Abstract
Background
Intravenous injection of narcotic stimulants affects many cellular functions relevant for the pathophysiological mechanisms of heart failure. There is considerable evidence that mast cells (MCs), TNF-α, and cell death play crucial roles in the pathogenesis and progression of cardiac arrest. In this study, we examined and compared the participation of MCs, TNF-α, apoptosis and necrosis in the heart of drug-related fatalities and the victims of sudden death due to the cardiac failure or aortic dissection.
Methods and results
Serum level of postmortem tryptase was determined in all study subjects that consisted of 50 autopsy cases: 30 drug overdose fatalities which were further divided into two groups with high and low level of tryptase, and 20 cases of sudden natural death (SND). The distribution profile of cardiac infiltrated-MCs and production patterns of TNF and C9 (necrotic marker) were investigated immunohistochemically. In situ-detection of apoptosis with TUNEL was applied to the heart sections. The level of tryptase was elevated (>45 μg/L) in the drug fatalities but remained below the cut-off value in SND. In the myocardium of overdose victims, MC-infiltration and degranulation were significantly increased as well as production of myocytic TNF-α compared with the SND cases. The expressions profile of myocytic TNF varied between the groups. Apoptotic myocytes were seen more frequently in the SND group while necrosis was more evident in the heart of drug-related fatalities.
Conclusion
Mast cells are recruited and activated in the heart of drug-associated deaths and the myocytes are the main source of TNF-α with the ability of different production patterns. The high degree of MC degranulation and the elevated levels of tryptase together with the pathological changes in heart of drug-related victims resemble that of the anaphylactic deaths as demonstrated in our previous study.
Keywords: mast cells, TNF-α, apoptosis, sudden death, narcotic stimulant
Introduction
Possible roles of cardiac mast cells (MCs), have been suggested in mediating injury in myocardial ischemia (Kovanen et al 1995; Somasundaram et al 2005) and involvement in regulating fibrous tissue deposition and scar formation in healing infarcts (Frangogiannis et al 2002; Somasundaram et al 2005). MCs are divided into three different types according to the content of tryptase (T) and Chymase (C); MCTC (mostly in connective tissue), MCT (in mucosal), and MCC that contains only chymase (Irani et al 1989; Yao et al 2003). Histochemical studies indicate rapid mast cell degranulation and mediator release after myocardial ischemia (Edston 1997). The complement split-product C5a, adenosine, and reactive oxygen may represent the stimuli responsible for initiation of mast cell degranulation (Linden 1994).
The symptoms of heroin intoxication with respiratory and circulatory shock and pulmonary edema, resembles anaphylaxis. The high concentration of postmortem blood tryptase (Edston et al 1997) in many heroin cases indicates that death was preceded by a systemic mast cell degranulation (Edston et al 1997; Schwartz et al 1989). However, the serum tryptase level remains normal in the victims of acute myocardial infarction and other causes of sudden cardiovascular deaths (van Haelst et al 2001). MCs are identified as the only source of preformed and immunologically induced tumor necrosis factor (TNF)-α, a proinflammatory mediator (Gordon et al 1990). MC degranulation appears to be confined to the ischemic area and results in rapid release of TNF-α and subsequent induction of an inflammatory cascade crucial for the pathophysiological mechanisms of cardiac arrest (Blancke et al 2005). Preliminary findings show that patients with heart failure have increased levels of TNF-α compared with healthy controls (Levine et al 1990) and this is associated with the severity of cardiac malfunction (Levine et al 1990; Anker et al 1997). However, to date there is no direct evidence that increased levels of TNF-α is due to the overproduction of cardiac myocytes.
The two major forms of cell death; apoptosis, which is the physiologic form of cell death; and necrosis, an accidental form of cell death; are both induced by the action of TNF-α. The evidence for a TNF-derived mast cell-mediated myocardial apoptosis in patients with heart failure supports the involvement of apoptosis in the pathophysiology of heart failure (Rossig et al 2000; Zheng et al 2006).
As potent sympathetic and/or parasympathetic stimulants, narcotic drugs are known to cause a broad range of long-term cardiac effects and/or acute cardiac arrest (Tong et al 2004; Lessa et al 2006). The aim of this study is to investigate the pathological changes in the heart of acute drug-related fatalities, with a focus on the intervention of cardiac MCs, TNF-α, and myocytic cell death compared with selected cases who died a sudden natural death (SND). To our knowledge, no studies have so far investigated the role of these factors in the heart of drug fatalities. Moreover, the observation of pulmonary edema and congestion that complicates heroin overdosage, in conjunction with increased postmortem levels of tryptase that hitherto has not been explained, encouraged us to investigate the distribution of infiltrated MCs also in the lung of drug fatalities, and compare with that of the cardiac tissue in the same group versus control group.
Materials and methods
Study subjects
A total number of 50 cases were studied in a retrospective case-control study: 30 drug fatalities in which toxicological investigation had revealed multiple drug intoxication; and 20 cases of SND revealed by autopsy or by witnesses and other circumstances. Sudden death was defined according to the World Health Organization criteria, ie, death within 24 h of the onset of symptoms. Before the final diagnoses were determined, the death scene circumstances as well as the autopsy, histological, and toxicological findings were evaluated. Infectious disease, asthma, and other immunologically compromising conditions were excluded. The time lapse after death until autopsy varied between 1 and 5 days during which time the bodies were stored at 4 °C. In all cases, toxicological analysis for alcohol and therapeutic drugs (screening test) were performed at the National Forensic Chemistry Laboratory Linköping, Sweden. Four autopsied individuals who died in a traffic accident, with no cardiac lesions as judged by histological examination, were included as negative controls for the source of myocardial TNF-α.
The autopsies were took place at the departments of Forensic Medicine in Linköping and Stockholm, Sweden. In all cases, femoral and heart blood were sampled at autopsy. Histological sections from the brain, myocardium, lungs, liver, kidneys, proximal coronary arteries from the three main branches, and laryngeal wall were stained with hematoxylin-erythrosin-saffron and Mallory’s PTAH. In drug victims, additional sections from the thyroid, adrenals, and spleen were also stained.
We measured tryptase postmortem in serum from femoral vein blood. In order to investigate its association to the number of infiltrated MCs, the drug-related fatalities were divided into two groups according to their level of blood tryptase. One group included 13 cases with elevated concentrations of femoral mast cell tryptase (α + β tryptase) with cut-off value set at 45 μg/l (HLT) (Edston et al 2006). The cut off value is based on statistical calculations of tryptase values in a larger control group. A value above 45 μg/l renders a 5% chance of being falsely positive. The other group contained 17 individuals with low levels of tryptase (LLT), ranging between 5–27 μg/l with a mean of 13.4 (± 1.3 SEM). The tryptase concentrations in the SND cases ranged between 3 and 21 μg/l with a mean of 11.6 (± 1.1 SEM).
Determination of tryptase, specific-, and total-IgE
Femoral blood was sampled in test tubes without additives. They were centrifuged, and the serum was frozen at −20 °C until analysis. Alpha and β-tryptase were measured with Tryptase FEIA (Pharmacia Diagnostics, Uppsala, Sweden). To enable exclusion of any possible immunological complications in drug addicts and SND cases, total serum-IgE was measured with Pharmacia CAP-system IgE-FEIA and morphine-specific IgE with Pharmacia CAP system specific IgE-FEIA (Pharmacia Diagnostics, Uppsala, Sweden). The analyses were done at the Laboratory of Clinical Immunology and Transfusion Medicine, Karolinska sjukhuset, Stockholm, Sweden.
Immunohistochemical analysis
For sequential double labelling with anti-tryptase monoclonal antibody (mab) and anti-chymase mab, a method of Irani and colleagues (1989), was used to display differentially stained MC subsets in the same tissue sections. Sections labeled with the antibodies were successively stained with the appropriate chromogens to distinguish tryptase and/or chymase containing cells by developing different colors. Exclusion of streptoavidine, and substitution of this step with a secondary antibody that directly conjugated to poly-peroxidase molecule, improved the staining. By using this technique we could differentiate between MCTC, MCT, and MCC among the subtypes of MCs.
For each case, four sequential heart-sections from the anterior and posterior walls of the left ventricle, the right ventricle, the septum, and four lung specimens of both lungs, were fixed in 4% phosphate-buffered formalin, embedded in paraffin, and cut into sections of 4 μm. All slides were deparaffinized and rehydrated with graded alcohols and finally in distilled water. Then they were incubated with 3% H2O2 in phosphate-buffer saline (PBS) for 10 min at room temperature (RT) and investigated with a retrieval technique: pretreated 20 min at RT with trypsine digestion (0.3%, pH 7.8) in the case of staining with anti-tryptase, and -chymase, while 10mM citrate buffer (pH 6.00) was used to reveal the epitope of TNF and laminB1 (a nuclear envelope protein). This step was followed by 30 min incubation of slides with blocking solution (5% bovine serum albumin in PBS) to block unspecific bindings. Mabs were applied to the sections at a dilution of 1:100 for anti-tryptase and chymase (NOVO Castra Laboratories LTD, UK), 1:50 for both anti-TNF-α (abcam, UK) and anti-laminB1 (Santa Cruz, INC). Necrosis was visualized with an antibody against the terminal complement complex, ie, C9 (NOVO Castra Laboratories LTD, UK), in a dilution of 1:50. All slides were incubated at RT for 60 min in a moist chamber. After rinsing in PBS, a peroxidase conjugated-mouse IgG (NOVO Castra Laboratories LTD, UK) as secondary antibody was applied at RT for 30 min. The peroxidase activity in tissue sections was visualized by utilizing a variety of chromogens. Diaminobenzidine (DAB) produces reddish brown precipitate in the sections. This was used to detect intracellular chymase, TNF-α, and laminB1 localization. Vector SG substrate developing a blue-black reaction product was used to visualize tryptase in the cell sections. Vector VIP peroxidase substrate, which produces an intense purple precipitate, was applied to distinguish C9 cell-attachment. All three chromogenic systems are available as kits and purchased from Vector Laboratories, Immunkemi, Järfälla, Sweden. The sections were counter-stained with light green in the case of MCs subtypes and C9, or Mayer’s Hematoxylin, for TNF-α and laminB1 staining. Sections without primary antibodies served as negative controls. To confirm the positive staining of MCs, TNF-α and laminB1, sections of spleen, lung, and human tonsil were processed in a similar manner as the respective experimental specimens.
To verify the results from immunohistochemical labelling of MCs, the tissues were simultaneously stained with Toluidine blue for counting mast cells. To confirm the results of immunohistochemical labelling of TNF-α, immunofluorescence staining of parallel sections was performed in an Avidin-Biotin system according to the manufacturer’s instructions (Vector Laboratories), and the results were evaluated in the same manner.
Detection of apoptotic cardiomyocytes
The ApopTag in situ apoptosis detection kit (Chemicon, CA) was used according to the manufacturer’s recommendations. Apoptotic cells were visualized by means of an anti-digoxigenin antibody conjugated to a peroxidase reporter molecule. Utilizing DAB as chromogen substrate, we localized the bound peroxidase antibody and counterstained these slides with a combination of methyl green in 0.1 M sodium acetate. The numbers of apoptotic nuclei of cardiomyocytes from the same tissue sections as for immunohistochemical analysis were determined in a sequence of high power field (HPF, X 400) covering an entire area of one square cm transverse section selected randomly prior to the microscopical examination on each slide. These numbers were reported as apoptotic nuclei per HPF. Considering the strength of the heart to adapt and modify a function or structure to tolerate different incidents and their consequences, including cell death, we defined a limit based on clinical observations and microscopical examination of myocardial apoptosis and necrosis. Equal or more than 3 ApopTag-positive myocytic nuclei/HFP, or ≥10 C9-positive myocytes throughout an area of 1 square centimeter, was considered as positive for apoptosis or necrosis, respectively.
As negative controls, sections were incubated in the absence of TdT enzyme. Sections that were digested with 1 μg DNase/ml buffer (Sigma) for 10 min were utilized as positive controls. Since nuclear Lamin B is fragmented as a consequence of apoptosis, immunological staining of Lamin B1 (Freude et al 2000) was performed to validate the result of ApopTag analysis. Cardiomyocyte nuclei negative for Lamin B1 were counted and calculated similarly as for ApopTag. In each case, two independent observers examined all tissue sections blindly.
Histomorphometry analysis of immunostaining
Fifty visual fields per each myocardial section in magnification × 400 were randomly selected, and all MCTC, MCT, and MCC (immunopositive stained cells) were counted by Planophotometry (DP-Soft Imaging System GmbH). The total numbers of immunopositive MCs throughout the entire heart sections were expressed as numbers of cells per 3 square millimeters. Each visual field per lung section in magnification × 400 was randomly chosen, and the numbers of the immunostained cells surrounding bronchioles in a total area of 1.5 square millimeters were counted. These cells were designated bronchial mast cells. Likewise, the equivalent areas in the same slides without bronchi were assessed, and the counted-immunoreative cells were regarded as parenchymal mast cells. To better evaluate the results obtained from the immunohistochemical analysis, a group of explanatory parameters were designed for each case (refers to Table 4). The sum of both MCTC and MCT per tissue area was considered as MCs. By lung MCTC, lung MCT, and lung MCC we considered the addition of amount of bronchial and parenchymal of these types of cells together. By lung MCs, we indicated the sum of both bronchial and parenchymal MCTC together with MCT and MCC per tissue area. By cardiac MCs we are referring to the sum of MCTC, MCC and MCT in heart tissue.
Table 4.
Mean number of mast cell-subtype in the lung and heart sections of study subjects
| Explanatory parameters | Total area (mm2) |
SND Mean ± SEM N = 20 |
HLT Mean ± SEM N = 13 |
LLT Mean ± SEM N = 17 |
|---|---|---|---|---|
| Bronchial MCs | 1.5 | 16.6 ± 1.2 | 31.3 ± 3.2 ** | 19.3 ± 1 |
| Parenchymal MCs | 1.5 | 11 ± 1 | 15.2 ± 1.2 | 13 ± 0.6 |
| Lung MCs | 3 | 27.6 ± 1 | 46.5 ± 2.2 ** | 32.3 ± 1.3 |
| Lung MCT | 3 | 5.6 ± 0.8 | 9.5 ± 1 | 5 ± 0.4 |
| Cardiac MCs | 3 | 12.8 ± 0.8 | 18.2 ± 2.8** | 17.6 ± 1.1** |
| Cardiac MCT | 3 | 0.5 ± 0.2 | 1.2 ± 0.9 | 1 ± 0.9 |
Abbreviations: SND, sudden natural death; HLT, high level of postmortal tryptase; LLT, low level of postmortal tryptase.
Note: Significant differences according to Kruskal-Wallis Test between three groups.
To estimate the cardiac production of TNF-α, four sequential sections of the heart of each case were examined using the same software (DP-Soft Imaging System GmbH). In each case, two independent observers examined all sections blindly.
Statistical analysis
All statistics were calculated using nonparametric tests. Computations of significances between two groups were performed using the Mann-Whitney U-test. Comparing several means, the Kruskal Wallis test was used. For correlations within groups, Spearman’s rank correlation test was used. All calculations were performed using the statistical package Statistica (Statsoft, Scandinavia AB). P < 0.05 was regarded as significant. The number of counted MCs was expressed as the mean ± SEM per tissue area.
Results
All drug deaths were poly-drug abusers who died from acute drug intoxication (Tables 2 and 3). Heroin was the last injected drug before death in 21 cases, amphetamine in 8, and methadone in a single case. Thus, 73% of all drug deaths were associated with recent intravenous injection of opioids. Concomitant use of other drugs and alcohol was a frequent finding (Table 2, and 3).
Table 2.
Data for the drug fatalities in HLT cases. Age, sex, and autopsy findings in drug fatalities with high levels of tryptase
| Case no. | Age/Gender | Heart weigh (g) | Recently injected drug | Other toxicological findings | Tryptase (μl/l) |
|---|---|---|---|---|---|
| 1 | 39/m | 312 | heroin | THC | 44 |
| 2 | 46/f | amphetamine | ethanol | 45 | |
| 3 | 36/m | 304 | amphetamine | benzodiazepines, THC | 46 |
| 4 | 29/m | 397 | heroin | amphetamine, THC | 48 |
| 5 | 31/m | 436 | heroin | benzodiazepines | 50 |
| 6 | 38/m | 434 | heroin | benzodiazepines, propoxyphene | 54 |
| 7 | 29/m | 292 | heroin | benzodiazepines, ethanol | 67.5 |
| 8 | 32/m | 335 | amphetamine | methadone, bensodiazepines | 71 |
| 9 | 36/m | 357 | heroin | amphetamine | 81 |
| 10 | 26/m | 400 | amphetamine | THC | 86 |
| 11 | 44/m | 390 | heroin | benzodiazepines, THC, ethanol | 90 |
| 12 | 35/m | 335 | methadone | benzodiazepines, THC | 240 |
| 13 | 24/m | 320 | heroin | amphetamine, cocaine, THC, benzodiazepines, stanozolol | 477 |
Abbreviations: THC, tetra-hydro-cannabinol (the active substance in hashish).
Table 3.
Data for the drug fatalities in LLT cases. Age, sex, and autopsy findings in drug fatalities with low levels of tryptase
| Case no. | Age/Gender | Heart weight (g) | Recently injected drug | Other toxicological findings | Tryptase (μl/l) |
|---|---|---|---|---|---|
| 1 | 24/m | 359 | heroin | benzodiazepines, THC | 5 |
| 2 | 26/m | 275 | heroin | benzodiazepines, cocaine, MDMA | 7 |
| 3 | 21/m | 394 | heroin | amphetamine, benzodiazepines, ethanol | 8 |
| 4 | 29/m | 304 | amphetamine | Ethanol | 9 |
| 5 | 21/m | 325 | heroin | Benzodiazepines | 10 |
| 6 | 21/m | 312 | heroin | benzodiazepines, ethanol | 11 |
| 7 | 51/m | 315 | heroin | benzodiazepines, THC, SRRI | 11 |
| 8 | 36/m | 335 | heroin | benzodiazepines, ethanol | 11 |
| 9 | 20/f | 231 | heroin | benzodiazepines, THC | 12 |
| 10 | 49/m | 410 | heroin | Ethanol | 13 |
| 11 | 30/m | 371 | amphetamine | benzodiazepines, THC, ethylmorphine | 14 |
| 12 | 25/m | 394 | heroin | benzodiazepines, THC, ethanol | 14 |
| 13 | 35/m | 408 | heroin | SRRI | 17 |
| 14 | 38/m | 361 | amphetamine | Tramadol | 18 |
| 15 | 27/m | 441 | heroin | MDMA, SRRI | 19 |
| 16 | 38/m | 430 | amphetamine | Cocaine | 22 |
| 17 | 26/m | 433 | heroin | metamphetamine, buprenorphine, SRRI | 27 |
Abbreviations: THC, tetra-hydro-cannabinol (the active substance in hashish); MDMA, 3,4-Methylendioxymetamphetamine (colloquially: Ecstasy); SSRI, Selective Serotonin Reuptake Inhibitor.
Morphopathological findings in SND and drug fatalities
In most drug deaths, the heart appeared to be within normal limits regarding configuration and with weights ranging between 231–436 g with a mean of 356 g (±10 SEM, Tables 2 and 3). In the controls, heart weight varied more (range 191–654 g) with a mean of 414 g (±29 SEM, Table 1). The difference between the groups was not significant (Kruskal-Wallis test p > 0.05) due to the wide range of the heart weight of the control group. In the myocardium, a small number of the drug-cases had slight, diffuse fibrosis and a few, probably insignificant, foci of inflammatory cells. Apart from congestion, the pathology in the control group was concentrated to the heart or the aorta (Table 1). In most drug cases, coronary artery disease was absent, and none had more than moderate coronary artery sclerosis.
Table 1.
Data for the SND cases. Age, sex, and autopsy findings in 20 cases of sudden natural death
| Case no. | Age/Gender | Heart weigh (g) | Diagnosis | Tryptase (μl/l) (α + β) |
|---|---|---|---|---|
| 1 | 52/m | 604 | AAD | 3 |
| 2 | 59/f | 363 | AMI | 3 |
| 3 | 54/m | 654 | AAD | 6 |
| 4 | 50/m | 440 | AVA | 6 |
| 5 | 40/f | 512 | HCM | 7 |
| 6 | 14/m | 312 | ARVD | 9 |
| 7 | 42/m | 370 | AMI | 9 |
| 8 | 47/f | 337 | AAD | 10 |
| 9 | 27/m | – | ARVD | 11 |
| 10 | 54/m | 364 | AMI | 11 |
| 11 | 45/m | 334 | MV PROLAPSE | 12 |
| 12 | 21/m | 389 | ARVD | 13 |
| 13 | 82/m | 621 | AVA | 13 |
| 14 | 42/m | 364 | AHF | 15 |
| 15 | 48/f | 318 | ARVD | 15 |
| 16 | 25/m | 323 | MYOCARDITIS | 15 |
| 17 | 49/m | 467 | AHF | 16 |
| 18 | 13/m | 284 | ARVD | 17 |
| 19 | 54/m | 610 | MYOCARDITIS | 17 |
| 20 | 48/m | 191 | ARVD | 21 |
Abbreviations: AHF, acute heart failure; AAD, acute aortic dissection; AVA, acute ventricular arrhythmia; ARVD, arrhythmogenic right ventricular dysplasia; AMI, acute myocardial infarction; MV, mitral valve; HCM, hypertrophic cardiomyopathy.
Analysis of tryptase
The results of the tryptase measurements are presented in Tables 1, 2, and 3. No specific IgE antibodies to morphine were detected in any case. There was a significant correlation between the number of infiltrated MCs in the lung sections of the HLT group and the femoral blood tryptase concentration (p < 0.05).
Morphometric analysis of the myocardium and lungs
In pulmonary and cardiac tissues, two populations of MCs were apparent: one resembled typical connective tissue type MCs that stained with both anti-tryptase and chymase (MCTC), and the other was atypical or mucosal MC that reacted only to anti-tryptase antibody. This population was seen in appreciable amounts in the lung but a minor number was found also in heart tissue. Due to a negligible quantity of tissue MCC, this subset was excluded from statistical analysis. Significantly higher amounts of infiltrated MCs were observed in the HLT and LLT-cardiac sections than the other groups (p < 0.015 Kruskal-Wallis test, Table 4). The number of MCTC predominated among the cardiac MCs. Moreover, we observed that in 10 cases of 13 (77%) and 4 cases of 17 (23.5%) in HLT- and LLT-heart sections, respectively, more than 60% (on average) of the infiltrated MCs were degranulated (Figure 1b), while none of SND-heart sections showed this phenomenon (Table 5). In lung sections of drug deaths with high levels of femoral tryptase (HLT), there were higher numbers of infiltrated MC-subsets at the site of bronchial smooth muscle (p < 0.05) and parenchymal tissues compared with the other two groups (Table 4). No significant differences were seen between the amount of infiltrated MCs in SND cases and drug deaths with low tryptase levels (LLT) (Table 4). Figure 1a, showing the infiltrated MC-subsets in the heart sections, illustrates the differences in immunostaining between these cells.
Figure 1.
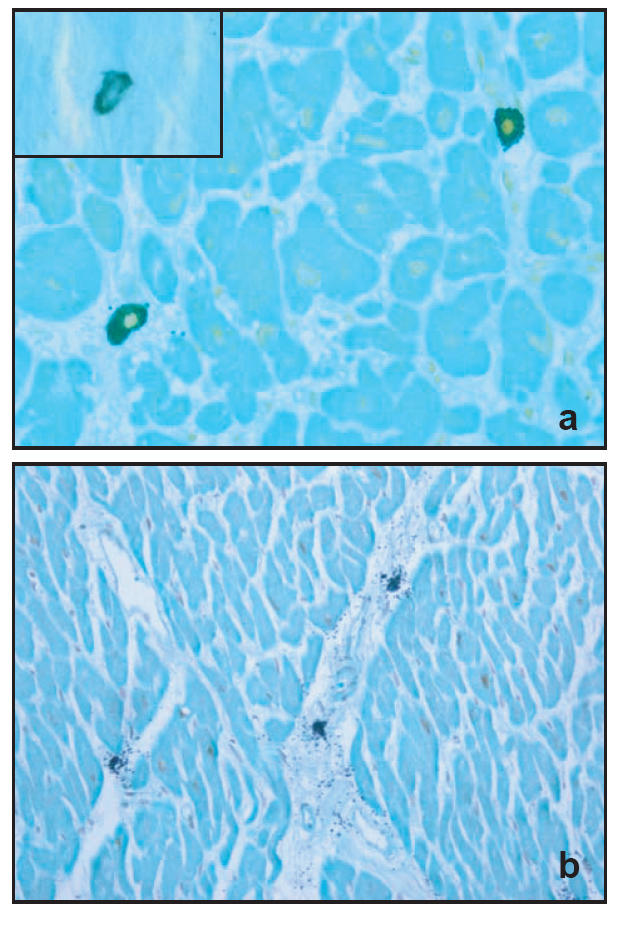
(a) Dual immunostaining of anti-tryptase and -chymase in the same mast cell located within the interior wall of right ventricle. The insert: Cardiac mast cell staining only with anti-tryptase, MCT. (b) Mast cell degranulation in the heart section of a drug victim. Original magnification × 400, in A and × 200 in b.
Table 5.
Characterization of TNF-α, cell death and MCs degranulation in the heart of study population
| Study subject | *Total production of myocytic TNFα/2mm2 mean ± SEM | Expression pattern of myocytic TNFα % Cases | Cell death | % Cases with MCs degranulation | |||
|---|---|---|---|---|---|---|---|
| I | II | III | **% Cases with myocytic apoptosis | ***% Cases with myocytic necrosis | |||
| SND (N = 20) | 10 ± 2.7 | 60 | 35 | 5 | 75 | 20 | 0 |
| HLT (N = 13) | 27.4 ± 5 | 15 | 0 | 84.6 | 23 | 30 | 77 |
| LLT (N = 17) | 34.5 ± 3 | 47 | 0 | 47 | 12 | 53 | 23.5 |
The mean number of granule-like structures of new synthesized intracelluar TNF-α.
≥3 apoptotic myocyte nuclei /HPF in a total area of 1 Cm2.
≥10 necrotic myocytes through an area of 1 Cm2.
I, Intracellular diffusely scattered pattern of TNF-αagranule-like structure.
II, Focal dense intracellular accumulation of TNF-α.
III, Focal, dense accumulation with an additional diffusely scattered intracellular distribution of TNF-α granule-like structures.
Furthermore, according to our results, the number of toluidine-stained MCs was underestimated by approximately 20% compared with the number of immunologically labelled cells. Thus the results rest on the immunological labeling of antibodies as it found more reliable markers.
Analysis and definition of expression patterns of TNF-α
An intriguing result of immunohistochemical analysis of TNF-α was the different expression patterns of myocytic TNF-α. Two distinctive patterns could be easily identified; one was a patchily dense intracellular production of TNF-α and the other a pattern of TNF-α granule-like structures spreading throughout the cardiomyocytes. Microscopical assessment of four sequential sections of the heart of each case revealed different morphological features. The following patterns expressing TNF-α production were found: I. An intracellular, diffusely scattered pattern of TNF-α granule-like structures; II. A focal, dense intracellular accumulation of TNF-α; III. A focal, dense accumulation with an additional, diffusely scattered intracellular distribution of TNF-α (Figures 2a, b, c). To evaluate changes in intracellular synthesis of TNF-α, we counted the number of TNF-α granule-like structures throughout the entire area of 2 mm2 of sections randomly and expressed the change as a percentage of mean of diffusely scattered TNF-α granules/2 mm2. In all cases of HLT and LLT in whom myocardial MCs were elevated, cardiomyocytic production of TNF-α was concomitantly increased (%27.4 ± 5 in HLT, and %34.5 ± 3 in LLT compared with SND cases %10 ± 2.7 (%mean ± SEM, Table 5). 11 of 13 HLT (84.6%) and 8 of 17 LLT cases (47%), but only 1 of 20 in the SND, group showed a TNF-α production according to pattern type III, indicating a considerable difference between these three groups (Table 5). In the SND group, 7 of 20 cases (35%) demonstrated focal, dense cardiomyocytic expression of TNF-α only (pattern type II), but neither the HLT- nor the LLT-heart sections expressed this pattern (Table 5). Additionally, all groups commonly showed a certain percentage of myocytic TNF-α according to the pattern type I (Table 5). Only one case in LLT stayed TNF-α negative. The corresponding cardiac sections obtained from the heart of four healthy controls that died in unnatural causes stained very few in one case and none in the other cases with anti-TNF-α (Figure 2d). Immunofluorescence staining of this cytokine in cardiac sections demonstrated the same pattern as the immunohistochemical staining. In addition, the endothelium of intramyocardial vessels and almost all MCs reacted with anti- TNF-α (Figure 2c).
Figure 2.
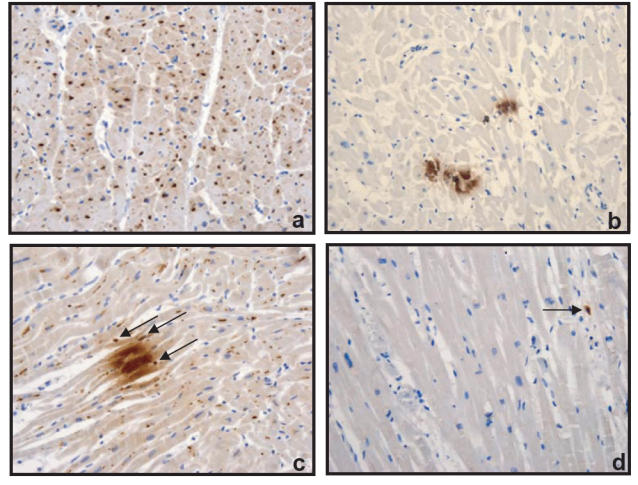
Immunohistochemical analysis of TNF-α in endomyocardial tissues obtained from drug-related victims (a and c) and SND (b). Overview of transverse sections with cytoplasmic localization of newly synthesized myocytic TNF-α of pattern type I (a) and II (b). The longitudinal section (c) showing intracellular production of TNF-α of pattern type III in cardiomyocytes. TNF- α protein was also present in cardiac MCs (arrows). Analysis of TNF-α in longitudinal section of endomyocardial tissues obtained from a healthy control that died in unnatural causes (d). Original magnification × 200.
Detection of apoptosis and necrosis
Classic ApopTag positivity was characterized by focal nuclear staining. In apoptotic myocytes nuclear and cell membrane integrity was intact (Figure 3a). The overall frequency of cardiomyocytic apoptosis was significantly higher in SND cases: 15 cases of 20 (75%) showed apoptotic nuclei (Figure 3a, Table 5). On the other hand, 10 of 13 (77%) drug victims with HLT and 16 of 17 (88%) drug overdose fatalities in LLT showed no nuclear staining in cardiomyocyte by ApopTag analysis (Table 5). Four heart sections of each case were then investigated for binding of C9 antibody to the sarcolemma. An intern positive control was found in the form of C9-positive material in the walls of the blood vessels in every section. Four of 20 (20%) SND cases underwent necrosis according to our definition, while this number was significantly higher in drug deaths with LLT, where 9 of 17 cases (53%) fulfilled the positive criteria and showed the attachment of C9 to the membrane of myocytes (Figure 3b). In drug victims with HLT, 4 of 13 cases (30%) were also classified as positive (Table 5).
Figure 3.
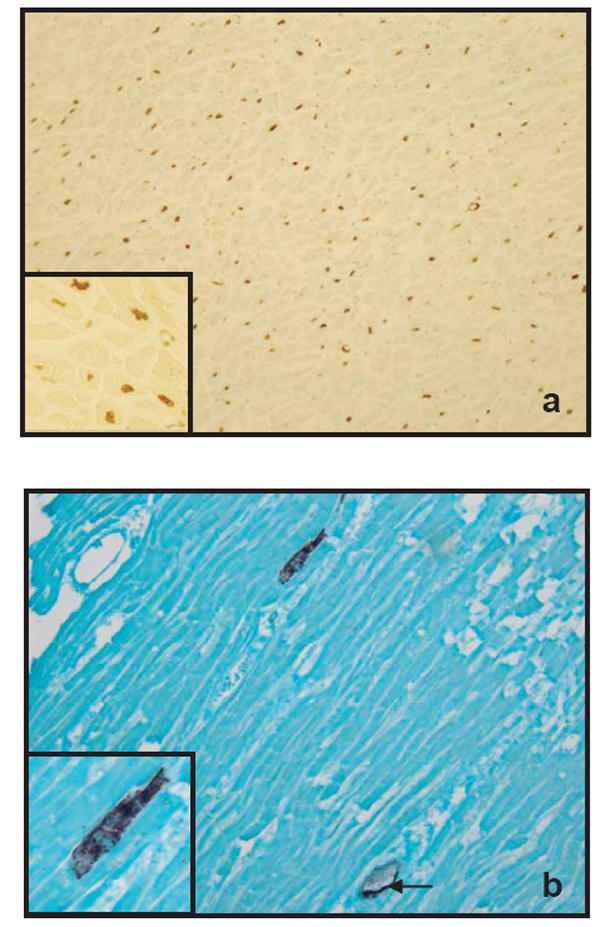
Figure 3 (a) TUNEL-positive myocytes nuclei (brown) demonstrated with the ApopTag kit. The transverse section is from the left ventricle in a case of SND (× 200 magnifications). The insert: Higher magnification (× 400) of the same section showing complete labelling of myocytes nuclei. (b) Myocytic necrosis visualized by the binding of antibody against the terminal complement complex (purple) to the sarcolemma. The longitudinal is from the left ventricle in a case of LLT group (× 200 magnification).Insert: Higher magnification (× 400) of the same section showing immunoreactivity of cardiac myocytes to the C9-mab. The endothelium of intramyocardial vessels also reacted with C9-ab and used as an internal control (arrow).
An excellent correlation was seen between the Apop-Tag and immunohistochemical analysis with anti-Lamin1B, indicating the reliability of the test. Moreover, by sequential immunostaining we examined if there was any accumulation of MCs in the vicinity of the ApopTag-positive cardiac myocytes in the heart sections. The results showed no significant accumulation of MCs into the neighborhood of the apoptotic myocytes.
Discussion
The most interesting result of this study is the production of cardiac TNF-α and different expression patterns of this cytokine, which was found to be synthesized in the cardiomyocytes. These findings are unexpected but logical, as it is known that TNF-α exerts endocrine, paracrine, and autocrine controls of inflammatory responses during the course of diseases (Sherry et al 1988). Moreover, according to our results healthy hearts showed almost absence of myocytic TNF-α expression.
An estimation of the correlations between the MCs, TNF-α, and apoptosis in the myocardium revealed that drug overdose victims exhibited significantly increased infiltration of MCs with a high degree of activation and degranulation, over-expression of cardiomyocytic TNF-α, mostly in patchy and diffuse form as defined in pattern type III, and an overall negativity for myocytic apoptosis. Moreover, the heart of the drug fatalities exhibited increased incidence of myocytic necrosis compared with SND. Examination of the myocardial sections in SND showed no degranulation of tissue MCs, relatively moderate TNF-α expression (%10 ± 2.7), and a significant number of cases exhibited myocytic apoptosis. The former result agrees with the result of one of our previous studies (Edston et al 2002).
Apoptosis is a time-dependent deterioration of the myocardium. Experimentally, after the initial stimulus, the first apoptotic phenomena are measurable 2 h after initiation, and the first signs of DNA fragmentation appear at 9 h after stimulation and subsequently, after 14 h apoptotic nuclei characterized by TUNEL/ApopTag (Rodriguez et al 2005). This indicates that in sudden death of cardiac or vascular origin, an ischemic or other challenge to the heart ought to have happened some time before the occurrence of the lethal ventricular arrhythmia. However, the lack of apoptosis in acute drug deaths indicates that such deaths occur very shortly after the drug injection. Unfortunately, those few cases of drug deaths that displayed some degree of apoptotic nuclei were unwitnessed deaths, where the duration of the agonal phase is not possible to ascertain. While the attachment and detachment of circulating complement components to cell surfaces occurs continuously, the binding to necrotic myocytes can be detected 1–3 h after the challenge (Edston 1997).
In addition, histopathological examination of the heart of SND cases demonstrated a significantly dissimilar profile despite the fact that the blood tryptase occasionally overlapped that of the drug victims with LLT. In accordance with the result of the previous study (Perskvist et al 2006), the mean number of MCs in the heart sections of victims who died from known causes, eg, suicide by hanging and traffic accidents, showed almost the same amount of infiltrated cells compared with that of the MCs in SND cases. Thus, the significant increase of infiltrated cells in the heart of drug victims might be a chronic effect of repeated intravenous drug injections.
Previously, we have demonstrated a significant augmentation of infiltrated MCs in heart sections of anaphylactic deaths, a high level of serum mast cell tryptase and a severe degree of pulmonary congestion and edema (Perskvist et al 2006). In this study histopathological analysis of HLT drug deaths shows similarities to that in victims of anaphylactic shock, supporting the hypothesis that the pathogenic mechanism behind the drug-related deaths involves systemic activation of mast cells, as the infiltration of MCs was significantly higher also in the heart and lung in this group. The degree of degranulation of MCs in the heart of the HLT was similar to that anaphylactic degranulation in the subjects of our previous study (Perskvist et al 2006). Thus, we suggest that in situ degranulation and discharge of MCs mediators into the interstitial spaces of the myocytes, where it may locally provoke coronary spasm, may contribute to more fatal cardiac disturbances than the density of the intact cells. Furthermore the lack of specific IgE against morphine in serum of heroin-related deaths gives no support for classic anaphylaxis in those cases. Also, the discovery that deaths by injection of psycho-stimulants (amphetamine) produced elevated tryptase values, and were morphologically indistinguishable from the heroin deaths, points to a non-IgE mediated mast cell activation. It cannot be ruled out that a few of our cases might have had an allergic disposition or an increased incidence of asthma. It has not been possible to extract such background information on the subjects of this study.
The action of TNF-α are mediated by its binding to one of two specific receptors, TNF-α receptor 1 and 2 express among the others on the surface of cardiomyocytes (Vassalli 1992). We propose that the different pattern of myocytic production of TNF-α may be dependent on the engagement or the level of expression of the type of TNF-α receptors, but this remains to be investigated.
In agreement with our finding, it has been demonstrated previously that the failing heart can produce TNF-α, which in excessive levels can promote left ventricular remodelling and may have negative inotropic effects (Torre-Amione 1995). Overproduction of TNF-α may aggravate the syndrome of heart failure by inducing peripheral deleterious responses. TNF-α receptors then can be detected and measured as soluble factors. These measurements give accurate estimation of the activity of TNF-α in chronic heart failure (Ferrari et al 1995). TNF-α is involved in the triggering and/or amplification of local inflammatory responses, eg, apoptotic cascade, related to ischemia-reperfusion injury in patients with acute myocardial infarction (Blancke et al 2005). We propose that repeated intravenous injection and drug intoxication both of opiates and psychostimulants induce a profound infiltration of MCs in the heart. In certain conditions an acute challenge may lead to fatal activation and degranulation of MCs, lung, and vascular tissues. Initially, release of histamine induces vasodilatation and increased vascular permeability causing hypotension and decreased cardiac output. Subsequently, vasoconstriction compounds are released locally and in the peripheral circulation. These mediators are able to induce coronary spasm progressing to acute myocardial ischemia, heart failure, and ventricular fibrillation. In addition, either the discharged MC-compounds or the circulating drug stimulate the overproduction of TNF-α in cardiomyocytes that contributes to the deterioration of the pumping function of the heart. Moreover, if the subject survives the initial events, TNF-α may induce an apoptotic and/or necrotic cascade of the myocytes and interstitial cells.
In conclusion the link between cardiac mast cells, TNF-α, and a number of pathological processes suggest that anti-histamine and anti-TNF-α therapies may have a beneficial effect on the cardiac effects and thus of morbidity and mortality after acute intravenous drug injection.
Acknowledgments
This study was supported by grants from the National Board of Forensic Medicine (no:X04-971126) and the Swedish Heart-Lung Foundation (no: 20060831). The study was approved by the Local Board of Medical Ethics no M116-05.
References
- Anker SD, Clark AL, Kemp M, et al. Tumor necrosis factor and steroid metabolism in chronic heart failure: possible relation to muscle wasting. J Am Coll Cardiol. 1997;30(4):997–1001. doi: 10.1016/s0735-1097(97)00262-3. [DOI] [PubMed] [Google Scholar]
- Blancke F, Claeys MJ, Jorens P, et al. Systemic inflammation and reperfusion injury in patients with acute myocardial infarction. Mediators Inflamm. 2005;2005(6):385–9. doi: 10.1155/MI.2005.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edston E. Evaluation of agonal artefacts in the myocardium using a combination of histological stains and immunohistochemistry. Am J Forensic Med Pathol. 1997;18(2):163–7. doi: 10.1097/00000433-199706000-00011. [DOI] [PubMed] [Google Scholar]
- Edston E, van Hage-Hamsten M. Anaphylactoid shock–a common cause of death in heroin addicts? Allergy. 1997;52:950–4. doi: 10.1111/j.1398-9995.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Edston E, Grontoft L, Johnsson J. TUNEL: a useful screening method in sudden cardiac death. Int J Legal Med. 2002;116:22–6. doi: 10.1007/s004140100254. [DOI] [PubMed] [Google Scholar]
- Edston E, Eriksson O, van Hage M. Mast cell tryptase in postmortem serum-reference values and confounders. Int J Legal Med. 2006;121(4):275–80. doi: 10.1007/s00414-006-0101-2. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53(1):31–47. doi: 10.1016/s0008-6363(01)00434-5. [DOI] [PubMed] [Google Scholar]
- Ferrari R, Bachetti T, Confortini R, et al. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92(6):1479–86. doi: 10.1161/01.cir.92.6.1479. [DOI] [PubMed] [Google Scholar]
- Freude B, Masters TN, Robicsek F, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32:197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346(6281):274–6. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Irani AM, Bradford TR, Kepley CL, et al. Detection of MCT and MCTC types of human mast cells by immunohistochemistry using new monoclonal anti-tryptase and anti-chymase antibodies. J Histochem Cytochem. 1989;37:1509–15. doi: 10.1177/37.10.2674273. [DOI] [PubMed] [Google Scholar]
- Kovanen PT, Kaartinen M, Paavonen T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation. 1995;92:1084–8. doi: 10.1161/01.cir.92.5.1084. [DOI] [PubMed] [Google Scholar]
- Lessa MA, Tibirica E. Pharmacologic evidence for the involvement of central and peripheral opioid receptors in the cardioprotective effects of fentanyl. Anesth Analg. 2006;103(4):815–21. doi: 10.1213/01.ane.0000237284.30817.f6. [DOI] [PubMed] [Google Scholar]
- Levine B, Kalman J, Mayer L, et al. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323(4):236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- Linden J. Cloned adenosine A3 receptors: pharmacological properties, species differences and receptor functions. Trends Pharmacol Sci. 1994;15(8):298–306. doi: 10.1016/0165-6147(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Perskvist N, Edston E. Differential accumulation of pulmonary and cardiac mast cell-subsets and eosinophils between fatal anaphylaxis and asthma death. postmortem comparative study. Forensic Sci Int. 2006;169(1):43–9. doi: 10.1016/j.forsciint.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Schaper J. Apoptosis: measurement and technical issues. J Mol Cell Cardiol. 2005;38:15–20. doi: 10.1016/j.yjmcc.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Rossig L, Haendeler J, Mallat Z, et al. Congestive heart failure induces endothelial cell apoptosis: protective role of carvedilol. J Am Coll Cardiol. 2000;36(7):2081–9. doi: 10.1016/s0735-1097(00)01002-0. [DOI] [PubMed] [Google Scholar]
- Schwartz LB, Yunginger JW, Miller J, et al. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989;83:1551–5. doi: 10.1172/JCI114051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B, Cerami A. Cachectin/tumor necrosis factor exerts endocrine, paracrine, and autocrine control of inflammatory responses. J Cell Biol. 1988;107(4):1269–77. doi: 10.1083/jcb.107.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram P, Ren G, Nagar H, et al. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J Pathol. 2005;205:102–11. doi: 10.1002/path.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W, Lima JA, Meng Q, et al. Long-term cocaine use is related to cardiac diastolic dysfunction in an African-American population in Baltimore, Maryland. Int J Cardiol. 2004;97(1):25–8. doi: 10.1016/j.ijcard.2003.06.024. [DOI] [PubMed] [Google Scholar]
- Torre-Amione G, Kapadia S, Lee J, et al. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92(6):1487–93. doi: 10.1161/01.cir.92.6.1487. [DOI] [PubMed] [Google Scholar]
- van Haelst PL, Timmer JR, Crijns HJ, et al. No long-lasting or intermittent mast cell activation in acute coronary syndromes. Int J Cardiol. 2001;78(1):75–80. doi: 10.1016/s0167-5273(00)00475-7. [DOI] [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–52. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Yao L, Baltatzis S, Zafirakis P, et al. Human mast cell subtypes in conjunctiva of patients with atopic keratoconjunctivitis, ocular cicatricial pemphigoid and Stevens-Johnson syndrome. Ocul Immunol Inflamm. 2003;11:211–22. doi: 10.1076/ocii.11.3.211.17353. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Ge JB, Chen JZ, et al. Mast cell contributes to cardiomyocyte apoptosis after coronary microembolization. J Histochem Cytochem. 2006;54(5):515–23. doi: 10.1369/jhc.5A6804.2005. [DOI] [PubMed] [Google Scholar]


