Abstract
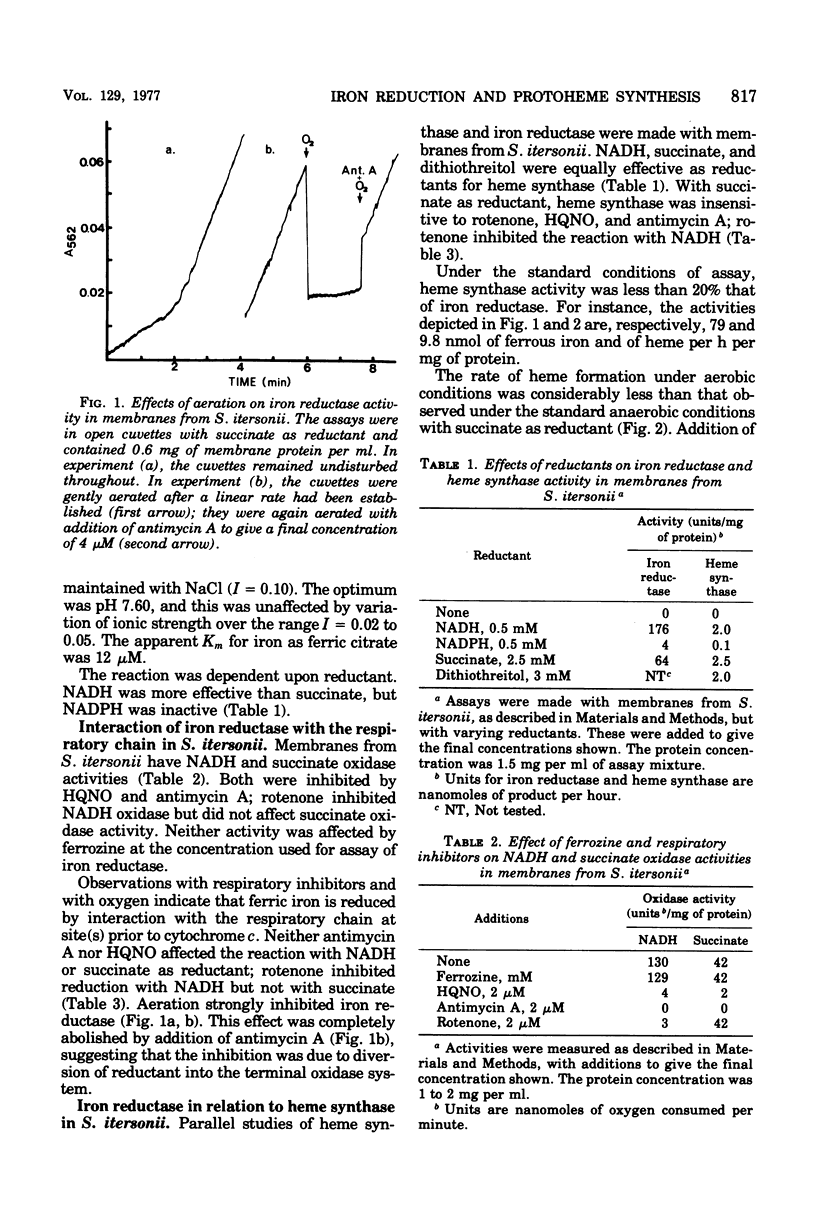
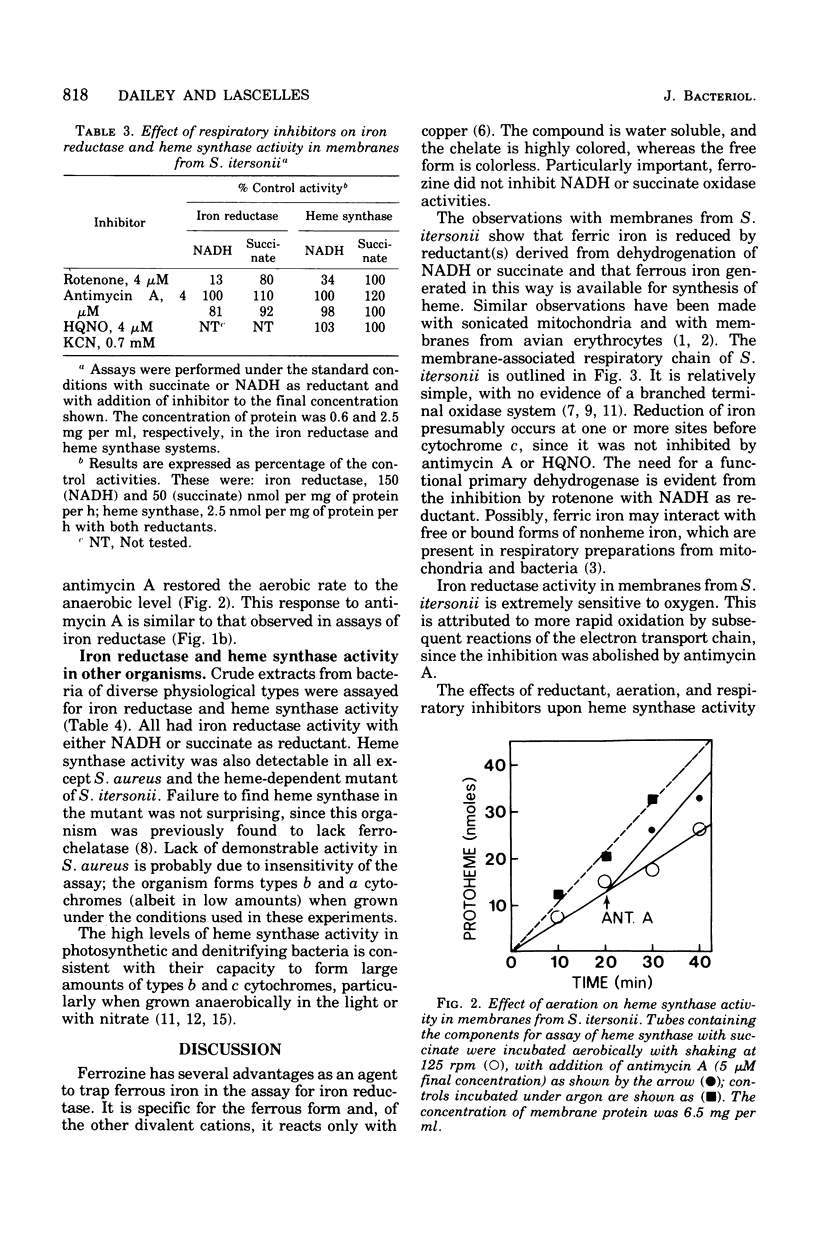
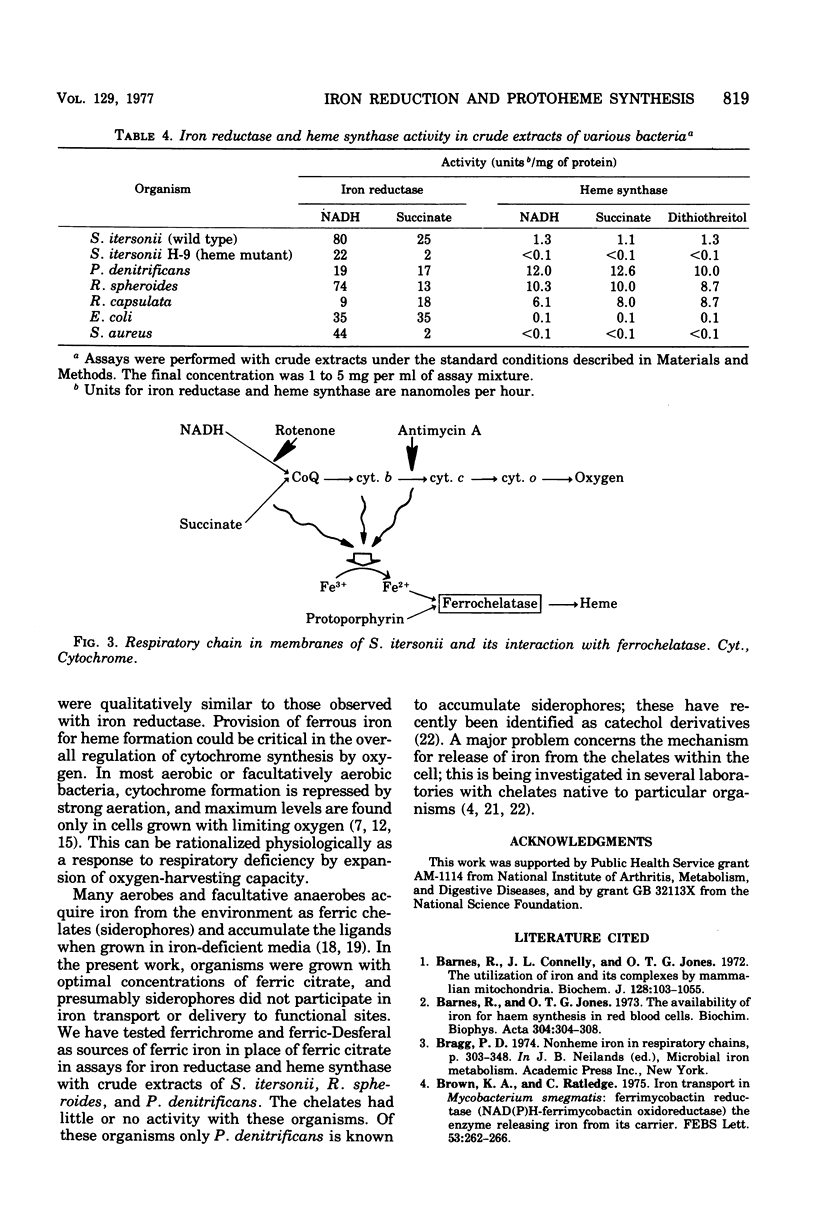
Membranes from Spirillum itersonii reduce ferric iron to ferrous iron with reduced nicotinamide adenine dinucleotide or succinate as a source of reductant. Iron reduction was measured spectrophotometrically at 562 nm using ferrozine, which chelates ferrous iron specifically. Reduced nicotinamide adenine dinucleotide or succinate was also effective as a source of iron. The effects of respiratory inhibitors suggested that reduction of iron occurs at one or more sites on the respiratory chain before cytochrome c. Reduction of iron and synthesis of protoheme with the physiological reductants were also observed with crude extracts of other bacteria, including Rhodopseudomonas spheroides, Rhodopseudomonas capsulata, Paracoccus denitrificans, and Escherichia coli. The effect of oxygen upon reduction of iron and formation of protoheme was examined with membranes from S. itersonii, using succinate as a source of reductant. Both systems were inhibited by oxygen, but this effect was completely reversed by addition of antimycin A. We conclude that reduced components of the respiratory chain serve as reductants for ferric iron, but with oxygen present they are oxidized preferentially by the successive members of the chain. This could be a mechanism for regulating synthesis of heme and cytochrome by oxygen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R., Connelly J. L., Jones O. T. The utilization of iron and its complexes by mammalian mitochondria. Biochem J. 1972 Aug;128(5):1043–1055. doi: 10.1042/bj1281043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes R., Jones O. T. The availability of iron for haem synthesis in red blood cells. Biochim Biophys Acta. 1973 Apr 28;304(2):304–308. doi: 10.1016/0304-4165(73)90248-1. [DOI] [PubMed] [Google Scholar]
- Brown K. A., Ratledge C. Iron transport in Mycobacterium smegmatis: ferrimycobactin reductase (nad(p)h:ferrimycobactin oxidoreductase), the enzyme releasing iron from its carrier. FEBS Lett. 1975 May 1;53(2):262–266. doi: 10.1016/0014-5793(75)80033-0. [DOI] [PubMed] [Google Scholar]
- Burke K. A., Lascelles J. Nitrate reductase system in Staphylococcus aureus wild type and mutants. J Bacteriol. 1975 Jul;123(1):308–316. doi: 10.1128/jb.123.1.308-316.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal Biochem. 1971 Apr;40(2):450–458. doi: 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Clark-Walker G. D., Rittenberg B., Lascelles J. Cytochrome synthesis and its regulation in Spirillum itersonii. J Bacteriol. 1967 Nov;94(5):1648–1655. doi: 10.1128/jb.94.5.1648-1655.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey H. A., Jr, Lascelles J. Ferrochelatase activity in wild-type and mutant strains of Spirillum itersonii. Solubilization with chaotropic reagents. Arch Biochem Biophys. 1974 Feb;160(2):523–529. doi: 10.1016/0003-9861(74)90429-9. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Jr Membrane-bound respiratory of Spirillum itersonii. J Bacteriol. 1976 Sep;127(3):1286–1291. doi: 10.1128/jb.127.3.1286-1291.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier D. K., Clark-Walker G. D., Garrard W. T., Jr, Lascelles J. Nitrate reductase and soluble cytochrome c in Spirillum itersonii. J Bacteriol. 1970 Jun;102(3):790–801. doi: 10.1128/jb.102.3.797-803.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. Ferrochelatase of Rhodopseudomonas spheroides. Biochem J. 1970 Sep;119(3):453–462. doi: 10.1042/bj1190453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. Ferrochelatase of spinach chloroplasts. Biochem J. 1968 Mar;107(1):113–119. doi: 10.1042/bj1070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lascelles J., Rittenberg B., Clark-Walker G. D. Growth and cytochrome synthesis in a hemin-requiring mutant of Spirillum itersonii. J Bacteriol. 1969 Jan;97(1):455–456. doi: 10.1128/jb.97.1.455-456.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascelles J. The regulation of heme and chlorophyll synthesis in bacteria. Ann N Y Acad Sci. 1975 Apr 15;244:334–347. doi: 10.1111/j.1749-6632.1975.tb41540.x. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra R. J., Langman L., Young I. G., Gibson F. The role of ferric enterochelin esterase in enterochelin-mediated iron transport and ferrochelatase activity in Escherichia coli. Arch Biochem Biophys. 1972 Nov;153(1):74–78. doi: 10.1016/0003-9861(72)90422-5. [DOI] [PubMed] [Google Scholar]
- Tait G. H. The identification and biosynthesis of siderochromes formed by Micrococcus denitrificans. Biochem J. 1975 Jan;146(1):191–204. doi: 10.1042/bj1460191. [DOI] [PMC free article] [PubMed] [Google Scholar]



