Abstract
The renin angiotensin system (RAAS) plays an important role in the pathophysiology of cardiovascular (CV) disease. Modulation of RAAS with angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), and aldosterone inhibitors reduces a range of adverse CV outcomes in patients with or at risk of CV disease. Currently, there is incomplete evidence to show all RAAS modulators provide vascular protection by reducing the incidence of myocardial infarction (MI), stroke and CV death. In patients at high risk for CV events, studies with ACEi designed to test for long-term vascular protection, showed benefit. In contrast, studies of ARBs in patients with hypertension, heart failure, and renal disease have not consistently shown a reduction of CV outcomes. However, none of these studies was specifically designed to examine the impact of ARBs on the vascular protective outcomes of CV death, non-fatal MI, and stroke. The ONTARGET and TRANSCEND studies are designed to determine whether the ARB telmisartan is similar (or non-inferior) or superior to the ACEi ramipril in the reduction of CV events in patients with established CV disease or diabetes with target organ damage. The ONTARGET study has enrolled 25,620, and TRANSCEND 5,776 subjects. The subjects in both trials are similar to those studied in the HOPE study, yet there is greater ethnic diversity, a higher proportion of patients with cerebro-vascular disease, and a greater use of beta blockers and lipid-lowering treatment. The studies completed recruitment in 2004, and are due to complete follow-up and report the results in 2008. The ONTARGET and TRANSCEND studies will provide valuable comparative data on the efficacy of telmisartan and ramipril and their combination in patients at high risk for CV events. Although it is possible that enhanced benefits will be observed with dual therapy, the outcomes with ARB monotherapy remain uncertain.
Keywords: RAAS modulation, ramipril, telmisartan, vascular protection
Role of angiotensin in the pathophysiology of cardiovascular disease
The renin angiotensin aldosterone system (RAAS) plays an important role in the development of cardiovascular (CV) disease. RAAS is a mediator for the development of atherosclerosis and atherothrombotic complications (Dzau 2001). In addition, RAAS activation promotes adverse remodeling of the damaged heart and the subsequent development of heart failure (Dzau 2005). Angiotensin II mediated stimulation of the AT1 receptor increases arterial pressure, promotes oxidative stress, stimulates an inflammatory response, and adversely alters the balance between the thrombotic and fibrinolytic state (Wagenaar et al 2002). AT1 receptors are upregulated in both experimental models and in patients with hypercholesterolemia (Strehlow et al 2000), thus enhancing the atherogenic state associated with hyperlipidemia.
Modulation of RAAS with either angiotensin-converting enzyme inhibitors (ACEi) or with AT1 receptors blockers (ARB) restrains several of the pathological processes that contribute to atherosclerosis and atherothrombosis (Dzau 1998). Blockade of the AT1 receptor reduces activation of pathways associated with the development of oxidative stress, diminishing activation of inflammatory cells, including monocyte migration and adhesion to endothelial cells (Grafe et al 1997; Dol et al 2001). In addition, both ACEi and ARBs have been shown to alter factors that promote fibrinolysis and reduce thrombosis (Vaughan 2001). ARBs, on the other hand might be pro-thrombotic by stimulating PAI-1 synthesis (Brown et al 2002) and encourage plaque rupture by enhancing MMP-1 activity (Kim et al 2005). Thus, experimental evidence suggests the both ACEi and the ARB classes of RAAS modulators have beneficial properties, which may reduce the development of atherosclerosis and its complications. Yet for the ARBs, vascular protective benefits remain uncertain until tested in a clinical trial.
Clinical trials in renin angiotensin system modulation and vascular protection
Angiotensin converting enzyme inhibition
The ACEi were initially introduced into the clinical arena for blood pressure control and management of heart failure. The SAVE (Pfeffer et al 1992) and SOLVD (The SOLVD Investigators 1992) trials of captopril and enalapril in patients with heart failure showed an important reduction of CV mortality and the progression of heart failure. Both of these heart failure trials observed that treatment with ACEi was associated with a 20%–25% reduction in the incidence of non-fatal myocardial infarction (MI) (Rutherford et al 1994). These observations lead to the HOPE trial (Yusuf et al 2000) in which high dose ACEi with ramipril 10 mg daily reduced the risk of MI by 20%, stroke by 32%, and CV mortality by 26% in patients at high risk for CV events but without heart failure or a low left ventricular ejection fraction. Subsequently the EUROPA study (The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators 2003) showed that perindopril 8 mg daily in patients with coronary artery disease reduced the endpoint of CV mortality, non-fatal MI, and cardiac arrest by 20%. Non-fatal MI was significantly reduced 22%, yet there were insufficient events to show a statistically significant reduction of CV mortality.
In contrast the PEACE trial (The PEACE Investigators 2004) failed to show a benefit from treatment with trandolapril 4 mg daily in patients with coronary artery disease. The study population was at low risk of CV events and the trial underpowered to show a benefit from the ACEi treatment. Coronary revascularization was the major contributor to the combined endpoint and occurred at similar rates in both treatment arms. In contemporary North American practice, aggressive coronary revascularization is probably a non-modifiable endpoint, as it is widely used independent of ischemia driven indications.
A combined analysis of the HOPE, EUROPA, and PEACE trials (Dagenais et al 2006) showed that ACEi significantly reduced all cause mortality (7.8 vs 8.9% p = 0.0004), CV mortality (4.3 vs 5.2% p = 0.0002), non-fatal MI (5.3 vs 6.4% p = 0.0001), stroke (2.2 vs 2.8% p = 0.0004), coronary artery bypass surgery (6.0 vs 6.9%), but not percutaneous coronary intervention (7.4 vs 7.6% p = 0.48). Similar reductions of CV mortality and MI are observed in the five trials in patients with heart failure or left ventricular systolic dysfunction (Danchin et al 2006).
The contribution of ACEi induced blood pressure reduction to the improved vascular outcomes remains controversial. In the HOPE study the average systolic and diastolic blood pressure reductions were 3/2 mmHg and in EUROPA 5/2 mmHg. Although blood pressure reduction may play a role, a high proportion of patients in both studies were normotensive and still received benefit from ACEi. Furthermore recent meta-regression analyses indicate that outcomes in patients receiving ACEi are better than would be expected from blood pressure lowering alone (Verdecchia et al 2005). A recent meta-regression analysis from the Blood Pressure Lowering Trialists with 140,000 subjects compared ACEi and ARB trials (Turnbull et al 2005). The study suggested that ACEi had a 9% greater effect on the reduction of MI and CV death, and benefits independent of blood pressure reduction. Similar blood pressure-independent effects of ARBs were not observed.
As a consequence of the HOPE and EUROPA studies, guidelines for the management of MI, heart failure hypertension, and diabetes recommend that ACEi should be considered in patients at a very high risk for recurrent vascular events.
Angiotensin receptor blockade
ARBs offer more effective interruption of the effects of angiotensin II on the AT1 receptor than ACEi. Current clinical trials indicate that for blood pressure control, prevention of heart failure mortality and morbidity, and retardation of the progression of diabetic nephropathy, ARBs appear to be as effective as ACEi. However, there is currently only limited evidence to show that ARBs reduce the incidence of MI. The ONTARGET and TRANSCEND studies will be the first to examine directly the vascular protective properties of ARBs used alone or in combination with ACEi.
Do the existing clinical trials suggest that ARBs have vascular protective properties similar to those demonstrated with ACEi? In contrast to the placebo-controlled ACEi trials described above, most ARB clinical trials have compared the ARB with another medication. In heart failure trials, the comparator was usually an ACEi, whereas in hypertension trials the ARB was compared with other medication strategies. The placebo-controlled clinical trials with clinical outcomes are either in ACEi intolerant patients (CHARM alternative) or in patients with diabetic nephropathy (RENAAL and IDNT).
ARBs are probably as effective as ACE inhibitors in reducing heart failure mortality (Pfeffer et al 2003b). Furthermore the combination of the ARB candesartan with an ACE inhibitor in the CHARM Added trial was associated with a 17% reduction of both CV mortality and admission to hospital for heart failure (McMurray et al 2003). In the CHARM overall trial (Demers et al 2004), patients receiving candesartan had a 23% reduction (95% CI 2%–40%) of non-fatal MI as well as a 12% reduction (95% CI 13%–21%) of CV death. The reduction of MI with candesartan was similar in patients receiving and not receiving an ACEi. Yet in the CHARM Alternative trial (Granger et al 2003) where candesartan was compared with placebo in patients intolerant of ACEi, there was a statistically significant 52% increase in the incidence of MI for patients receiving candesartan compared with those receiving placebo. However, the risk of CV death was decreased by 20% (p = 0.02 after covariate adjustment).
ARBs are effective and well tolerated antihypertensive agents. The LIFE trial (Dahlof et al 2002) showed the ARB losartan compared with atenolol resulted in a greater reduction of the combined end-point of CV death, MI, or stroke after equal blood pressure control, for the management of the older patient with moderately severe hypertension and left ventricular hypertrophy. For the individual components of the primary endpoint, losartan reduced stroke, but there was no impact on either CV mortality or MI. In patients with diabetes, losartan reduced CV and total mortality, but not MI. Furthermore, atenolol is not the optimal antihypertensive comparator in an older population, as a recent meta-analysis showed a higher mortality with atenolol than with other anti-hypertensive medications (Carlberg et al 2004).
In the VALUE trial (Julius et al 2004), valsartan was not as effective as an antihypertensive agent as amlodipine, yet there was no difference in the primary combined endpoint of CV mortality and morbidity. Acute vascular events (MI and CVA) were less frequent in the amlodipine-treated patients. It seems unlikely that a 2–4 mmHg higher systolic blood pressure in the valsartan-treated patients during the first few months of treatment could account for a 19% increase in MI (p = 0.02) and 15% (p = 0.08) increase in stroke throughout the course of the trial.
Following acute MI, the major threat to survival and future morbidity is from recurrent acute coronary events, the development of heart failure and life threatening arrhythmias. ACE inhibition reduces the risk of death by 26%, major non-fatal CV events such as hospital admission for heart failure by 27%, and recurrent MI by 20% (Flather et al 2000). The VALIANT (Pfeffer et al 2003a) trial showed the ARB valsartan was not inferior to captopril in the reduction of all-cause mortality in patients with heart failure or left ventricular ejection fraction <40%. MI rates were similar in the valsartan- and captopril-treated patients.
The diabetic patient with micro- or macroalbuminuria is at a high risk for adverse CV outcomes. Although the diabetic nephropathy trials RENAAL (Brenner et al 2001) and IDNT (Parving et al 2001) did not have CV events as primary outcomes, it is possible to gain some insight of the value of ARBs relative to placebo in such a high risk population. Both trials showed that the ARB successfully slowed the progression of renal failure. An analysis of the CV outcomes in the IDNT trial (Berl et al 2003) also showed that congestive heart failure was less frequent in the irbesartan compared with placebo or amlodipine-treated patients. However, there was no reduction of CV mortality, MI, cardiac revascularization, or stroke rates in the irbesartan group. Yet there was a strong trend to less MI for patients receiving amlodipine compared with those randomized to placebo or irbesartan.
In the RENAAL trial (Brenner et al 2001), there was a trend towards less non-fatal MI for patients receiving losartan (relative risk reduction [RRR] 28% p < 0.08). The number of patients requiring at least one admission for heart failure was significantly reduced in the losartan group (RRR 32% p = 0.005). ARBs prevent the progression of diabetic nephropathy and reduce the combined endpoint of total mortality and progression to end-stage renal failure. Yet the ARB nephropathy trials do not show any significant reduction of CV events with the exception of heart failure admissions. As many more patients have CV events than progress to dialysis-dependent renal failure, it is surprising that the ARB-treated patients did not have a reduction of CV events.
A recent discussion paper (Strauss and Hall 2006) has suggested that ARBs may increase the incidence of MI. This hypothesis, supported by a meta-analysis of over 50,000 patients in clinical trials that compared ARBs with placebo, non-ACEi comparators and ACEi suggested there was a significant 8% increase in MI for ARB-treated patients compared with the comparator. An alternative explanation is that the comparators that included ACEi and beta-adrenergic blockers had a more powerful effect than the ARB in reducing coronary events. Only a randomized trial such as ONTARGET and TRANSCEND will answer this controversy.
Potential differences between the effects of ACEi inhibitors and ARB
ACE inhibition reduces angiotensin II available to stimulate the AT receptors. Alternative pathways for AII synthesis, as mediated by other proteolytic enzyme such as chymase, explain the failure of ACEi to reduce circulating AII during long-term treatment. However, in tissues, different mechanisms for AII synthesis may be in play, as ACE and not chymase co-localizes with angiotensin II (Ohishi M et al 1999) in atherosclerotic plaque. Consequently, it is possible there is no breakthrough synthesis of angiotensin II in the atherosclerotic plaque in the presence of an effective ACEi. ACE not only promotes the synthesis of angiotensin II, but the same enzyme also inactivates the vasoactive peptide bradykinin and inhibition of ACE with ACEi increases the availability of bradykinin (Hornig et al 1997). Bradykinin is not only a vasodilator peptide, but is also a powerful stimulant of endothelial nitric oxide synthesis which inhibits cell proliferation and an inflammatory response (Murphey et al 2003). Bradykinin restores a favorable fibrinolytic balance by stimulating the synthesis of tissue plasminogen activator (tPA) (Vaughan 2001).
Long-term AT1 inhibition results in a several-fold increase in plasma angiotensin II and the unopposed stimulation of AT2 receptors (Horiuchi et al 1999). Early studies suggested that stimulation of the AT2 receptor was beneficial, with effects that counterbalanced the effects of AT1 stimulation (Matsubara 1998). Consequently, unopposed AT2 stimulation was considered to be one of the potential benefits of interruption of the effects of angiotensin II with an ARB. Yet recent research has not only questioned the role of the AT2 receptor in adult human vasculature, but also suggested that AT2 over-stimulation may be detrimental. Levy, in a recent review (Levy 2004), suggested that AT2 stimulation can in certain situations result in cellular proliferation, an increase in fibrosis, inappropriate apoptosis and an associated anti-angiogenic effect: all effects that might result in increased atherogenesis and a poor outcome following vascular occlusion. Clearly, the biology of AT2 receptors is much more complex than we had previously believed, and there is insufficient evidence to show AT2 receptor stimulation during ARB therapy is equivalent to the effects of ACEi on bradykinin and nitric oxide.
Both the ACEi and the ARB class of agents have properties that would be expected to reduce CV events. However, both the ACEi and ARBs have limitations that might translate into a smaller benefit than might be predicted from their effect on RAAS modulation. Hence, the need for a clinical trial to explore the benefits of each agent and in combination for patients at high risk of CV mortality, MI, and stroke.
The ONTARGET and TRANSCEND trials
The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) and Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (TRANSCEND) trials will examine the role of ARBs when used alone or in combination with an ACEi in high risk individuals with controlled hypertension (Teo et al 2004). The primary objective of the ONTARGET trial is to determine whether the combination of telmisartan 80 mg (Micardis®, Boehringer Ingelheim) and ramipril 10 mg (Altace®, Sanofi-Aventis, King) is more effective than ramipril alone and to assess whether telmisartan alone is at least as effective as ramipril. The TRANSCEND trial will determine whether telmisartan 80 mg reduces vascular endpoints compared with placebo in patients intolerant of ACEi. The dose of ramipril is the dose shown to have vascular protective benefit in the HOPE study (Yusuf et al 2000). The telmisartan dose is the dose that is effective and well tolerated in hypertensive trials. The primary endpoint for both trials is the combination of CV death, non-fatal MI, stroke, or hospitalization for heart failure. The two studies included patients at high risk for coronary, peripheral or cerebrovascular events as summarized in Table 1. Recruitment from 730 centres in 40 countries for ONTARGET (n = 25,620) was completed in July 2003, and TRANSCEND (n = 6,000) in May 2004. The study results will be reported in 2008.
Table 1.
Inclusion and exclusion criteria for ONTARGET and TRANSCEND trials
| Inclusion criteria | |
| Individuals >55 years of age with one of the following: | |
| Coronary artery disease | Prior AMI (>2 days post uncomplicated AMI) |
| Stable angina | |
| Unstable angina (>30 days before + documented CAD) | |
| Multivessel PCI (>0 days before) | |
| Multivessel CABG (>4 years before or with recurrent angina) | |
| Peripheral artery disease | Prior limb vascular surgery or angioplasty |
| Limb/foot amputation | |
| Intermittent claudication (ABIa < 0.80) | |
| Significant peripheral vascular stenosis (>50%) by angiography | |
| Cerebrovascular disease | Previous stroke |
| TIA after >7 days and <1 year | |
| Diabetes mellitus | High risk patient with evidence of end organ damage |
| Exclusion criteria | |
| Medication use | Inability to discontinue ACEi or ARB |
| Known hypersensitivity or intolerance to ARB or ACEi (except TRANSCEND) | |
| CVD | Symptomatic heart failure |
| Significant valvular heart disease | |
| Pericardial constriction | |
| Complex congenital heart disease | |
| Syncopal episodes of unknown aetiology | |
| Planned PCI or CABG < 3 months after randomization | |
| Uncontrolled hypertension (BP >160/100) | |
| Heart transplant recipient | |
| Stroke due to subarachnoid hemorrhage | |
| Others (examples) | Significant renal disease |
| Proteinuria (TRANSCEND only) | |
| Hepatic dysfunction |
Ankle brachial index
The study design is shown in Figure 1. After evaluation for eligibility, subjects entered a 3-week run-in period receiving open label study drugs in escalating doses. Patients who were adherent to and tolerated the study medication were randomized to the treatment groups as shown in Figure 1. During the follow-up period of 3.5–5.5 years, events comprising the primary, secondary, and other outcomes are recorded and confirmed by a central adjudicator. The study has an 89% power of showing the non-inferiority of telmisartan compared with ramipril that ensures telmisartan has at least 50% of the ramipril effect at the upper 95th% confidence limit.
Figure 1.
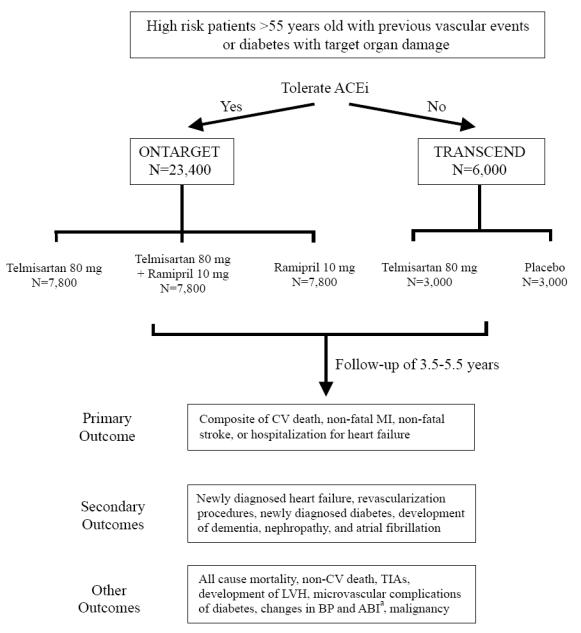
ONTARGET and TRANSCEND study designs. aAnkle brachial index.
The study is designed to have a 93% power of showing a 13% superiority of telmisartan plus ramipril vs ramipril alone. However, a non-inferiority analysis will be performed initially prior to an analysis for superiority. In the TRANSCEND study with 3,000 patients in each group the study has a 94% power to show a 19% superiority of telmisartan over placebo.
Baseline characteristics in ONTARGET and TRANSCEND: a comparison with HOPE
The major baseline characteristics of the ONTARGET and TRANSCEND studies compared with those from the HOPE study are shown in Table 2. A complete list is available in the design and baseline characteristics publication (Teo et al 2004). The age of the patients in the ONTARGET and TRANSCEND studies is slightly older than in the HOPE trial. A greater proportion of female subjects are entered into the TRANSCEND trial than was seen in either ONTARGET or HOPE. A similar higher proportion of female subjects (31.8%) was observed in the ACEi intolerant arm of the CHARM study compared with the 21.2% in the overall CHARM trial. Diabetes was present at a similar prevalence in the ONTARGET and TRANSCEND studies as in HOPE. In the current trials the use of ACEi and statins prior to enrollment was considerably greater than reported in HOPE. Blood pressures in the ONTARGET and TRANSCEND patients are slightly higher than in HOPE at the time of initial assessment.
Table 2.
Baseline characteristics in ONTARGET, TRANSCEND and HOPE trials
| ONTARGET | TRANSCEND | HOPE | |
|---|---|---|---|
| Age (y) | 66.4 | 66.9 | 65.9 |
| Male (%) | 73.3 | 57.1 | 73.3 |
| Prior MI (%) | 48.7 | 46.2 | 52.8 |
| BMI | 28.2 | 28.3 | 27.2 |
| Revascularization (%) | |||
| PCI | 28.9 | 26.0 | 18.0 |
| CABG | 22.1 | 18.9 | 26.0 |
| Risk factors (%) | |||
| Hypertension | 68.3 | 75.9 | 46.5 |
| Diabetes | 37.3 | 35.4 | 38.3 |
| Current smoker | 12.5 | 9.8 | 14.1 |
| Medication (%) | |||
| ACEi | 57.5 | 58.1 | 11.6 |
| ARB | 8.6 | 29.9 | – |
| Statins | 60.7 | 54.5 | 28.9 |
| BP at run-in | 143/82 | 142/82 | 139/79 |
Adapted from Teo et al (2004).
What can we expect from the ONTARGET and TRANSCEND trials
Placebo-controlled clinical trials have shown that long-term treatment with ACE inhibition is effective for the management of disease across the CV continuum (Table 3). ARBs are as effective as ACEi in the management of heart failure, and left ventricular systolic dysfunction after MI. Although ACEi prevent acute MI in patients with and without systolic dysfunction, it remains controversial whether ARBs have similar vascular protective benefits.
Table 3.
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) in cardiovascular disease: strength of evidence
| ACE Inhibition | ARB | |
|---|---|---|
| Heart failure management | +++ | +++ |
| Heart failure prevention | +++ | + |
| Stroke | +++ | +++ |
| Myocardial infarction | +++ | + |
| Atrial fibrillation | +++ | ++ |
| Cardiovascular mortality | +++ | + |
| Diabetic nephropathy | ++ | +++ |
There are several possible scenarios. Firstly the effect of the combination of ACE inhibitor and ARB is likely to show enhanced benefits for the primary outcome compared with monotherapy. An ACEi and ARB combination in patients with heart failure in the Randomized Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) (McKelvie et al 1999) showed that the combination of enalapril and candesartan resulted in significantly lower systolic and diastolic blood pressures compared with either medication alone. In addition, combined ACEi ARB therapy further reduced neurohormone levels and improved cardiac function. Consequently, it is likely that the combined treatment will reduce vascular and heart failure endpoints more than either ramipril or telmisartan as monotherapy. How much of the benefit will be from greater blood pressure lowering and how much from non-BP lowering effects will be difficult to assess.
The comparison between the ACEi ramipril with the ARB telmisartan is designed to show non-inferiority of telmisartan. In other words, is “most” of ramipril’s effect vs placebo preserved by telmisartan? In ONTARGET, the non-inferiority margins for the comparison have been set to assure that telmisartan retains at least half of the ramipril effect. Thus if telmisartan has vascular protective properties, it is likely that it will be shown to be non-inferior to ramipril.
For the comparison of telmisartan with placebo in ACEi-intolerant patients in TRANSCEND, the study is powered to show a 19% reduction of event rates. As event rates of patients with vascular disease have been reduced by a more aggressive approach vascular protection with the greater use of statins revascularization and lifestyle measures, it is possible that the event rates in the ONTARGET and TRANSCEND population will be below the 3.97% observed in HOPE. If the event rates are low, it is likely that the TRANSCEND study, with a total of 6,000 patients, will be underpowered to show a benefit for telmisartan.
Class effect of ARBs or benefit from only telmisartan
Should the ARB telmisartan be shown to be non-inferior or superior to the ACEi ramipril, are the results generalizable to all ARBs or are they a specific benefit of the ARB telmisartan? Telmisartan has a longer half-life than other ARBs. Consequently, once-daily treatment results in blood pressure lowering throughout the next 24 hours after receiving the medication. Recent pre-clinical studies have suggested that telmisartan may have properties associated with vascular protection beyond those achievable with angiotensin receptor blockade alone (Kurtz and Pravenek 2004). Telmisartan acts as a selective partial agonist of peroxisome proliferator-activated receptor-gamma (PPAR-γ). This nuclear receptor influences the expression of multiple genes involved in lipid and carbohydrate metabolism. PPAR-γis therefore a potential therapeutic target for modulation of insulin resistance, treatment of diabetes, and prevention of atherosclerosis. In clinical studies (DeRosa et al 2004) telmisartan improved carbohydrate and lipid metabolism without the side effects associated with the full PPAR-γactivators such as the thiazolidinediones (TZDs). With more effective 24-hour blood pressure lowering and different pleiotropic effects, any observed benefits of telmisartan may not be generalizable to the other ARBs. Furthermore the effective vascular protective dose of other ARBs would be no more than an intelligent guess.
Conclusion
The ONTARGET and TRANSCEND studies will provide important information to support or refute the hypothesis that ARBs have similar vascular protective properties as the ACEi. ARBs are generally tolerated better than ACEi. Consequently should the ONTARGET and TRANSCEND studies show the non-inferiority of telmisartan to ramipril, it is likely that telmisartan, the ARB with evidence, will become the favored treatment for vascular protection.
References
- Berl T, Hunsicker LG, Lewis JB, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003;138:542–9. doi: 10.7326/0003-4819-138-7-200304010-00010. [DOI] [PubMed] [Google Scholar]
- Brenner BM, Cooper ME, de Z, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- Brown NJ, Kumar S, Painter CA, et al. ACE inhibition versus angiotensin type 1 receptor antagonism: differential effects on PAI-1 over time. Hypertension. 2002;40:859–65. doi: 10.1161/01.hyp.0000040264.15961.48. [DOI] [PubMed] [Google Scholar]
- Carlberg B, Samuelsson O, Hjalmar Lindholm L. Atenolol in hypertension: is it a wise choice? Lancet. 2004;364:1684–9. doi: 10.1016/S0140-6736(04)17355-8. [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Pogue J, Fox K, et al. Angiotensin-converting-enzyme inhibitors in stable vascular disease without left ventricular systolic dysfunction or heart failure: a combined analysis of three trials. Lancet. 2006;368:581–8. doi: 10.1016/S0140-6736(06)69201-5. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Danchin N, Cucherat M, Thuillez C, et al. Angiotensin-converting enzyme inhibitors in patients with coronary artery disease and absence of heart failure or left ventricular systolic dysfunction: an overview of long-term randomized controlled trials. Arch Intern Med. 2006;166:787–96. doi: 10.1001/archinte.166.7.787. [DOI] [PubMed] [Google Scholar]
- Demers C, McMurray JJ, Svedberg K, et al. Impact of Candesartan in Preventing Myocardial Infarction: Results of the Candesartan in Heart failure Assessment of Reduction in Mortality and Morbidity (CHARM) Programme. Circulation. 2004;110(Suppl III):III–514. [Google Scholar]
- DeRosa G, Ragonesi PD, Mugellini A, et al. Effects of telmisartan compared with eprosartan on blood pressure control, glucose metabolism and lipid profile in hypertensive, type 2 diabetic patients: a randomized, double- blind, placebo-controlled 12-month study. Hypertens Res. 2004;27:457–64. doi: 10.1291/hypres.27.457. [DOI] [PubMed] [Google Scholar]
- Dol F, Martin G, Staels B, et al. Angiotensin AT1 receptor antagonist irbesartan decreases lesion size, chemokine expression, and macrophage accumulation in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol. 2001;38:395–405. doi: 10.1097/00005344-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. Tissue angiotensin and the pathobiology of vascular disease: a unifying hypothesis. Hypertension. 2001;37:1047–52. doi: 10.1161/01.hyp.37.4.1047. [DOI] [PubMed] [Google Scholar]
- Dzau VJ. The cardiovascular continuum and renin-angiotensin-aldosterone system blockade. J Hypertens. 2005;23(Suppl 1):S9–S17. [PubMed] [Google Scholar]
- Dzau VJ. Mechanism of protective effects of ACE inhibition on coronary artery disease. Eur Heart J. 1998;19(Suppl J):J2–J6. [PubMed] [Google Scholar]
- Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group [see comments] Lancet. 2000;355:1575–81. doi: 10.1016/s0140-6736(00)02212-1. [DOI] [PubMed] [Google Scholar]
- Grafe M, Auch-Schwelk W, Zakrzewicz A, et al. Angiotensin II-induced leukocyte adhesion on human coronary endothelial cells is mediated by E-selectin. Circ Res. 1997;81:804–11. doi: 10.1161/01.res.81.5.804. [DOI] [PubMed] [Google Scholar]
- Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet. 2003;362:772–6. doi: 10.1016/S0140-6736(03)14284-5. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Akishita M, Dzau VJ. Recent progress in Angiotensin type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- Hornig B, Kohler C, Drexler H. Role of bradykinin in mediating vascular effects of angiotensin-converting enzyme inhibitors in humans. Circulation. 1997;95:1115–18. doi: 10.1161/01.cir.95.5.1115. [DOI] [PubMed] [Google Scholar]
- Julius S, Kjeldsen SE, Weber M, et al. Outcomes in patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–31. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- Kim MP, Zhou M, Wahl LM. Angiotensin II increases human monocyte matrix metalloproteinase-1 through the AT2 receptor and prostaglandin E2: implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Pravenek M. Antidiabetic mechanisms of ACE inhibitors and AII receptor antagonists: beyond the renin-angiotensin system. J Hypertens. 2004;22:2253–61. doi: 10.1097/00004872-200412000-00003. [DOI] [PubMed] [Google Scholar]
- Levy BI. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implicvations for the therapeutic blockade of the renin angiotensin system. Circulation. 2004;109:8–13. doi: 10.1161/01.CIR.0000096609.73772.C5. [DOI] [PubMed] [Google Scholar]
- Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal disease. Circ Res. 1998;83:1182–91. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- McKelvie RS, Yusuf S, Pericak D, et al. Comparison of candesartan, enalapril, and their combination in congestive heart failure: randomized evaluation of strategies for left ventricular dysfunction (RESOLVD) pilot study. The RESOLVD Pilot Study Investigators. Circulation. 1999;100:1056–64. doi: 10.1161/01.cir.100.10.1056. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–71. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- Murphey L, Vaughan D, Brown N. Contribution of bradykinin to the cardioprotective effects of ACE inhibitors. Euro Heart J Suppl. 2003;5(Suppl A):A37–A41. [Google Scholar]
- Ohishi M, Ueda M, Rakugi H, et al. Relative localization of angiotensin-converting enzyme, chymase and angiotensin II in human coronary atherosclerotic lesions. J Hypertens. 1999;17:547–53. doi: 10.1097/00004872-199917040-00013. [DOI] [PubMed] [Google Scholar]
- Parving HH, Lehnert H, Brochner M, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345:870–8. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after f infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–77. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003a;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003b;362:759–66. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- Rutherford JD, Pfeffer MA, Moye LA, et al. Effects of captopril on ischemic events after myocardial infarction. Results of the Survival and Ventricular Enlargement trial. SAVE Investigators. Circulation. 1994;90:1731–8. doi: 10.1161/01.cir.90.4.1731. [DOI] [PubMed] [Google Scholar]
- Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–54. doi: 10.1161/CIRCULATIONAHA.105.594986. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Wasserman S, Bohm M, et al. Angiotensin AT1 receptor over expression in hypercholesterolemia. Ann Med. 2000;32:386–9. doi: 10.3109/07853890008995944. [DOI] [PubMed] [Google Scholar]
- Teo K, Yusuf S, Sleight P, et al. Rationale, design, and baseline characteristics of 2 large, simple, randomized trials evaluating telmisartan, ramipril, and their combination in high-risk patients: the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial/Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease (ONTARGET/TRANSCEND) trials. Am Heart J. 2004;148:52–61. doi: 10.1016/j.ahj.2004.03.020. [DOI] [PubMed] [Google Scholar]
- The EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomized, double-blind, placebo-controlled, multicentre trial (the EUROPAstudy) Lancet. 2003;362:782–8. doi: 10.1016/s0140-6736(03)14286-9. [DOI] [PubMed] [Google Scholar]
- The PEACE Investigators. Angiotensin-Converting–Enzyme Inhibitionin Stable Coronary Artery Disease. N Engl J Med. 2004;351:2058–68. doi: 10.1056/NEJMoa042739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- Turnbull F, Neal B, Algert C, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–9. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]
- Vaughan DE. Angiotensin, fibrinlysis and vascular homeostasis. Am J Cardiol. 2001;87:24. doi: 10.1016/s0002-9149(01)01509-0. [DOI] [PubMed] [Google Scholar]
- Verdecchia P, Reboldi G, Angeli F, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention [In Process Citation] Hypertension. 2005;46:386–92. doi: 10.1161/01.HYP.0000174591.42889.a2. [DOI] [PubMed] [Google Scholar]
- Wagenaar LJ, Voors AA, Buikema H, et al. Angiotensin receptors in the cardiovascular system. Can J Cardiol. 2002;18:1339. [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]


