Abstract
Background
Albuminuria and glomerular filtration rate (GFR), two factors linked to kidney and vascular function, may influence longitudinal blood pressure (BP) responses to complex antihypertensive drug regimens.
Methods
We reviewed the clinic records of 459 patients with hypertension in an urban, academic practice.
Results
Mean patient age was 57-years, 89% of patients were African American, and 69% were women. Mean patient systolic/diastolic BP (SBP/DBP) at baseline was 171/98 mmHg while taking an average of 3.3 antihypertensive medications. At baseline, 27% of patients had estimated (e)GFR <60 ml/min/1.732, 28% had micro-albuminuria (30–300 mg/g) and 16% had macro-albuminuria (>300 mg/g). The average longitudinal BP decline over the observation period (mean 7.2 visits) was 25/12 mmHg. In adjusted regression models, macro-albuminuria predicted a 10.3 mmHg lesser longitudinal SBP reduction (p < 0.001) and a 7.9 mmHg lesser longitudinal DBP reduction (p < 0.001); similarly eGFR <60 ml/min/1.732 predicted an 8.4 mmHg lesser longitudinal SBP reduction (p < 0.001) and a 4.5 lesser longitudinal DBP reduction (p < 0.001). Presence of either micro- or macro-albuminuria, or lower eGFR, also significantly delayed the time to attainment of goal BP.
Conclusions
These data suggest that an attenuated decline in BP in drug-treated hypertensives, resulting in higher average BP levels over the long-term, may mediate a portion of the increased risk of cardiovascular-renal disease linked to elevated urinary albumin excretion and reduced eGFR.
Keywords: albuminuria, glomerular filtration rate, blood pressure, antihypertensive drug therapy
Introduction
Level of kidney function may influence blood pressure (BP) responses to antihypertensive drug therapies given that diminished glomerular filtration predisposes to sodium retention unless dietary sodium intake is restricted. Moreover, vasodilator drugs – especially those that increase the capacitance of venous vessels (Abshagen and von Mollendorff 1986; Taylor 1988) – can promote salt and water retention, which may attenuate BP reductions with these agents when dietary sodium intake is ad lib (Weir et al 1997, 1998). Elevated urinary albumin excretion, even amongst normotensives, has been linked to attenuated endothelial and non-endothelial mediated arterial dilatory capacity (Clausen et al 2001). Other studies have linked increased urinary albumin excretion to endothelial dysfunction (Pedrinelli et al 1994), more rapid loss of kidney function (Bianchi et al 1997, 1999; Pinto-Sietsma et al 2000; Campbell et al 2003), increased left ventricular mass and cardiac structural changes (Leoncini et al 2002; Plavnik et al 2002), sodium sensitivity (Imanishi et al 2001; Cubeddu et al 2003) and increased global cardiovascular risk (Pedrinelli et al 2002). Patients with microalbuminuria have been observed to have higher systolic, diastolic, and mean arterial BP compared to patients without microalbuminuria (Cruz et al 1997).
Thus, we sought to examine whether albuminuria and estimated glomerular filtration rate (eGFR) attenuate the longitudinal BP response to complex antihypertensive drug regimens. We hypothesized that albuminuria might represent a state of diminished vascular response to vasculoactive drugs and would therefore blunt longitudinal BP responses to complex antihypertensive drug regimens. Conversely, we postulated that eGFR would directly correlate with longitudinal BP reductions. If our hypotheses were correct, then higher BP levels over the long-term would be at least one mechanism through which albuminuria and/or reduced eGFR contribute to the heightened cardiovascular-renal risks associated with reductions in kidney function and/or albuminuria.
Methods
The research design was an observational study of hypertensive patients in an urban academic hypertension clinic in the Department of Internal Medicine, at Wayne State University, Detroit Michigan. Patients with a baseline evaluation and at least one post-baseline clinic visit, who satisfied co-morbidity specific BP criteria for the diagnosis of hypertension according to published guidelines from the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC) (JNC VI 1997; JNC VII 2003), who had baseline data on albuminuria and eGFR status, and who were not at JNC goal BP at baseline were included. Data collected during clinic visits occurring from January 2001 through mid-May 2006 were considered. Antihypertensive therapy and lifestyle interventions were prescribed in accordance with routine clinical practice as guided by either JNC-VI (prior to mid-August 2003) or JNC-VII (from mid-August 2003) target BP goals depending on the time frame of observation (JNC VI 1997; JNC VII 2003). Patient demographic, clinical (including medication), and laboratory data were collected and maintained using an Institutional Review Board approved electronic medical record/clinical decision support system – MedTrace (MedTrace Inc., Orchard Lake, MI, USA). Data were stripped of individual patient identifiers and imported into a research database for analysis. This project was reviewed and approved by the Wayne State University, Institutional Review Board.
Patients were characterized by age, gender, and race (African American, non-African American). Diabetes was defined based on use of insulin and/or oral hypoglycemic agents or elevated glucose levels (fasting glucose > 126 mg/dL or random glucose > 200 mg/dL). Obesity status was determined from calculation of body mass index (BMI) [weight (Kg)/ height (m2)], with obesity defined as BMI > 30.0 kg/m2. Anti-hypertensive therapeutic intensity scores (daily dose divided by maximum FDA approved dose, summed across medication class) were calculated from visit-specific prescribed therapeutic choices. The Modified Diet and Renal Disease (MDRD) formula ([eGFR] = 186.3* [serum reatinine−1.154]*[age−0.203]*0.742 for women; *1.21 for African Americans) (Levy et al 1999) was used to calculate eGFR at baseline. eGFR values <60 ml/min/1.73m2 (Kidney Disease Outcomes Quality Initiative [K/DOQI] categories 3–5) were considered abnormal, and indicated at least moderately reduced kidney function (NKF 2002). Albuminuria status at baseline was determined from random spot urine albumin:creatinine ratio measurements and categorized as normal (<30 mg/g), microalbuminuria (30–300 mg/g), or macroalbuminuria (>300 mg/g) (Keane and Eknoyan 1999).
Longitudinal BP response was defined as baseline systolic (SBP) or diastolic blood pressure (DBP) minus visit-specific SBP or DBP, respectively, and calculated at all post-baseline visits. In addition, patients attaining co-morbidity specific (JNC VI or JNC VII) goal BP levels at least once during follow-up were identified. The BP goal for patients with proteinuria > 1 gram/24 hours was <125/75 mmHg using JNC VI criteria and <130/80 mmHg using JNC VII criteria; the BP goal for patients with diabetes mellitus, heart failure, and/or renal insufficiency was <130/85 mmHg using JNC VI criteria and <130/80 mmHg using JNC VII criteria (except heart failure); the BP goal for all others was <140/90 mmHg using either criteria. Differences from BP goal at baseline were calculated as baseline SBP or DBP minus goal SBP or DBP, respectively.
Statistical analysis
Statistical analyses were performed using SAS statistical software (SAS, version 9.1, SAS Institute, Cary, NC). Population characteristics, including demographics, BP response, and attainment of BP goal, were evaluated by eGFR and albuminuria cut-points using chi-square statistics for categorical variables and Wilcoxon rank sum tests and ANOVA techniques for continuous values. Repeated measures linear mixed effects regression models, with appropriate correlation structures to account for within-subject correlation across multiple observations on individual patients, were used to examine the influence of eGFR and albuminuria categories on visit-specific SBP and DBP response; models were adjusted for patient demographics (age, gender, race), diabetes and obesity status, difference between baseline BP and goal BP, and visit-specific anti-hypertensive therapeutic intensity scores. Time to BP goal attainment was evaluated using survival analysis with comparison of hazard curves stratified by eGFR and albuminuria categories; similarly, factors associated with goal attainment with calculation of hazard ratios were determined using Cox proportional hazards models.
Results
Data were available on 459 patients meeting the inclusion criteria, including 437 patients who were not at systolic BP goal and 365 patients who were not at diastolic goal. The average age of participants was 57-years (range 19–88-years); 89% (n = 408) of the study population was African American and 69% (n = 315) were women. Thirty-four percent (n = 157) of patients had diabetes, and 59% (n = 262) were obese (BMI > 30.0 kg/m2). Mean baseline SBP/DBP was 171/98 mmHg; mean SBP/DBP difference from goal BP at baseline was 39/18 mmHg. Participating patients were taking an average of 3.3 (range 0–7) antihypertensive medications at baseline, and had a mean baseline anti-hypertensive therapeutic intensity score of 2.0 (range 0–11.8). Mean baseline eGFR was 75 ml/min/1.732 (range 14–23) and 27% (n = 125) of the population had eGFR values <60 ml/min/1.732. Mean baseline urine albumin:creatinine ratio was 254 mg/g (range 0–10,333) and 43% (n = 198) of patients had either micro- or macro-albuminuria.
During the study period, patients had an average of 7.2 clinic visits (range 2–44) over a mean of 16.8-months (range 0.23–56). During follow-up patients were prescribed an average of 3.4 (range 0–7) antihypertensive medications with a mean anti-hypertensive therapeutic intensity score of 2.2 (range 0–10.7). The mean longitudinal decline in SBP during follow-up was 25.4 mmHg (SD = 24.6) and the mean longitudinal decline in DBP was 12.2 mmHg (SD = 12.7). Fifty-five percent (n = 252) of patients attained both systolic and diastolic BP goals during follow-up; mean follow-up time to goal attainment was 4.4 visits over an average of 7.0-months.
In Table 1, patient baseline characteristics and BP responses were examined by albuminuria status. Likelihood of micro- or macro-albuminuria significantly varied by age and race, but not by gender or obesity status. As expected, albuminuria was more prevalent in patients with diabetes and those with lower eGFR values. Mean baseline SBP (but not mean baseline DBP) and mean levels above systolic and diastolic JNC BP goals were progressively greater (versus hypertensives without albuminuria) in those with micro- and macro-albuminuria; however, unadjusted mean post-baseline systolic and diastolic BP responses did not significantly vary by albuminuria status. Patients with albuminuria were significantly less likely than those without albuminuria to attain JNC BP goals. At baseline, therapeutic intensity scores and number of antihypertensive medications at baseline significantly differed by albuminuria status; both were higher among patients with albuminuria compared to those without albuminuria.
Table 1.
Characteristics of 459 patients with hypertension, who were not at goal blood pressure (either or both of systolic or diastolic BP) at baseline, stratified by albuminuria status
| Characteristic | Normal (n = 261) |
Albuminuria Status* | p-value | |
|---|---|---|---|---|
| Micro-albuminuria (n = 126) |
Micro-albuminuria (n = 72) |
|||
| Mean age (SD) | 55.4 (12.9) | 57.4 (13.1) | 59.7 (13.8) | 0.046 |
| African American race, n (%) | 225 (86.2) | 113 (89.7) | 70 (97.2) | 0.030 |
| Male gender, n (%) | 72 (27.6) | 43 (34.1) | 29 (40.3) | 0.09 |
| BMI > 30 kg/m2, n (%) | 140 (55.8) | 79 (63.7) | 43 (60.6) | 0.32 |
| Diabetes, n (%) | 54 (20.7) | 58 (46.0) | 45 (62.5) | <0.001 |
| Mean baseline therapeutic intensity score (SD) | 1.8 (1.1) | 2.3 (1.3) | 2.3 (1.0) | <0.0001 |
| Mean baseline number of antihypertensive medications (SD) | 3.0 (1.4) | 3.5 (1.3) | 3.8 (1.1) | <0.0001 |
| Mean baseline SBP, mmHg (SD) | 164.9 (22.8) | 178.0 (28.5) | 181.0 (27.7) | <0.001 |
| Mean difference from SBP goal (SD) | 31.4 (22.3) | 45.7 (27.6) | 50.8 (27.8) | <0.001 |
| Mean baseline DBP, mmHg (SD) | 96.9 (15.0) | 100.7 (17.3) | 96.7 (20.9) | 0.053 |
| Mean difference from DBP goal (SD) | 14.4 (11.8) | 20.2 (14.0) | 24.1 (17.5) | <0.001 |
| Mean eGFR [ml/min/1.73 m2] (SD) | 80.9 (23.6) | 72.7 (24.4) | 54.1 (20.3) | <0.001 |
| eGFR < 60 ml/min/1.73 m2, n (%) | 46 (17.6) | 38 (30.2) | 41 (56.9) | <0.001 |
| Mean post-baseline SBP response (SD) | 26.6 (21.4) | 27.8 (28.2) | 24.7 (26.7) | 0.80 |
| Mean post-baseline DBP response (SD) | 14.3 (11.5) | 15.0 (13.1) | 15.8 (13.7) | 0.50 |
| Attained S/DBP Goal, n (%) | 168 (64.4) | 59 (46.8) | 25 (34.7) | <0.001 |
Note: Normal = <30 mg/g creatinine; micro-albuminuria = 30–300 mg/g creatinine; macro-albuminuria = >300 mg/g creatinine.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; Goal BP, comorbidity-specific goal blood pressure as defined by JNC-VII guidelines.
Patients with eGFR <60 ml/min/1.732 were older, and were more likely to have diabetes and either micro- or macro-albuminuria than those with higher eGFR values, but were equally likely to be male, African American or obese (Table 2). In these unadjusted analyses, patients with eGFR <60 ml/min/1.732 had reduced post-baseline systolic and diastolic BP responses compared to those with higher eGFR values, and were significantly less likely to attain JNC BP goals. At baseline, therapeutic intensity scores and number of antihypertensive medications at baseline significantly differed by eGFR status; both were higher among patients with reduced eGFR.
Table 2.
Characteristics of 459 patients with hypertension, who were not at goal blood pressure (either or both of systolic or diastolic BP) at baseline, stratified by eGFR status
| Characteristic | eGFR < 60 ml/min/1.73 m2 (n = 125) |
eGFR ≥ 60 ml/min/1.73 m2 (n = 334) |
p-value |
|---|---|---|---|
| Mean age (SD) | 62.7 (12.2) | 54.4 (12.8) | <0.001 |
| African American race, N (%) | 112 (89.6) | 296 (88.6) | 0.77 |
| Male gender, n (%) | 40 (32.0) | 104 (31.1) | 0.86 |
| BMI > 30 kg/m2, n (%) | 68 (55.3) | 194 (60.1) | 0.36 |
| Diabetes, n (%) | 55 (44.0) | 102 (30.5) | 0.007 |
| Mean baseline therapeutic intensity score (SD) | 2.4 (1.4) | 1.9 (1.1) | <0.0001 |
| Mean baseline number of antihypertensive medications (SD) | 3.7 (1.2) | 3.1 (1.4) | <0.0001 |
| Mean baseline SBP, mmHg (SD) | 173.7 (27.2) | 170.0 (25.8) | 0.14 |
| Mean difference from SBP goal (SD) | 45.5 (25.9) | 36.1 (25.7) | <0.001 |
| Mean baseline DBP, mmHg (SD) | 93.4 (17.2) | 99.6 (16.2) | <0.001 |
| Mean difference from DBP goal (SD) | 18.4 (14.6) | 17.2 (13.6) | 0.57 |
| Mean urine albumin: creatinine mg/g (SD) | 663.1 (1439.7) | 100.2 (273.0) | <0.001 |
| Albuminuria status* | <0.001 | ||
| Normal | 46 (36.8) | 215 (64.4) | |
| Micro-albuminuria | 38 (30.4) | 88 (26.4) | |
| Macro-albuminuria | 41 (32.8) | 31 (9.3) | |
| Mean post-baseline SBP response (SD) | 22.6 (23.5) | 28.1 (24.5) | 0.06 |
| Mean post-baseline DBP response (SD) | 12.2 (11.6) | 15.5 (12.4) | 0.040 |
| Attained BP Goal, N (%) | 58 (46.4) | 194 (58.1) | 0.025 |
Note: Normal = <30 mg/g creatinine; micro-albuminuria = 30–300 mg/g creatinine; macro-albuminuria = >300 mg/g creatinine.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; Goal BP, comorbidity-specific goal blood pressure as defined by JNC-VII guidelines.
Table 3 presents results from two repeated measures linear regression models examining the influence of albuminuria and eGFR on visit-specific SBP and DBP responses during post-baseline visits. Macro-albuminuria predicted a 10.3 mmHg lesser longitudinal SBP reduction (95% CI = −15.3, −5.4 p < 0.001) and micro-albuminuria predicted a 5.1 mmHg lesser reduction (95% CI = −8.6, −1.6, p = 0.005); similarly, macro-albuminuria predicted a 7.9 mmHg lesser longitudinal DBP reduction (95% CI = −11.1, −4.6, p < 0.001) and micro-albuminuria predicted a 3.9 mmHg lesser reduction (95% CI = −6.2, −1.6, p = 0.001). Lower eGFR also had a significant impact on reducing longitudinal BP response; eGFR <60 ml/min/1.73 m2 predicted an 8.4 mmHg lesser longitudinal SBP reduction (95% CI = −12.6, −4.3, p < 0.001) and a 4.5 mmHg lesser longitudinal DBP reduction (95% CI = −7.1, −1.8, p < 0.001). These models were adjusted for patient characteristics and anti-hypertensive therapeutic intensity; in both models higher therapeutic intensity was significantly associated with greater reduction in longitudinal BP.
Table 3.
Results from two repeated measures linear mixed effects regression models examining the influence of eGFR and albuminuria on systolic and diastolic blood pressure (BP) response*
| Covariate | Systolic blood pressure response | Diastolic blood pressure response | ||||
|---|---|---|---|---|---|---|
| Estimate^ | 95% CI | p-value | Estimate^ | 95% CI | p-value | |
| Age (years) | −0.28 | −0.43, −0.13 | <0.001 | 0.08 | −0.01, 0.17 | 0.11 |
| Male gender | −1.14 | −5.04, 2.76 | 0.57 | −0.38 | −2.36, 1.94 | 0.75 |
| African American race | −5.11 | −11.33, 1.11 | 0.11 | −2.83 | −6.75, 1.09 | 0.16 |
| Diabetes | −5.68 | −9.55, −1.81 | 0.004 | −0.89 | −3.31, 1.53 | 0.47 |
| BMI > 30 kg/m2 | −3.71 | −7.00, −0.42 | 0.027 | −1.48 | −3.58, 0.62 | 0.17 |
| Difference from SBP or DBP goal at baseline | 0.68 | 0.61, 0.75 | <0.001 | 0.51 | 0.43, 0.59 | <0.001 |
| Therapeutic intensity score# | 2.09 | 0.95, 3.23 | <0.001 | 1.27 | 0.50, 1.34 | 0.001 |
| eGFR < 60 ml/min/1.73 m2 | −8.44 | −12.63, −4.25 | <0.001 | −4.48 | −7.12, −1.84 | <0.001 |
| Albuminuria status†: | ||||||
| Macro-albuminuria | −10.32 | −15.28, −5.36 | <0.001 | −7.88 | −11.14, −4.62 | <0.001 |
| Micro-albuminuria | −5.08 | −8.63, −1.55 | 0.005 | −3.88 | −6.18, −1.58 | 0.001 |
Note: Visit-specific change in BP from baseline (baseline BP minus visit-specific BP calculated at each post-baseline visit).
With normal albumin as reference. Normal = <30 mg/g creatinine; micro-albuminuria = 30–300 mg/g creatinine; macro-albuminuria = >300 mg/g creatinine.
Visit-specific anti-hypertension therapeutic intensity score: calculated as daily dose/maximum FDA approved dose, summed across medication class.
Estimate (beta coefficient) deviation from expected (mean [intercept]) BP response in mmHg for participants with this characteristic compared to those without, given inclusion of all other variables in adjusted model. Positive values indicate greater reductions in BP.
Abbreviations: eGFR, estimated glomerular fi ltration rate; BMI, body mass index; BP, blood pressure.
Table 4 presents results from two proportional hazards models examining the influence of albuminuria and eGFR on likelihood of attaining systolic and diastolic BP goals during follow-up. Patients with micro- (HR = 0.71, 95% CI = 0.53–0.97, p =0.032) or macro-albuminuria (HR = 0.54, 95% CI = 0.35–0.83, p = 0.005) were significantly less likely than patients without albuminuria to attain their systolic BP goal; patients with eGFR <60 ml/min/1.73 m2 were also less likely to attain systolic BP goals but not significantly. In contrast, neither reduced eGFR nor the presence of either micro- or macro-albuminuria were significantly associated with likelihood of attaining diastolic BP goal. Higher therapeutic intensity was not significantly associated with increased likelihood of either systolic or diastolic goal attainment. In fact, mean therapeutic intensity scores (2.3 vs 2.6, p = 0.001) and mean number of antihypertensive medications (3.4 vs 3.9, p < 0.001) were both significantly lower for patients who attained SBP goal (calculated at the time of goal attainment) compared to those patients who never reached SBP goal (calculated at last follow-up visit). Similarly, therapeutic intensity (2.2 vs 2.4, p = 0.06) and number of medications (3.3 vs 3.6, p = 0.015) were lower for patients attaining DBP goal.
Table 4.
Results from two proportional hazards models examining the influence of eGFR and albuminuria status, on likelihood of attaining systolic and diastolic blood pressure goals during follow-up
| Covariate | Attainment of SBP goal | Attainment of DBP goal | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | |
| Age (years) | 0.99 | 0.98–1.00 | 0.17 | 1.00 | 0.99–1.01 | 0.64 |
| Male gender | 0.71 | 0.54–0.95 | 0.020 | 0.77 | 0.58–1.03 | 0.07 |
| African American race | 1.02 | 0.67–1.55 | 0.91 | 1.06 | 0.69–1.63 | 0.80 |
| Diabetes | 0.83 | 0.62–1.12 | 0.23 | 1.31 | 0.96–1.78 | 0.09 |
| BMI > 30 kg/m2 | 0.76 | 0.58–1.00 | 0.048 | 0.67 | 0.50–0.90 | 0.008 |
| Therapeutic intensity score# | 1.00 | 0.90–1.12 | 0.98 | 1.02 | 0.91–1.14 | 0.75 |
| Difference from SBP or DBP goal at baseline | 0.99 | 0.98–0.99 | <0.001 | 0.97 | 0.96–0.98 | <0.001 |
| eGFR <60 ml/min/1.73 m2 | 0.74 | 0.54–1.02 | 0.07 | 0.85 | 0.61–1.18 | 0.32 |
| Albuminuria status*: | ||||||
| Macro-albuminuria | 0.54 | 0.35–0.83 | 0.005 | 0.87 | 0.56–1.36 | 0.54 |
| Micro-albuminuria | 0.71 | 0.53–0.97 | 0.032 | 0.82 | 0.60–1.13 | 0.23 |
Note: Model adjusted for visit month (time).
With normal albumin as reference. Normal = <30 mg/g creatinine; micro-albuminuria = 30–300 mg/g creatinine; macro-albuminuria = >300 mg/g creatinine.
Anti-hypertension therapeutic intensity score: calculated as daily dose/maximum FDA approved dose, summed across medication class.
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
Results from analyses of time-to-attainment of JNC BP goal stratified by albuminuria categories (Figure 1) indicated significant (Log-rank test p < 0.001) variation, with a significantly longer time to BP goal attainment for hypertensives with micro-albuminuria (median time 12.9-months [95% CI = 10.3 to 17.5]) or macro-albuminuria (median time 24.6-months [95% CI = 9.6 to infinity]) compared with those without albuminuria (median time 4.9-months [95% CI = 3.6 to 7.6]). Similarly, time-to-attainment of BP goal stratified by eGFR status (Figure 2) indicated significantly (Log-rank test p = 0.012) greater time to goal attainment for patients with eGFR <60 ml/min/1.732 (median time 12.9-months [95% CI = 9.1 to 18.4]) compared with those with values ≥60 ml/min/1.732 (median time 7.6-months [95% CI = 5.1 to 10.3]).
Figure 1.
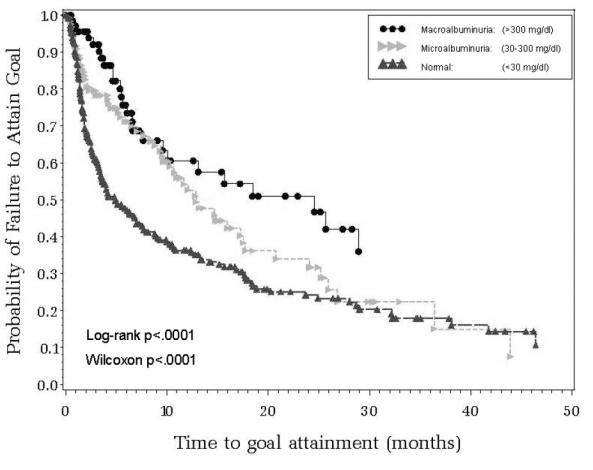
Time to attainment of systolic and diastolic blood pressure goal (JNC-VII guidelines) by albuminuria status.
Figure 2.
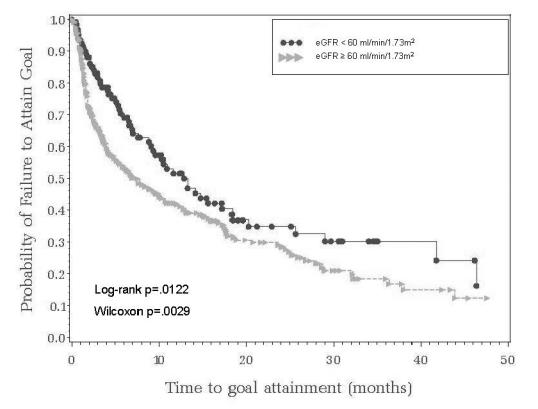
Time to attainment of systolic and diastolic blood pressure goal (JNC-VII guidelines) by eGFR status.
Discussion
We examined the influence of albuminuria and eGFR on longitudinal BP responses to antihypertensive therapy using data derived from a population of mostly African American patients with hypertension who were managed in an urban academic hypertension clinic. Our results demonstrated that the presence of micro- or macro-albuminuria was linked to an attenuation of the longitudinal BP response to complex antihypertensive drug regimens. In our analysis, the impact of albuminuria on longitudinal responses and attainment of JNC goal BP levels was independent of the level of kidney function (eGFR). Albuminuria resulted in a significantly lower proportion of hypertensive patients attaining JNC goal BP levels and also prolonged the time to attainment of goal BP. Similarly, lower eGFR values (<60 ml/min/1.732) were also linked to attenuation of longitudinal BP response to antihypertensive therapy and with increased time to attainment of JNC goal BP levels. Typically, patients with the highest baseline BP levels, experience the greatest reductions in BP over time (MacFayden et al 1991; Mokwe et al 2004), an observation attributable in part to regression to the mean. However, we observed a lesser, rather than a greater decline in BP in hypertensive patients with albuminuria or low eGFR values, lending credence to our thesis that albuminuria and reduced eGFR confer true resistance to antihypertensive drug therapy.
Microalbuminuria has been linked to glomerular hyperfiltration, increased glomerular permeability and increased intraglomerular pressure (Keane and Eknoyan 1999). Glomerular injury, almost irrespective of etiology, typically leads to a rise in systemic BP. Thus, an association between albuminuria and elevated BP is not surprising (Gosling and Beevers 1989; Jensen et al 1995; Cruz et al 1997). Albuminuria appears to be a marker for global vascular dysfunction extending beyond the kidney. There is clear evidence that albuminuria is linked to endothelial and non-endothelial arterial dilatory capacity (Pedrinelli et al 1994; Clausen et al 2001). The level of circulating von Willebrand factor, a marker of vascular endothelial damage and predictor of cardiovascular events, is elevated in hypertensive patients with micro-albuminuria (Pedrinelli et al 1994).
On the arterial side, vascular dysfunction might lead to vasoconstriction and diminished responsiveness to vasculoactive antihypertensive agents and therefore lesser reductions in BP over the long-term relative to drug-treated hypertensives without albuminuria. On the venous side of the vascular system, which contains 75%–80% of the blood volume, endothelial dysfunction has been linked to reduced venous capacitance and centralization of the blood volume (Francis and Cohn 1990; Abrams 1996). Patients with albuminuria are more salt-sensitive than non-albuminuric patients (Campese 1994; Nesovic et al 1996). Salt sensitivity has, in turn, been linked with higher antihypertensive medication requirements in drug-treated hypertensives (Hooper et al 2004). Thus, it is plausible to speculate that some of the resistance to drug therapy we observed in our hypertensive patients may have been explained by enhanced renal absorption of sodium. Renal tubular binding of diuretics to filtered proteins is another possible mechanism of resistance to antihypertensive drug therapy in patients with albuminuria. Accordingly, proteinuria has also been shown to result in decreased levels of diuretic drugs reaching their respective active sites within the renal tubules (Kirchner et al 1992). Nevertheless, we were unable to directly test mechanistic explanations; therefore we can only speculate about the mechanisms of resistance to therapies in hypertensive patients with albuminuria.
There are several important considerations when interpreting our study results. First, our study was observational and therefore subject to biases that are inherent in non-randomized study designs. An important limitation of our study is that our referral cohort of hypertensive patients had higher BP levels at baseline, was more intensively treated than the majority of drug-treated hypertensives, and was disproportionately African American women. The fact that we classified eGFR and albuminuria status on the basis of single serum creatinine and urinary albumin:creatinine ratio determinations, respectively, likely caused misclassification of these covariates across time. However, this would have lessened rather than increased the probability of positive findings when longitudinal BP response data were contrasted across albuminuria categories. Another potential weakness in our analysis is that we did not adjust for the effect of specific anti-hypertensive therapies. Patients like those studied here typically require, and are very likely already taking, multiple antihypertensive drug therapies, initiated and subsequently changed based on clinician decisions rather than in a systematic fashion. In order to adjust multivariate models for the effect of anti-hypertensive medication use on blood pressure response, we calculated visit-specific anti-hypertensive therapeutic intensity scores (daily dose divided by maximum FDA approved dose, summed across medication class); in both models examining longitudinal blood pressure response, higher therapeutic intensity was significantly associated with greater reduction in longitudinal BP.
We believe that our observations are potentially important for several reasons. Measurement of urine albumin excretion is readily available to clinicians (and is specifically recommended for measurement in all hypertensive patients for risk stratification in the JNC VII report) from random spot urine samples that are reasonably well correlated with estimates obtained from 24-hour collections (Ng et al 2000; Claudi and Cooper 2001); similarly eGFR can be calculated from serum creatinine values (Levey et al 1999). Second, though the mechanisms through which albuminuria increases cardiovascular-renal risk are only partially understood (Raynaud et al 1998), our data suggest that a well established and modifiable risk factor, BP elevations, may explain part of the risk imparted by albuminuria. Though other studies have shown higher levels of BP in patients with albuminuria/proteinuria, they have not reported the influence of albuminuria on longitudinal BP responses (Hunsicker et al 1997; Agodoa et al 2001). The commingling of albuminuria with BP complicates the interpretation of clinical studies that report endpoints according to albuminuria status, unless this fact is taken into account in the design and/or analysis of the data. It is our hope that this report will stimulate reanalysis of the influence of higher levels of BP on the risk that has been attributed to albuminuria, per se.
In summary, the presence of albuminuria and reduced eGFR appears to identify patients who will manifest attenuated responses to antihypertensive pharmacotherapy. BP elevations over the long-term may be one mechanism through which albuminuria and reduced eGFR mediate an increased risk of cardiovascular-renal disease among patients receiving various complex antihypertensive regimens. Our findings need to be confirmed in other longitudinal clinical data sets.
References
- Abrams J. Beneficial actions of nitrates in cardiovascular disease. Am J Cardiol. 1996;77:31C–37C. doi: 10.1016/s0002-9149(96)00186-5. [DOI] [PubMed] [Google Scholar]
- Abshagen U, von Mollendorff E. Haemodynamic abnormalities in hypertensive patients: a review of the influence of vasodilating drugs. J Int Med Res. 1986;14:289–98. doi: 10.1177/030006058601400601. [DOI] [PubMed] [Google Scholar]
- Agodoa LY, Appel L, Bakris GL, et al. African American Study of Kidney Disease and Hypertension (AASK) Study Group et al: effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285:2719–28. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- Bianchi S, Bigazzi R, Campese VM. Microalbuminuria in essential hypertension. J Nephrol. 1997;10:216–19. [PubMed] [Google Scholar]
- Bianchi S, Bigazzi R, Campese VM. Microalbuminuria in essential hypertension: significance, pathophysiology, and therapeutic implications. Am J Kidney Dis. 1999;34:973–95. doi: 10.1016/S0272-6386(99)70002-8. [DOI] [PubMed] [Google Scholar]
- Campbell RC, Ruggenenti P, Remuzzi G. Proteinuria in diabetic nephropathy: treatment and evolution. Curr Diab Rep. 2003;3:497–504. doi: 10.1007/s11892-003-0014-0. [DOI] [PubMed] [Google Scholar]
- Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension. 1994;23:531–50. doi: 10.1161/01.hyp.23.4.531. [DOI] [PubMed] [Google Scholar]
- Claudi T, Cooper JG. Comparison of urinary albumin excretion rate in overnight urine and albumin creatinine ratio in spot urine in diabetic patients in general practice. Scand J Prim Health Care. 2001;19:247–8. doi: 10.1080/02813430152706774. [DOI] [PubMed] [Google Scholar]
- Clausen P, Jensen JS, Jensen G, et al. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–74. doi: 10.1161/01.cir.103.14.1869. [DOI] [PubMed] [Google Scholar]
- Cruz HM, Cruzera AB, Cruz J. Microalbuminuria in essential hypertensives in treatment for hypertension. Rev Hosp Clin Fac Med Sao Paulo. 1997;52:258–62. [PubMed] [Google Scholar]
- Cubeddu LX, Hoffmann IS, Aponte LM, et al. Role of salt sensitivity, blood pressure, and hyperinsulinemia in determining high upper normal levels of urinary albumin excretion in a healthy adult population. Am J Hypertens. 2003;16:343–9. doi: 10.1016/s0895-7061(03)00057-8. [DOI] [PubMed] [Google Scholar]
- Francis GS, Cohn JN. Heart failure: mechanisms of cardiac and vascular dysfunction and the rationale for pharmacologic intervention. FASEB J. 1990;4:3068–75. doi: 10.1096/fasebj.4.13.2210153. [DOI] [PubMed] [Google Scholar]
- Gosling P, Beevers DG. Urinary albumin excretion and blood pressure in the general population. Clin Sci (Lond) 1989;76:39–42. doi: 10.1042/cs0760039. [DOI] [PubMed] [Google Scholar]
- Hooper L, Bartlett C, Davey SG, et al. Advice to reduce dietary salt for prevention of cardiovascular disease. Cochrane Database Syst Rev. 2004;1:CD003656. doi: 10.1002/14651858.CD003656.pub2. [DOI] [PubMed] [Google Scholar]
- Hunsicker LG, Adler S, Caggiula A, et al. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 1997;51:1908–19. doi: 10.1038/ki.1997.260. [DOI] [PubMed] [Google Scholar]
- Imanishi M, Yoshioka K, Okumura M, et al. Sodium sensitivity related to albuminuria appearing before hypertension in type 2 diabetic patients. Diabetes Care. 2001;24:111–16. doi: 10.2337/diacare.24.1.111. [DOI] [PubMed] [Google Scholar]
- Jensen JS, Borch-Johnsen K, Jensen G, et al. Atherosclerotic risk factors are increased in clinically healthy subjects with microalbuminuria. Atherosclerosis. 1995;112:245–52. doi: 10.1016/0021-9150(94)05420-n. [DOI] [PubMed] [Google Scholar]
- JNC VI Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. (JNC VI) Arch Intern Med. 1997;157:2413–66. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- JNC VII Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. (JNC VI) JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–10. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- Kirchner KA, Voelker JR, Brater DC. Tubular resistance to furosemide contributes to the attenuated diuretic response in nephrotic rats. J Am Soc Nephrol. 1992;2:1201–7. doi: 10.1681/ASN.V271201. [DOI] [PubMed] [Google Scholar]
- Leoncini G, Sacchi G, Viazzi F, et al. Microalbuminuria identifies overall cardiovascular risk in essential hypertension: an artificial neural network-based approach. J Hypertens. 2002;20:1315–21. doi: 10.1097/00004872-200207000-00018. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, et al. a more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- MacFadyen RJ, Bainbridge AD, Lees KR, et al. The response to the first dose of an angiotensin converting enzyme inhibitor in uncomplicated hypertension – a placebo controlled study utilising ambulatory blood pressure recording. Br J Clin Pharmacol. 1991;32:393–8. doi: 10.1111/j.1365-2125.1991.tb03918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokwe E, Ohmit SE, Nasser SA, et al. Determinants of blood pressure response to quinapril in black and white hypertensive patients: the Quinapril Titration Interval Management Evaluation trial. Hypertension. 2004;43:1202–7. doi: 10.1161/01.HYP.0000127924.67353.86. [DOI] [PubMed] [Google Scholar]
- National Kidney Foundation. Clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kid Disease. 2002;39(Suppl 1):S1–S266. [PubMed] [Google Scholar]
- Nesovic M, Stojanovic M, Nesovic MM, et al. Microalbuminuria is associated with salt sensitivity in hypertensive patients. J Hum Hypertens. 1996;10:573–6. [PubMed] [Google Scholar]
- Ng WY, Lui KF, Thai AC. Evaluation of a rapid screening test for microalbuminuria with a spot measurement of urine albumin-creatinine ratio. Ann Acad Med Singapore. 2000;29:62–5. [PubMed] [Google Scholar]
- Pedrinelli R, Dell’Omo G, Di Bello V, et al. Microalbuminuria, an integrated marker of cardiovascular risk in essential hypertension. J Hum Hypertens. 2002;16:79–89. doi: 10.1038/sj.jhh.1001316. [DOI] [PubMed] [Google Scholar]
- Pedrinelli R, Giampietro O, Carmassi F, et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet. 1994;344:14–18. doi: 10.1016/s0140-6736(94)91047-2. [DOI] [PubMed] [Google Scholar]
- Pinto-Sietsma SJ, Janssen WM, Hillege HL, et al. Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol. 2000;11:1882–8. doi: 10.1681/ASN.V11101882. [DOI] [PubMed] [Google Scholar]
- Plavnik FL, Silva MA, Kohlmann NE, et al. Relationship between microalbuminuria and cardiac structural changes in mild hypertensive patients. Braz J Med Biol Res. 2002;35:799–804. doi: 10.1590/s0100-879x2002000700006. [DOI] [PubMed] [Google Scholar]
- Raynaud E, Brun JF, Fedou C, et al. Is microalbuminuria, an early marker of clinincal nephropathy, also a cardiovascular risk factor? Ann Biol Clin (Paris) 1998;56:671–9. [PubMed] [Google Scholar]
- Taylor SH. Pharmacotherapeutic stature of doxazosin and its role in coronary risk reduction. Am Heart J. 1988;116:1735–47. doi: 10.1016/0002-8703(88)90223-2. [DOI] [PubMed] [Google Scholar]
- Weir MR, Chrysant SG, McCarron DA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. 1998;31:1088–96. doi: 10.1161/01.hyp.31.5.1088. [DOI] [PubMed] [Google Scholar]
- Weir MR, Hall PS, Behrens MT, et al. Salt and blood pressure responses to calcium antagonism in hypertensive patients. Hypertension. 1997;30:422–7. doi: 10.1161/01.hyp.30.3.422. [DOI] [PubMed] [Google Scholar]


