hyp.32.3.560. [DOI] [PubMed] [Google Scholar]
- Benetos A, Safar M, Rudnichi A, et al. Pulse pressure:a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–5. doi: 10.1161/01.hyp.30.6.1410. [DOI] [PubMed] [Google Scholar]
- Blacher J, Guerin AP, Pannier B, et al. Impact of aortic stiffness on survival in end-stage renal disease. Circulation. 1999;99:2434–9. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- Blacher J, Staessen J, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Int Med. 2000;160:1085–9. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- Bonapace S, Rossi A, Cicoira M, et al. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation. 2003;107:1603–8. doi: 10.1161/01.CIR.0000051458.39176.43. [DOI] [PubMed] [Google Scholar]
- Boutouyrie P, Tropeano AI, Asmar R, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- Bowman AJ, Chen CP-H, Ford GA. Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol. 1994;38:199–204. doi: 10.1111/j.1365-2125.1994.tb04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breithaupt-Grogler K, Leschinger M, Belz GG, Butzer R, Erb K, de Mey C, Sinn W. Influence of antihypertensive therapy with cilazapril and hydrochlorothiazide on the stiffness of the aorta. Cardiovasc Drugs Ther. 1996;10:49–57. doi: 10.1007/BF00051130. [DOI] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, et al. Endothelial function and dysfunction. Part II: association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–46. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Carlberg B, Samuelsson O, Lindholm LH. Atenolol in hypertension: is it a wise choice? Lancet. 2004;364:1684–9. doi: 10.1016/S0140-6736(04)17355-8. [DOI] [PubMed] [Google Scholar]
- Chen CP-H, Ting C-T, Lin S-J, et al. Different effects of fosinipril and atenolol on wave reflection in hypertensive patients. Hypertension. 1995;25:1034–41. doi: 10.1161/01.hyp.25.5.1034. [DOI] [PubMed] [Google Scholar]
- Cleophas TJ, Agrawal R, Lichtenthal A, et al. Nationwide efficacy-safety study of nebivolol in mildly hypertensive patients. Am J Ther. 2006;13:192–7. doi: 10.1097/01.mjt.0000149923.39085.44. [DOI] [PubMed] [Google Scholar]
- Cleophas TJ, Grabowsky I, Niemeyer MG, et al. Long-term efficacy of nebivolol monotherapy in patients with hypertension. Curr Ther Res. 2001;62:451–61. [Google Scholar]
- Cockcroft JR, Chowienczyk PJ, Brett SE, et al. Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther. 1995;274:1067–71. [PubMed] [Google Scholar]
- Cockcroft JR, Wilkinson IB, Webb DJ. The Trevor Howell Lecture: age, arterial stiffness and the endothelium. Ageing. 1997;26:53–60. doi: 10.1093/ageing/26.suppl_4.53. [DOI] [PubMed] [Google Scholar]
- Cruickshank JM, Prichard BNC. Beta-blockers in clinical practice. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Cruickshank K, Riste L, Anderson SG, et al. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance. An integrated index of vascular function? Circulation. 2002;106:2085–90. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- Dawes M, Brett SE, Chowienczyk PJ, Mant TGK, et al. The vasodilator action of nebivolol in forearm vasculature of subjects with essential hypertension. J Clin Pharmacol. 1999;48:460–3. doi: 10.1046/j.1365-2125.1999.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cree J, Geukens H, Verhaegen H. Non-invasive cardiac haemodynamics of nebivolol. Clin Drug Investig. 1991;3(Suppl 1):25–30. [Google Scholar]
- Dhakam Z, McEniery CM, Yasmin, et al. Atenolol and eprosartan:differential effects on central blood pressure and aortic pulse wave velocity. Am J Hypertens. 2006;19:214–9. doi: 10.1016/j.amjhyper.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Dreary AJ, Schumann AL, Murfet H, et al. Influence of drugs and gender on the arterial pulse wave and natiuretic peptide secretion in untreated patients with essential hypertension. Clin Sci. 2002;103:493–9. doi: 10.1042/cs1030493. [DOI] [PubMed] [Google Scholar]
- Erdogan D, Gullu H, Caliskan M, et al. Nebivolol improves coronary flow reserve in patients with idiopathic dilated Cardiomyopathy. Heart. 2007;93:319–24. doi: 10.1136/hrt.2006.091751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallois JV, Faulhaber H-D. Nebivolol, a third generation beta-blocker:the current treatment of arterial hypertension. Praxis. 2001;90:435–41. [PubMed] [Google Scholar]
- Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–25. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- Fogari R, Zoppi A, Lazzari P, et al. Comparative effects of nebivolol and atenolol on blood pressure and insulin sensitivity in hypertensive patients with type II diabetes. J Hum Hypertens. 1997;11:753–7. doi: 10.1038/sj.jhh.1000533. [DOI] [PubMed] [Google Scholar]
- Gao Y, Nagao T, Bond RA, et al. Nebivolol induces endothelium-dependent relaxations of canine coronary arteries. J Cardiovasc Pharmacol. 1991;17:964–9. doi: 10.1097/00005344-199106000-00016. [DOI] [PubMed] [Google Scholar]
- Kamp O, Sieswerda GT, Visser CA. Comparison of effects on systolic and diastolic left ventricular function of nebivolol versus atenolol in patients with uncomplicated essential hypertension. Am J Cardiol. 2003;92:344–8. doi: 10.1016/s0002-9149(03)00645-3. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Wilson PW, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J. 1991;121:1268–73. doi: 10.1016/0002-8703(91)90432-h. [DOI] [PubMed] [Google Scholar]
- Kass DA. Age-related changes in venticular-arterial coupling: pathophysiologic implications. Heart Fail Rev. 2002;7:51–62. doi: 10.1023/a:1013749806227. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Hay I, Fetics B, et al. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- Kelly R, Daley J, Avolio A, et al. Arterial dilation and reduced wave reflection. Benefit of dilevalol in hypertension. Hypertension. 1989;14:14–21. doi: 10.1161/01.hyp.14.1.14. [DOI] [PubMed] [Google Scholar]
- Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–53. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- Lacourciere Y, Arnott W. Placebo-controlled comparison of the effects of nebivolol and low-dose hydrochlorothiazide as monotherapies and in combination on blood pressure and lipid profile in hypertensive patients. J Hum Hypertens. 1994;8:283–8. [PubMed] [Google Scholar]
- Lacourciere Y, Poirier L, Lefebvre J. Comparative effects of a new cardioselective beta-blocker nebivolol and nifedipine sustained-release on 24-hour ambulatory blood pressure and plasma lipoproteins. J Clin Pharmacol. 1992;32:660–6. doi: 10.1002/j.1552-4604.1992.tb05778.x. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Lindholm LH, Carlberg B, Samuelsson O. Should beta-blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet. 2005;366:1545–53. doi: 10.1016/S0140-6736(05)67573-3. [DOI] [PubMed] [Google Scholar]
- Lombardo RM, Reina C, Abrignani MG, et al. Effects of nebivolol versus carvedilol on left ventricular function in patients with chronic heart failure and reduced left ventricular systolic function. Am J CardiovascDrugs. 2006;6:259–63. doi: 10.2165/00129784-200606040-00006. [DOI] [PubMed] [Google Scholar]
- Mason RP. Nitric oxide mechanisms in the pathogenesis of global risk. J Clin Hypertens (Greenwich) 2006;8:31–8. doi: 10.1111/j.1524-6175.2006.05838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Kalinowski L, Jacob RF, et al. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation. 2005;112:3795–801. doi: 10.1161/CIRCULATIONAHA.105.556233. [DOI] [PubMed] [Google Scholar]
- Mazza A, Gil-Extremera B, Maldonato A, et al. Nebivolol vs amlodipine as first-line treatment of essential arterial hypertension in the elderly. Blood Press. 2002;11:182–8. doi: 10.1080/080370502760050421. [DOI] [PubMed] [Google Scholar]
- McEniery CM, Schmitt M, Qasem A, et al. Nebivolol increases arterial distensibility in vivo. Hypertension. 2004;44:305–10. doi: 10.1161/01.HYP.0000137983.45556.6e. [DOI] [PubMed] [Google Scholar]
- Meaume S, Benetos A, Henry OF, et al. Aortic pulse wave velocity predicts cardiovascular mortality in subjects. >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- Millar JA, Lever AF, Burke V. Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens. 1999;17:1065–72. doi: 10.1097/00004872-199917080-00004. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Moye LA, Braunwald E, et al. Sphygomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. Circulation. 1997;96:4254–60. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Tardif JC, Arnold JM, et al. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–9. doi: 10.1161/hy1201.098298. [DOI] [PubMed] [Google Scholar]
- Moncada S. Nitric oxide. J Hypertens Suppl. 1994;12:S35–S9. [PubMed] [Google Scholar]
- Mottram PM, Haluska BA, Leano R, et al. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–6. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE. Hypertension: Management of Hypertension in Adults in Primamry care – Partial update. NICE. 2006 [Google Scholar]
- O’Rourke MF, Kelly RP, Avolio AP, et al. Effects of arterial dilator agents on central systolic pressure and on left ventricular hydraulic load. Am J Cardiol. 1989;63:381–441. doi: 10.1016/0002-9149(89)90127-6. [DOI] [PubMed] [Google Scholar]
- Palatini P, Casiglia E, Julius S. High heart rate: a risk factor for cardiovascular death in elderly men. Arch Int Med. 1999;159:585–92. doi: 10.1001/archinte.159.6.585. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Ashton DS, Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–6. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Pedersen ME, Cockcroft JR. The latest generation of beta-blockers:new pharmacologic properties. Curr Hypertens Rep. 2006;8:279–86. doi: 10.1007/s11906-006-0065-0. [DOI] [PubMed] [Google Scholar]
- Redfield MM, Jacobsen SJ, Borlaug BA, et al. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–62. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- Resnick LM, Lester MH. Differential effects of antihypertensive drug therapy on arterial compliance. Am J Hypertens. 2002;15:1096–100. doi: 10.1016/s0895-7061(02)03058-3. [DOI] [PubMed] [Google Scholar]
- Ritter JM. Nebivolol: endothelium-mediated vasodilating effect. J Cardiovasc Pharmacol. 2001;38(Suppl 3):S13–S16. doi: 10.1097/00005344-200112003-00003. [DOI] [PubMed] [Google Scholar]
- Robertson JIS, Ball SG. Hypertension for the clinician. London: W.B. Saunders; 1994. [Google Scholar]
- Schmitt M, Avolio A, Qasem A, et al. Basal NO locally modulates human iliac artery function in vivo. Hypertension. 2005;46:227–31. doi: 10.1161/01.HYP.0000164581.39811.bd. [DOI] [PubMed] [Google Scholar]
- Shibata M, Flather M, Wang W. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Failure. 2001;3:351–7. doi: 10.1016/s1388-9842(01)00144-1. [DOI] [PubMed] [Google Scholar]
- Staessen J, Wang J-G, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until March 2003. Hypertension. 2003;21:1055–76. doi: 10.1097/00004872-200306000-00002. [DOI] [PubMed] [Google Scholar]
- Steiner SS, Friedhoff AJ, Wilson BL, et al. Antihypertensive therapy and quality of life: comparison of atenolol, enalapril and propanolol. J Hum Hypertens. 1990;4:217–25. [PubMed] [Google Scholar]
- Stoleru L, Wijns W, van Eyll C, et al. Effects of d-nebivolol and l-nebivolol on left ventricular systolic and diastolic function: comparison with dl-nebivolol and atenolol. J Cardiovasc Pharmacol. 1993;22:183–90. doi: 10.1097/00005344-199308000-00002. [DOI] [PubMed] [Google Scholar]
- Tzemos N, Lim PO, MacDonald TM. Nebivolol reverses endothelial dysfunction in essential hypertension. Circulation. 2001;104:511–14. doi: 10.1161/hc3001.094207. [DOI] [PubMed] [Google Scholar]
- Uhlir O, Fejifusa M, Havranek K. Nebivolol versus metoprolol in the treatment of hypertension. Clin Drug Investig. 1991;3(Suppl 1):107–10. [Google Scholar]
- Van Bortel LM, Bulpitt CJ, Fici F. Quality of life and antihypertensive effect with nebivolol and losartan. Am J Hypertens. 2005;18:1060–6. doi: 10.1016/j.amjhyper.2005.03.733. [DOI] [PubMed] [Google Scholar]
- Van Bortel LMAB, Breed JGS, Joosten J, et al. Nebivolol in hypertension: a double-blind placebo-controlled multicenter study assessing its antihypertensive efficacy and impact on quality of life. J Cardiovasc Pharmacol. 1993;21:856–62. [PubMed] [Google Scholar]
- Van Bortel LMAB, de Hoon JNJM, Kool MJ, et al. Pharmacological properties of nebivolol in man. Eur J Clin Pharmacol. 1997;51:379–84. doi: 10.1007/s002280050217. [DOI] [PubMed] [Google Scholar]
- Van Bortel LMAB, Kool MJ, Boudier HA, et al. Effects of antihypertensive agents on local arterial distensibility and compliance. Hypertension. 1995;26:531–4. doi: 10.1161/01.hyp.26.3.531. [DOI] [PubMed] [Google Scholar]
- Van Bortel LMAB, Van Baak MA. Exercise tolerance with nebivolol and atenolol. Cardiovasc Drugs Ther. 1992;6:239–47. doi: 10.1007/BF00051145. [DOI] [PubMed] [Google Scholar]
- Van de Water A, Janssens WJ, Van Nueten L, et al. Pharmacological and hemodynamic profile of nebivolol, a chemically novel, potent and selective beta1-adrenergic antagonist. J Cardiovasc Pharmacol. 1988;11:552–63. doi: 10.1097/00005344-198805000-00007. [DOI] [PubMed] [Google Scholar]
- Van Merode T, Van Bortel LMAB, Smeets FA. Verapamil and nebivolol improve carotid artery distensibility in hypertensive patients. J Hypertens. 1989;(Suppl 7):S262–S3. doi: 10.1097/00004872-198900076-00127. [DOI] [PubMed] [Google Scholar]
- Van Nueten L, De Cree J. Nebivolol: a comparison of the effects of dl-nebivolol, d-nebivolol, l-nebivolol, atenolol, and placebo on exercise-induced increases in heart rate and systolic blood pressure. Cardiovasc Drugs Therap. 1998;12:339–44. doi: 10.1023/a:1007760515117. [DOI] [PubMed] [Google Scholar]
- Van Nueten L, Dupont AG, Vertommen C, et al. A dose-response trial of nebivolol in essential hypertension. J Hum Hypertens. 1997a;11:139–44. doi: 10.1038/sj.jhh.1000392. [DOI] [PubMed] [Google Scholar]
- Van Nueten L, Lacourciere Y, Vyssoulis G, et al. Nebivolol versus nifedipine in the treatment of essential hypertension: a double-blind randomised comparative trial. Am J Therap. 1998a;5:237–43. doi: 10.1097/00045391-199807000-00006. [DOI] [PubMed] [Google Scholar]
- Van Nueten L, Schelling A, Vertommen C, et al. Nebivolol versus enalapril in the treatment of essential hypertension:a double-blind randomised trial. J Hum Hypertens. 1997b;11:813–19. doi: 10.1038/sj.jhh.1000550. [DOI] [PubMed] [Google Scholar]
- Van Nueten L, Taylor FR, Robertson JI. Nebivolol vs atenolol and placebo in essential hypertension: a double-blind randomised trial. J Hum Hypertens. 1998b;12:135–40. doi: 10.1038/sj.jhh.1000571. [DOI] [PubMed] [Google Scholar]
- Van Nueten L, Taylor FR, Robertson JIS. Nebivolol versus atenolol and placebo in essential hypertension: a double-blind randomised trial. J Hum Hypertens. 1998c;12:135–40. doi: 10.1038/sj.jhh.1000571. [DOI] [PubMed] [Google Scholar]
- van Popele NM, Mattace-Raso FU, Vliegenthart R, et al. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24:2371–6. doi: 10.1097/01.hjh.0000251896.62873.c4. [DOI] [PubMed] [Google Scholar]
- WHO-ISH. Hypertension guidelines. J Hypertens. 1999;17:151–83. [PubMed] [Google Scholar]
- Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–6. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, McEniery CM, Cockcroft JR. Atenolol and cardiovascular risk: an issue close to the heart. Lancet. 2006;367:627–9. doi: 10.1016/S0140-6736(06)68238-X. [DOI] [PubMed] [Google Scholar]
- Wilkinson IB, Qasem A, McEniery CM, et al. Nitric oxide regulates local arterial distensibility in vivo. Circulation. 2002;105:213–7. doi: 10.1161/hc0202.101970. [DOI] [PubMed] [Google Scholar]
- Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- Yambe M, Tomiyama H, Hirayama Y, et al. Arterial stiffening as a possible risk factor for both atherosclerosis and diastolic heart failure. Hypertens Res. 2004;27:625–31. doi: 10.1291/hypres.27.625. [DOI] [PubMed] [Google Scholar]


