Abstract
The Group I family of metabotropic glutamate receptors includes subtype 1 (mGlu1) and subtype 5 (mGlu5) receptors. This family of receptors has generated interest as potential targets for different areas of therapeutic development, including intervention for alcohol and drug abuse. Most of this interest is driven by findings showing involvement of mGlu5 receptors in the regulation of drug self-administration; however, studies examining the role of mGlu1 receptors in drug self-administration are limited. The purpose of this work was to examine the role of mGlu1 receptor antagonism in the maintenance of ethanol self-administration and the self-administration of an alternate non-drug reward, sucrose. Male alcohol-preferring inbred (iP) rats were trained to self-administer ethanol (15% v/v) vs. water on a concurrent schedule of reinforcement, and the effect of the mGlu1 receptor antagonist JNJ16259685 (0.1 - 1.0 mg/kg IP) was evaluated on self-administration. The rats were then trained to self-administer sucrose (0.4% w/v) vs. water, and the same dose range of JNJ16259685 was tested. Locomotor activity was tested in a separate assessment to evaluate potential non-specific motor effects of the antagonist. Ethanol self-administration was dose-dependently reduced by JNJ16259685. This reduction was likely due to a motor impairment as the lowest effective dose (0.1 mg/kg) significantly reduced locomotor behavior. Sucrose self-administration was reduced by the highest JNJ16259685 dose (1.0 mg/kg), and this reduction was also likely due to a motor impairment. Interestingly, ethanol self-administration was more sensitive to mGlu1 receptor antagonism than sucrose self-administration as lower JNJ16259685 doses reduced ethanol reinforced-responding and motor behavior. Together these results suggest that mGlu1 receptors do not play a specific role in modulating ethanol self-administration, or the self-administration of an alternate non-drug reward (i.e., sucrose).
Keywords: ethanol self-administration, P-rat, JNJ16259685, mGlu1 antagonist, glutamate, reinforcement
Introduction
The Group I family of metabotropic glutamate receptors is made up of subtype 1 (mGlu1) and subtype 5 (mGlu5). These receptor subtypes stimulate phospholipase C and phosphoinositide hydrolysis (Abe et al., 1992; Houamed et al., 1991) and share common agonist pharmacological profiles (Conn and Pin, 1997). Group I mGlu receptors are widely expressed throughout the brain as determined from in situ hybridization and immunohistochemical work (for review see Ferraguti and Shigemoto, 2006). For example, mGlu1 receptors show intense expression in the cerebellum, and are also abundant in other regions such as the lateral septum, ventral pallidum, and thalamic nuclei (Hubert et al., 2001; Lavreysen et al., 2004a; Martin et al., 1992). mGlu5 receptors show intense expression in corticolimbic regions such as the nucleus accumbens, lateral septum, striatum, and hippocampus (Abe et al., 1992; Romano et al., 1995; Shigemoto et al., 1993).
Group I metabotropic glutamate receptors have generated interest as potential targets for different areas of therapeutic development (for reviews see Bordi and Ugolini, 1999; Gasparini et al., 2002). For example, antagonists of the Group I mGlu receptors show anxiolytic properties (Spooren et al., 2000; Steckler et al., 2005a), positive effects in models of nociception (Bhave et al., 2001; Sevostianova and Danysz, 2006), and may possess neuroprotective properties (Makarewicz et al., 2006; Szydlowska et al., 2007). In addition, mGlu5 receptors may be a viable target for therapeutic interventions in drug abuse as mGlu5 receptors have been shown to modulate cocaine, nicotine, and ethanol reinforcement (Bespalov et al., 2005; Hodge et al., 2006; Paterson et al., 2003; Tessari et al., 2004) and drug seeking behavior in reinstatement models (Backstrom et al., 2004; Backstrom and Hyytia, 2006; Bespalov et al., 2005; Schroeder et al., 2005).
In relation to drug reinforcement, studies examining the role of mGlu1 receptors are very limited. Antagonism of mGlu1 receptors with EMQMCM has been shown to reduce both cue-induced reinstatement and nicotine priming-induced reinstatement in rats trained to self-administer nicotine (Dravolina et al., 2007). In regard to ethanol reinforcement, the data published to date have shown mixed results. Work from our laboratory has shown no effect of mGlu1 receptor antagonism by CPCCOEt on ethanol self-administration in alcohol-preferring (P) rats and in mice (Hodge et al., 2006; Schroeder et al., 2005). In contrast, others have shown that CPCCOEt reduced ethanol self-administration in mice (Lominac et al., 2006). These studies do not conclusively suggest a role for mGlu1 receptors in ethanol reinforcement, and thus, further study is warranted.
Therefore, the goal of the present work was to further characterize the role of mGlu1 receptors in ethanol reinforcement using the recently available mGlu1 receptor antagonist 3,4-dihydro-2H-pyrano[2,3]b quinolin-7-yl) (cis-4-methoxycyclohexyl) methone (JNJ16259685). JNJ16259685 is a potent non-competitive mGlu1 receptor antagonist (Ki = 0.34 nM) that displays >1,000-fold selectivity over mGlu5 receptors in a Ca2+ mobilization assay (Lavreysen et al., 2004b). JNJ16259685 also displays no agonist, antagonist, or allosteric activity at mGlu2, mGlu3, mGlu4, or mGlu6 receptors (Lavreysen et al., 2004b). JNJ16259685 is centrally active after systemic administration and has been shown to modulate learning and memory, and anxiety, at doses ranging from 0.63 - 10 mg/kg (Steckler et al., 2005a; Steckler et al., 2005b). Thus, examining the effects of a highly potent, selective, and behaviorally active mGlu1 antagonist has the potential to further elucidate the role of these receptors in ethanol reinforcement. In addition, the mGlu1 receptor antagonist was also tested on sucrose reinforcement as a measure of reinforcer specificity. Finally, locomotor assessments were conducted to assess potential motor disturbances by mGlu1 receptor antagonism alone and to address possible pharmacological interactions with ethanol.
Materials and Methods
Subjects
Male alcohol-preferring inbred (iP) rats (n=10) were derived from a line provided by Indiana University. This stock of inbred P rats (5B substrain) was derived from breeders of the selected line of P rats originally provided in 1999 by Indiana University (courtesy of Dr. T.K. Li) and has been bred on-site at the University of North Carolina at Chapel Hill. The rats were housed in pairs in Plexiglas cages with water and food available continuously unless otherwise mentioned. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. At the beginning of testing, rats weighed 514 ± 6.09 g (mean ± S.E.M.). All procedures were carried out in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Research Council, National Academy Press, 1996) and institutional guidelines.
Apparatus
The self-administration chambers (30.5 cm × 24.1 cm × 21.0 cm; Med Associates, Georgia, VT) were located within sound-attenuating cubicles. Each cubicle was equipped with an exhaust fan that provided ventilation and masked external sounds. The left and right wall of each chamber contained one liquid receptacle and a response lever (Med Associates). Lever press responses activated a syringe pump (Med Associates) that delivered 0.1 ml of solution into the receptacle across a 1.66-s period. A stimulus light located above each response lever was illuminated during pump activation. Lever presses during reinforcer delivery were recorded, but produced no programmed consequence. The chambers were interfaced (Med Associates) to a computer that was programmed to control sessions and record data.
Four clear Plexiglas chambers (48 × 26 × 20 cm) were used to assess locomotor activity. Each chamber was divided into 4 equal sections by markings on the outside wall of the length of the chamber. The locomotor sessions were videotaped and the number of line crosses was subsequently quantified by an observer blind to drug treatment.
Procedure
Ethanol self-administration training sessions (30 min) were conducted 5 days per week (M-F). Rats were trained using a sucrose fading method to self-administer ethanol (15% v/v) vs. water on a concurrent FR1-FR1 schedule of reinforcement. That is, one lever response activated a syringe pump that delivered 0.1 ml of the appropriate solution into a liquid receptacle (e.g., left lever responses resulted in ethanol delivery; right lever responses resulted in water delivery). At the time of testing, the rats had 68 sessions of ethanol 15% (v/v) vs. water self-administration.
Effect of mGluR1 Antagonism on ethanol self-administration and motor activity
Rats were administered JNJ16259685 (0, 0.1, 0.3, 1.0 mg/kg, IP) and returned to the home cage. 40 min later, rats were placed in the chambers for a self-administration session (30 min). These test sessions were interspersed with training sessions with at least 2 self-administration sessions between tests. The order of JNJ16259685 dose was randomized.
After the JNJ16259685 evaluation on ethanol self-administration was completed, locomotor activity was tested in a separate assessment. The lowest effective JNJ16259685 dose (0.1 mg/kg) that reduced ethanol self-administration was evaluated to determine whether the reductions in ethanol self-administration were the result of a motor impairment and/or a result of a pharmacological interaction between the consumed ethanol and the antagonist. To examine the possibility of whether the antagonist interacted with the consumed ethanol, the dose of consumed ethanol at the time at which the antagonist effect emerged was estimated. That is, the reductions in ethanol self-administration emerged after 15 min (between 15 min - 20 min), corresponding to an approximate ethanol intake of 0.73 g/kg. Rats were injected with vehicle or JNJ16259685 (0.1 mg/kg) and returned to the home cage. 50 min later, rats were injected with saline or ethanol (0.7 g/kg) and returned to the home cage for 10 min. The goal of this injection protocol was to parallel the conditions of the self-administration session as closely as possible. Thus, the rats were injected with saline/ethanol at what would correspond to min 10 of the self-administration session and returned to the home cage for 10 min to allow for a rise in blood ethanol, and then locomotor activity was tested for 10 min (e.g. correspond to min 20 - 30 of the self-administration session the time at which the antagonist reduced ethanol responding). Rats experienced each drug combination in a random order such that activity wasmonitored in 4 separate sessions. These locomotor test sessions were interspersed with self-administration sessions with at least 2 days between tests. Self-administration sessions were withheld on the days of the locomotor assessments.
Effect of mGluR1 Antagonism on sucrose self-administration and motor activity
Once testing of the antagonists on ethanol self-administration and locomotor activity was complete, sucrose self-administration training began. That is, sucrose replaced ethanol as the reinforcer and from this point forward the rats no longer received exposure to ethanol. All parameters for the sucrose self-administration sessions were identical to the ethanol self-administration sessions and sucrose reinforcement was paired with the same lever with which ethanol had been paired. At the time of antagonist testing, rats had 56 sessions of sucrose (0.4%, w/v) self-administration. This concentration of sucrose was chosen because it produced comparable levels of responding as 15% (v/v) ethanol (see later). The effects of JNJ16259685 (0, 0.1, 0.3, 1 mg/kg IP) were tested on sucrose self-administration (30 min session). The same testing protocol as described for ethanol self-administration was used for these tests.
After the JNJ16259685 evaluation on sucrose self-administration was completed, locomotor activity was tested. Given that the dose of JNJ16259685 (0.1 mg/kg) that reduced locomotor activity did not alter sucrose self-administration, we sought to determine whether the rats overcame a motor deficit during the sucrose self-administration sessions, or whether reinforcer history changed locomotor response to JNJ16259685. Rats were injected with vehicle or JNJ16259685 (0.1 mg/kg) and returned to the home cage. 1 h later rats were placed in the locomotor monitoring chambers and activity was monitored for 10 min (i.e., duration between antagonist administration and placement in the chamber is identical to the initial assessment). In order to determine whether the JNJ16259685 (1.0 mg/kg)-induced reduction in sucrose self-administration was due to a motor impairment, that dose was also tested using the same testing protocol.
Follow-up ethanol self-administration assessment
After 74 sucrose self-administration (0.4%, w/v) sessions, rats were returned to ethanol self-administration (15% v/v). All parameters for these self-administration sessions were identical to the previous ethanol and sucrose self-administration sessions and ethanol reinforcement was paired with the same lever sucrose had been paired and ethanol had been previously paired. After 15 ethanol self-administration sessions, a lower dose range of JNJ16259685 was tested (0, 0.01, 0.03, 0.3 mg/kg IP). The same testing procedures as previously described were used for this follow-up assessment.
Drugs
For self-administration, ethanol (95% w/v) was diluted in distilled water. For systemic injection, ethanol (95% w/v) was diluted in saline (0.9%) to 20% (v/v) and injected at different volumes to achieve the appropriate dosage (0.7 g/kg). JNJ16259685 (Tocris, Ellisville, MO) was suspended in a 0.1% carboxymethylcellulose vehicle and injected IP at a volume of 1 ml/kg. JNJ16259685 dose selection and pretreatment interval was made based on published work (Steckler et al., 2005a; Steckler et al., 2005b).
Data Analysis
For self-administration tests, two-way repeated measures (RM) analysis of variance (ANOVA) were used to analyze total session responses and cumulative responses across the sessions. Ethanol intake (g/kg) was determined from body weight and the number of reinforcers delivered and was analyzed by a one-way RM ANOVA. A one-way RM ANOVA was used to compare baseline ethanol/water and sucrose/water responses. For locomotor assessments, RM ANOVAs were used to examine JNJ16259685/ethanol effects on locomotor behavior (i.e., number of line crosses). Tukey post hoc comparisons were conducted to extract significant main effects and interactions. Statistical significance was declared at p<0.05.
Results
Ethanol reinforcement and motor activity
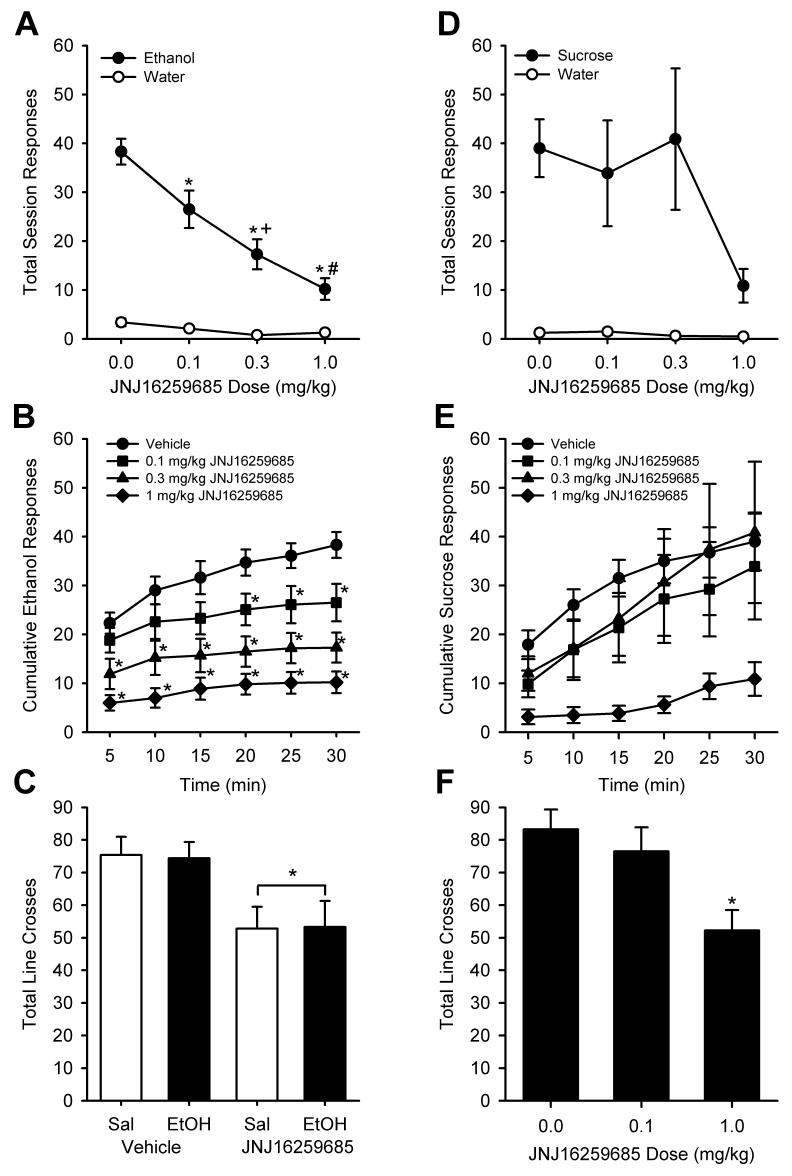
Average baseline data (mean ± S.E.M.) for the 2 days preceding testing of JNJ16259685 on ethanol self-administration was as follows: ethanol responses (30.75 ± 3.50), water responses (7.45 ± 1.45), ethanol intake (0.65 ± 0.07 g/kg). JNJ16259685 significantly reduced total session responding for ethanol (15% v/v) reinforcement (Figure 1A). The two-way RM ANOVA showed a significant main effect of lever with greater responses on the ethanol lever (F(1,9)=117.01, p<0.001), a main effect of JNJ16259685 pretreatment (F(3,27)=23.80, p<0.001), and a significant interaction (F(3,27)=17.75, p<0.001). JNJ16259685 significantly reduced ethanol responses at every dose tested relative to vehicle treatment (ps<0.001). Further, this reduction in ethanol-reinforced responding was dose-dependent (ps<0.05). No changes in water responses were evident. Ethanol intake (g/kg) was also reduced by JNJ16259685 pretreatment (F(3,33)=15.78, p<0.001), with significantly reduced ethanol intake at the 0.3 (0.32 ± 0.07 g/kg) and 1 mg/kg (0.18 ± 0.04 g/kg) JNJ16259685 doses relative to vehicle (0.69 ± 0.1 g/kg). To examine the pattern of ethanol responding across the 30-min session, cumulative ethanol responses were examined (Figure 1B). There was a significant main effect of time (F(5,45)=37, p<0.001), a significant main effect of JNJ16259685 dose (F(3,27)=20.01, p<0.001), and a significant interaction (F(15,35)=5.49, P<0.001). The two highest doses of JNJ16259685 (0.3 and 1 mg/kg) significantly reduced ethanol responding throughout the session (ps<0.02). The lowest JNJ16259685 dose (0.1 mg/kg) induced a reduction in ethanol responses that emerged after 15 min and continued for the duration of the 30-min session (ps<0.03). In the locomotor assessment, JNJ16259685 (0.1 mg/kg) significantly reduced the number of line crosses (F(1,9)=11.78, p=0.007; Figure 1C). Ethanol (0.7 g/kg) did not alter motor activity. Further, the lack of a significant interaction shows that JNJ16259685 (0.1 mg/kg) treatment reduced motor behavior in general, and did not produce a behavioral interaction with ethanol.
Figure 1.
Panel A. Mean (± SEM) responses on the ethanol (15% v/v) and water levers after JNJ16259685 pretreatment (N=10). Panel B. Mean (± SEM) cumulative ethanol responses across the 30-min self-administration session after JNJ16259685 pretreatment (N=10). Panel C. Mean (± SEM) line crosses during the 10-min locomotor assessment after pretreatment with vehicle/JNJ16259685 (0.1 mg/kg) followed by saline/ethanol (0.7 g/kg) administration (N=10). Panel D. Mean (± SEM) responses on the sucrose (0.4% w/v) and water levers after JNJ16259685 pretreatment (N=8). Panel E. Mean (± SEM) cumulative sucrose responses across the 30-min self-administration session after JNJ16259685 pretreatment (N=8). Panel F. Mean (± SEM) line crosses during the 10-min locomotor assessment after pretreatment with JNJ16259685 (N=8). * Indicates significant difference from vehicle; + indicates significant difference from 0.1 mg/kg JNJ16259685; # indicates significant difference from 0.3 mg/kg JNJ16259685 (Tukey, p<0.05).
Sucrose reinforcement and motor activity
Average baseline data (mean ± S.E.M.) for the 2 days preceding testing of JNJ16259685 on sucrose self-administration was as follows: sucrose responses (41.05 ± 4.58), water responses (1.90 ± 0.56). In comparing the baseline responses between ethanol and sucrose training, the RM ANOVA showed a significant difference in responses (F(3,26)=37.92, p<0.001), which was driven by significantly greater responses on the ethanol and sucrose levers relative to the water levers (ps<0.001). Importantly, there was no difference between the number of ethanol and sucrose reinforced responses or the corresponding water responses under the two conditions. Total session sucrose (0.4% w/v) and water responses after JNJ16259685 pretreatment are shown in Figure 1D. Due to problems with the water delivery system on one of the cages, data from 2 rats are not included due to inconsistent performance on 2 test days and intervening training sessions. The two-way RM ANOVA showed a significant main effect of lever with greater responses on the sucrose lever (F(1,7)=26.54, p=0.001). Cumulative sucrose responses were examined across the 30-min JNJ16259685 test sessions (Figure 1E). There was a significant main effect of dose (F(3,21)=3.53, p=0.03), with a significant reduction in sucrose-reinforced responding by the highest JNJ16259685 dose (1 mg/kg) relative to vehicle (p=0.03). There was also a significant main effect of time (F(5,35)=18.18, p<0.001). There was no significant interaction. Locomotor activity as measured by the number of line crosses was significantly reduced by 1 mg/kg JNJ16259685 (F(2,14)=11.46, p=0.001; Figure 1F).
Follow-up ethanol reinforcement assessment
On the first day of return to ethanol self-administration, total responses (mean ± S.E.M.) on the ethanol and water levers were 42.7 ± 5.27 and 1.6 ± 0.9, respectively, with mean (± S.E.M.) ethanol intake of 0.77 (± 0.05 g/kg). The number of ethanol responses did not differ from ethanol responses on the final day of ethanol self-administration exposure (mean ± S.E.M.; 47.3 ± 5.55). Baseline responding (mean ± S.E.M.) on the 2 ethanol self-administration sessions preceding testing of JNJ16259685 was as follows: ethanol responses (27.1 ± 3.33); water responses (5.75 ± 4.37). Ethanol and water responses with JNJ16259685 pretreatment are shown in Table 1. The two-way RM ANOVA showed a significant main effect of lever (F(1,8)=219.85, p<0.001), and a significant main effect of JNJ16259685 dose (F(3,24)=5.39, p=0.006), with a significant reduction in self-administration by 0.3 mg/kg JNJ16259685 relative to vehicle (p<0.003), consistent with the previous assessment. No other JNJ16259685 dose produced a reduction in ethanol self-administration.
Table 1.
Total session lever responses for the follow-up ethanol reinforcement assessment (mean ± S.E.M.)
| JNJ16259685 Dose (mg/kg) | ||||
|---|---|---|---|---|
| Lever | 0 | 0.01 | 0.03 | 0.3* |
| Ethanol | 26.40 ± 3.46 | 23.30 ± 3.25 | 21.30 ± 2.74 | 14.80 ± 2.74 |
| Water | 2.5 ± 0.87 | 1.00 ± 0.70 | 2.0 ± 0.56 | 0.60 ± 0.31 |
Denotes significant main effect of JNJ 16259685 dose relative to vehicle (Tukey, p<0.05).
Discussion
In the present work, blockade of mGlu1 receptors with JNJ16259685 reduced ethanol self-administration at doses (0.1 and 0.3 mg/kg JNJ16259685) that did not alter sucrose self-administration. This data pattern suggests that ethanol-reinforced responding is more sensitive to mGlu1 receptor antagonism than sucrose-reinforced responding. However, the doses of the mGlu1 receptor antagonist that reduced ethanol and sucrose self-administration also reduced locomotor activity, suggesting that the reductions in self-administration of both reinforcers were likely due to non-specific motor impairments. Interestingly, in addition to differential effects on ethanol and sucrose self-administration, differential response to the same dose of the antagonist in the locomotor assessments was also observed. Together, these findings suggest that rather than a selective reduction of the antagonist to ethanol reinforcement, the differential response can be explained by differential locomotor response to the antagonist.
Antagonism of mGlu1 receptors produced a significant reduction in ethanol self-administration. Examination of the pattern of ethanol responding across the 30-min session showed that reductions in ethanol self-administration by the two highest JNJ16259685 doses (0.3 and 1 mg/kg) were evident by the first 5 min of the session and continued throughout the session. In contrast, reductions in ethanol self-administration induced by the lowest JNJ16259685 dose (0.1 mg/kg) did not emerge until after the first 15 min of the self-administration session. A possible explanation for this reduction in self-administration later into the session is that the antagonist may have interacted with the ethanol that had been consumed during the session. A pharmacological interaction between the consumed ethanol and the antagonist may have produced an alteration in the stimulus properties of the consumed ethanol or a significant reduction in motor behavior, both of which could result in a reduction in ethanol self-administration. Indeed, antagonism of mGlu5 receptors (also a member of the Group I mGluR family) has been shown to inhibit the discriminative stimulus properties of self-administered and investigator-administered ethanol (Besheer and Hodge, 2005; Besheer et al., 2006); however, to date, the role of mGlu1 receptors has not been examined.
Given the high level of mGlu1 expression in the cerebellum, the separate evaluation of motor behavior is critical to the interpretation of the results of the present study. Indeed, antagonism of mGlu1 receptors by JNJ16259685 has been reported to produce motor impairments (Steckler et al., 2005a). Accordingly, the purpose of the locomotor assessment was two-fold: 1) to test if JNJ16259685 (0.1 mg/kg) interacted with ethanol (0.7 g/kg - the estimated consumed dose) to produce a motor impairment, and 2) to test if JNJ16259685 (0.1 mg/kg) alone produced a motor impairment. Importantly, to obtain a relevant assessment of motor activity the assessment occurred at the time point during which reductions in ethanol-reinforced responding emerged (i.e., 20 min after the start of the session). JNJ16259685 (0.1 mg/kg) produced a motor impairment which was not altered by ethanol administration, suggesting that the antagonist did not interact with the consumed ethanol to reduce motor behavior. However, given the overall reduction in motor behavior by JNJ16259685 administration, we conclude that the JNJ16259685 (0.1 mg/kg)-induced reduction in ethanol-reinforced responding that emerged after the first 15 min of the session was likely due to a motor impairment.
In order to assess the effects of mGlu1 antagonism on an alternate reinforcer, JNJ16259685 was tested on the maintenance of sucrose self-administration. Sucrose-reinforced responding was reduced by the highest JNJ16259685 dose (1.0 mg/kg). However, the JNJ16259685-induced reduction in sucrose-reinforced responding was likely due to a motor impairment, as that dose (1.0 mg/kg) significantly reduced locomotor behavior. Thus, as with ethanol self-administration, the reduction in sucrose self-administration cannot be dissociated from a non-specific motor impairment.
Interestingly, ethanol self-administration was more sensitive to mGlu1 receptor antagonism than sucrose self-administration. That is, the JNJ16259685 dose that reduced ethanol self-administration and motor activity (0.1 mg/kg), had no effect on sucrose self-administration or motor activity. This finding suggests that sensitivity to the antagonist may have changed. A change in sensitivity to the antagonist may be due to repeated testing of the antagonist. However, this explanation is unlikely given that upon re-exposure to ethanol self-administration in the follow-up experiment JNJ16259685 (0.3 mg/kg) reduced self-administration as in the initial assessment. Another plausible explanation is that reinforcer history (i.e., ethanol self-administration) produced changes in mGlu1 receptor systems and/or function which could consequently alter sensitivity to the antagonists. Indeed, chronic exposure to ethanol (i.e., liquid diet) has been shown to reduce mGlu1 receptor mRNA levels (Simonyi et al., 2004; Simonyi et al., 1996); however, the functionality of these changes has not yet been determined. Future experiments examining the effects of ethanol self-administration and abstinence on mGlu1 receptor expression/function may provide insight into differential response to an mGlu1 receptor antagonist.
A limitation of the present work is that in the initial assessment on ethanol self-administration, a dose of JNJ16259685 that did not alter self-administration was not tested. This became especially relevant after we found that the effective JNJ16259685 dose (0.1 mg/kg) was ineffective in altering sucrose reinforced-responding or motor activity. In the follow-up experiment (i.e., return to ethanol self-administration) lower doses of JNJ16259685 (0.01 and 0.03 mg/kg) did not reduce ethanol self-administration, while confirming that ethanol self-administration was reduced by 0.3 mg/kg JNJ16259685 as in the initial assessment.
A unique feature of this experiment is that ethanol self-administration was assessed after a period of abstinence from ethanol that occurred without a concurrent absence from the environment. In the follow-up experiment (i.e., return to ethanol self-administration), on the first ethanol re-exposure day, ethanol self-administration returned to previous baseline levels. An increase in ethanol self-administration after a period of abstinence as predicted by the alcohol deprivation effect (Sinclair and Senter, 1968) was not observed. However, given the length of absence from ethanol self-administration (90 self-administration sessions), an alcohol deprivation effect may not be predicted (see Rodd et al., 2003). Thus, an advantage of the present design is that there was no corresponding absence from the self-administration environment/situation which parallels the demonstrations of the alcohol deprivation effect using home cage two-bottle drinking protocols (Colombo et al., 2004; McKinzie et al., 1998; Vengeliene et al., 2005), in which increased ethanol drinking has been shown in P rats after 8 weeks of abstinence from alcohol (Rodd-Henricks et al., 2000). However, in home cage studies animals are not given access to an alternate reinforcer (e.g., sucrose) during the abstinence period as was the procedure in the present work (i.e., sucrose self-administration).
In sum, antagonism of mGlu1 receptors by JNJ16259685 reduced ethanol and sucrose self-administration. Interestingly, the antagonist produced differential effects on ethanol and sucrose self-administration as well as motor activity, with increased sensitivity to the antagonist during ethanol self-administration and the subsequent motor assessment. This data pattern suggests a change in sensitivity to the antagonist which could be due to neuroadaptations in mGlu1 receptor systems produced by ethanol self-administration; however, this explanation will have to be examined in future studies. Importantly, the antagonist-induced reductions in ethanol- and sucrose-reinforced responding cannot be dissociated from non-specific motor effects as the effective doses also produced motor impairments in separate assessments. Thus, under the present conditions, mGlu1 receptors do not play a major role in modulating ethanol self-administration, or the self-administration of an alternate non-drug reward (i.e., sucrose).
Acknowledgements
This work was supported by Grants AA016009 to JB and AA014983 and AA011605 to CWH from the National Institute on Alcohol Abuse and Alcoholism and by the Bowles Center for Alcohol Studies. The authors would like to thank Dr. David H. Overstreet for breeding and providing the P-rats.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kimpel MW, Rodd ZA, Strother WN, Bai F, Peper CL, Mayfield RD, Lumeng L, Crabb DW, McBride WJ, Witzmann FA. Protein expression changes in the nucleus accumbens and amygdala of inbred alcohol-preferring rats given either continuous or scheduled access to ethanol. Alcohol. 2006;40:3–17. doi: 10.1016/j.alcohol.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;(49 Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau R. W. t. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bordi F, Ugolini A. Group I metabotropic glutamate receptors: implications for brain diseases. Prog Neurobiol. 1999;59:55–79. doi: 10.1016/s0301-0082(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Colombo G, Addolorato G, Agabio R, Carai MA, Pibiri F, Serra S, Vacca G, Gessa GL. Role of GABA(B) receptor in alcohol dependence: reducing effect of baclofen on alcohol intake and alcohol motivational properties in rats and amelioration of alcohol withdrawal syndrome and alcohol craving in human alcoholics. Neurotox Res. 2004;6:403–414. doi: 10.1007/BF03033315. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Floyd DW, Friedman DP, Daunais JB, Pierre PJ, Grant KA, McCool BA. Long-term ethanol self-administration by cynomolgus macaques alters the pharmacology and expression of GABAA receptors in basolateral amygdala. J Pharmacol Exp Ther. 2004;311:1071–1079. doi: 10.1124/jpet.104.072025. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Kuhn R, Pin JP. Allosteric modulators of group I metabotropic glutamate receptors: novel subtype-selective ligands and therapeutic perspectives. Curr Opin Pharmacol. 2002;2:43–49. doi: 10.1016/s1471-4892(01)00119-9. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O’Hara PJ, Mulvihill ER, Almers W, Hagen FS. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J Neurosci. 2001;21:1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavreysen H, Pereira SN, Leysen JE, Langlois X, Lesage AS. Metabotropic glutamate 1 receptor distribution and occupancy in the rat brain: a quantitative autoradiographic study using [3H]R214127. Neuropharmacology. 2004a;46:609–619. doi: 10.1016/j.neuropharm.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Lavreysen H, Wouters R, Bischoff F, Nobrega Pereira S, Langlois X, Blokland S, Somers M, Dillen L, Lesage AS. JNJ16259685, a highly potent, selective and systemically active mGlu1 receptor antagonist. Neuropharmacology. 2004b;47:961–972. doi: 10.1016/j.neuropharm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Makarewicz D, Duszczyk M, Gadamski R, Danysz W, Lazarewicz JW. Neuroprotective potential of group I metabotropic glutamate receptor antagonists in two ischemic models. Neurochem Int. 2006;48:485–490. doi: 10.1016/j.neuint.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, Li TK. The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- Nestby P, Vanderschuren LJ, De Vries TJ, Mulder AH, Wardeh G, Hogenboom F, Schoffelmeer AN. Unrestricted free-choice ethanol self-administration in rats causes long-term neuroadaptations in the nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 1999;141:307–314. doi: 10.1007/s002130050838. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, Li TK. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcohol Clin Exp Res. 2000;24:8–16. [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O’Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neuroscience Letters. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Christian MR, Sun AY, Sun GY. Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcohol Clin Exp Res. 2004;28:1419–1423. doi: 10.1097/01.alc.0000139825.35438.a4. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Zhang JP, Sun AY, Sun GY. Chronic ethanol on mRNA levels of IP3R1, IP3 3-kinase and mGluR1 in mouse Purkinje neurons. Neuroreport. 1996;7:2115–2118. doi: 10.1097/00001756-199609020-00010. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Spooren WP, Vassout A, Neijt HC, Kuhn R, Gasparini F, Roux S, Porsolt RD, Gentsch C. Anxiolytic-like effects of the prototypical metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)pyridine in rodents. J Pharmacol Exp Ther. 2000;295:1267–1275. [PubMed] [Google Scholar]
- Steckler T, Lavreysen H, Oliveira AM, Aerts N, Van Craenendonck H, Prickaerts J, Megens A, Lesage AS. Effects of mGlu1 receptor blockade on anxiety-related behaviour in the rat lick suppression test. Psychopharmacology (Berl) 2005a;179:198–206. doi: 10.1007/s00213-004-2056-7. [DOI] [PubMed] [Google Scholar]
- Steckler T, Oliveira AF, Van Dyck C, Van Craenendonck H, Mateus AM, Langlois X, Lesage AS, Prickaerts J. Metabotropic glutamate receptor 1 blockade impairs acquisition and retention in a spatial Water maze task. Behav Brain Res. 2005b;164:52–60. doi: 10.1016/j.bbr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Kaminska B, Baude A, Parsons CG, Danysz W. Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur J Pharmacol. 2007;554:18–29. doi: 10.1016/j.ejphar.2006.09.061. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]