Abstract
Background
Prazosin, a CNS active alpha-1 adrenoreceptor antagonist, has reduced nightmares and sleep disturbance in placebo controlled studies of combat-related PTSD. We evaluated objective sleep parameters and PTSD symptoms in a placebo-controlled prazosin trial for civilian trauma-related PTSD.
Methods
Thirteen outpatients with chronic civilian trauma PTSD, frequent nightmares and sleep disturbance participated in a randomized placebo-controlled crossover trial of prazosin. Sleep parameters were quantified at home with the REMView. PTSD symptoms were quantified with the CAPS “recurrent distressing dreams” and “disturbed sleep” items, a non-nightmare distressed awakenings scale, the PTSD Dream Rating Scale (PDRS), the PTSD Checklist-Civilian (PCL-C) and the Clinical Global Impression of Improvement (CGI-I).
Results
Prazosin compared to placebo significantly increased total sleep time by 94 minutes; increased REM sleep time and mean REM period duration without altering sleep onset latency; significantly reduced trauma-related nightmares, distressed awakenings and total PCL scores; significantly improved CGI-I scores; and changed PDRS scores towards normal dreaming.
Conclusions
Prazosin reductions of nighttime PTSD symptoms in civilian trauma PTSD are accompanied by increased total sleep time, REM sleep time and mean REM period duration in the absence of a sedative-like effect on sleep onset latency.
Trauma-related nightmares and sleep disturbance are distressing and often treatment resistant symptoms of posttraumatic stress disorder (PTSD) (1, 2, 3). Increased central nervous system (CNS) adrenergic activity may contribute to the pathophysiology of PTSD in general (4, 5) and to PTSD nighttime symptoms specifically (6). Increased CNS adrenergic activity in PTSD could interfere with normal REM sleep (7) as suggested by increased phasic motor activity, stage shifts and arousals during REM sleep (8, 9) and REM fragmentation during early PTSD development (10). REM disruption could contribute to trauma nightmares (10, 11) and interfere with the normal cognitive processing of traumatic events (12).
Prazosin is a generic alpha-1 adrenergic antagonist that is centrally active (13). Prazosin is a promising treatment for trauma nightmares and sleep disturbance in PTSD (14, 15, 16). However, prazosin effects on objective measures of sleep in PTSD have not been investigated. We used the REMView portable sleep assessment device to quantify effects of prazosin on sleep physiology (17, 18).
Methods and Materials
This study ran from 2004 to 2005 and was approved by the Western Institutional Review Board and subjects provided written informed consent. Subjects were outpatients in the clinic of F.T. who met DSM-IV criteria for PTSD, scored at least 40 on the PTSD Checklist-Civilian Version (PCL-C) (19), at least 4 (of a maximum of 8) on the Clinician Administered PTSD Scale (CAPS) (20) “recurrent distressing dreams” item, and at least 4 on the CAPS “difficulty falling asleep/staying asleep” item. Medical history and examination revealed all subjects in good general health and without restless leg syndrome, narcolepsy, and alcohol or other substance abuse for at least three months.
To eliminate subjects likely to develop alpha-1 adrenoreceptor antagonist “first dose hypotension” (21), each consented subject was prescribed a single blind 1 mg “test dose” of prazosin one week prior to randomization. Of 18 subjects, two experienced subjectively uncomfortable orthostatic dizziness following the “test dose” and were excluded. Three subjects withdrew consent prior to randomization because they decided against research participation (n = 2) or because they left the area (n = 1).
All 13 randomized subjects (11 women; 49 ± 10 years of age [mean age ± SD]) completed ratings in both conditions. Most relevant traumas included childhood sexual abuse (n = 5), childhood physical abuse (n = 3), adult assault (n = 3), rape (n = 1), and life-threatening motor vehicle accident (n = 1). Comorbid psychiatric disorders included major depressive disorder (n = 10, 3 in remission), alcohol abuse in remission (n = 3, mean remission duration = 8.5 ± 7.4 years), obsessive-compulsive disorder (n = 1), and panic disorder (n = 1). Mean PCL-C score at entry was 59 ± 13. All 13 randomized subjects maintained ongoing psychotherapy unaltered through the trial. Eleven subjects were receiving one or more maintenance psychotropic medications. These included sertraline (n = 8, mean dose = 100 ± 41 mg/day), duloxetine (n = 2, mean dose = 70 ± 46 mg/day), and alprazolam (n = 3, mean dose = 0.38 ± 0.18 mg/day). Doses had been stable for at least six weeks and continued unchanged during the trial.
This 7-week 2-period 2-treatment study satisfied criterion for a classic crossover design (22). Subjects completed random order 3-week treatments of indistinguishable capsules of prazosin and placebo separated by a 1-week washout period. To maintain the blind, random allocation, sequence and medications were determined, distributed and tracked by the study coordinator (PM). Drug was initiated at 1 mg of prazosin (or placebo) at bedtime. Over the next 10 days prazosin (or placebo) were titrated upward by 1 mg increments every two to three days to achieve a therapeutic effect with a minimum of adverse effects. Rapid titration was to enhance subject retention. Prazosin dose achieved (mean 3.1 ± 1.3 mg, range 2–6 mg) or placebo dose achieved (equivalent to 3.2 ± 1.2 mg, range 2–5 mg) were then maintained for 11 days. Phone contact for adverse effect query was made on the morning after drug initiation and after each dose increase through titration phases.
During the last three nights of each treatment, participants wore a REMView (Respironics, Inc.). This battery-operated portable 4.5 ounce device includes head and eye movement sensors that define sleep vs. awake and REM vs. non-REM sleep stages. It has high agreement with polysomnography measures (17, 18). Total sleep time, REM sleep time and the number of REM sleep periods were generated by REMView software.
Psychiatric and behavioral rating scales (relevant to previous week) were performed by the blinded clinician (FBT) at the first baseline (prior to the first drug trial arm), on the last day of the first drug trial arm, at the second baseline (last day of the washout period) and on the last day of the second drug trial arm. Rating scales included the CAPS “recurrent distressing dreams” (primary measure) and “sleep disturbance” items, a non-nightmare distressed awakenings (NNDA) scale, (a modification of the CAPS “distressing dreams” item that substitutes “NNDA” for “distressing dreams”) (23), the subject-rated PCL-C, the Clinical Global Impression-Improvement (CGI-I) (24), and the civilian version of the PTSD Dream Rating Scale (PDRS) (25) that assesses pathologic PTSD dream content vs. normal dream content. Adverse effects were assessed with the Systematic Assessment for Treatment Emergent Events (SAFTEE) (26). Sitting heart rate, systolic and diastolic blood pressure were obtained on the last day of each treatment condition.
All three nights of home REMView data in each treatment condition were averaged. Fifty-five nights (29 during prazosin, and 26 during the placebo condition) of interpretable REMView data were available for 10 of the 13 participants. Three subjects’ data were unavailable because of incorrect lead placement, technical failure or battery power loss.
Sample size was estimated by examining the number of subjects used to achieve the effect size in our 2003 crossover study (16). Repeated-measures analyses of variance (ANOVAs) were performed to evaluate the interaction of change over time with treatment condition. Significance level was p < 0.05. We looked for violations of assumptions of the repeated measures ANOVA test, including order, carryover, and treatment by period effects, and none were found. Effect sizes were calculated using Cohen’s d.
Results
Prazosin Effects on REMView Sleep Parameters
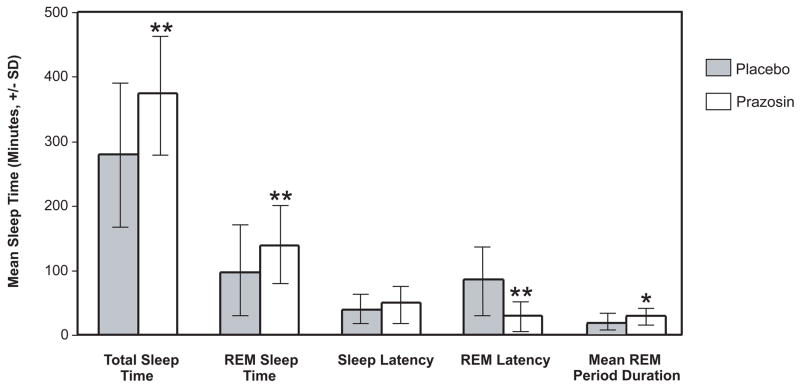
Total sleep time was greater in the prazosin than placebo condition (374 ± 86 vs. 280 ± 105 minutes, p < 0.01, Cohen’s d = .98) as was REM sleep time (138 ± 63 vs. 97 ± 70 minutes, p < 0.01 Cohen’s d = .62) (Figure 1). REM latency was less in the prazosin condition (85 ± 62 minutes vs. 30 ± 20 minutes, p < 0.05, Cohen’s d = 1.2). REM period duration (total REM time/night divided by number of REM periods/night) was greater in the prazosin than in the placebo condition (27 ± 9 vs. 18 ± 9 minutes, p < 0.05). Sleep onset latency did not differ between conditions.
Figure 1.
Effects of Prazosin vs. Placebo on Sleep Measures in PTSD Subjects (n=10)
* Significant difference between prazosin and placebo groups by repeated measures ANOVA, *p<0.05; **p<0.01.
Prazosin Effects on Nighttime and Overall PTSD Symptoms
Reductions from baseline were greater in the prazosin than placebo condition for the CAPS distressing dreams item, NNDA, and total PCL-C score (all p < 0.05) (Table 1). The CGI-I also favored prazosin (p < 0.05). PDRS scores indicated a significant change toward normal dream content during prazosin.
Table 1.
Treatment Effects on Clinical Outcome Measures in 13 Persons with Civilian Trauma PTSD
| Outcome Measure (mean ± SD) | Placebo Baseline | Placebo End | Prazosin Baseline | Prazosin End | P valuea | Effect size (Cohen’s d) |
|---|---|---|---|---|---|---|
| CAPS “recurrent distressing dreams” (item #2) | 3.9 ± 2.3 | 3.9 ± 1.9 | 4.8 ± 1.7 | 3.3 ± 2.3 | 0.04 | .96 |
| CAPS “difficulty falling asleep/staying asleep” (item #13) | 6.1 ± .8 | 5.6 ± 1.4 | 6.0 ± 1.0 | 4.8 ± 1.8 | .35 | .50 |
| Non-nightmare distressed awakenings | 3.3 ± 3.4 | 3.2 ± 3.8 | 4.6 ± 3.5 | 1.8 ± 1.7 | 0.05 | 1.2 |
| CGI-I scoresb | 4.1 ± 1.1 | 2.6 ± 0.9 | .002 | 1.5 | ||
| PTSD Dream Rating Scale | 14.9 ± 9.6 | 14.4 ± 10.0 | 22.3 ± 6.8 | 11.7 ± 9.4 | .006 | 1.4 |
| PCL-C | 56 ± 16 | 55 ± 15 | 58 ± 13 | 51 ± 14 | .025 | .79 |
Analysis of variance, significance of group by time interaction.
1 = markedly improved, 2 = moderately improved, 3 = minimally improved, 4 = unchanged, 5 = minimally worse, 6 = moderately worse, 7 = markedly worse.
Adverse events did not differ between conditions. Dizziness occurred 3 times in both the placebo and prazosin conditions. Sitting systolic blood pressure at end treatment did not differ between prazosin and placebo conditions (140 ± 23 vs. 133 ± 14 mmHg, p = 0.5), but diastolic blood pressure was lower (78 ± 6 vs. 82 ± 5 mmHg, p = 0.03) and resting heart rate higher (73 ± 3 vs. 70 ± 3, p = 0.03) in the prazosin condition.
Discussion
PTSD symptomatic and sleep physiology results during prazosin treatment are consistent with involvement of enhanced responsiveness of CNS alpha-1 adrenoreceptors in PTSD trauma nightmares and sleep disruption. These prazosin effects on sleep physiology are consistent with several studies in animals. Disruption of REM sleep by the alpha-1 adrenoreceptor agonist methoxamine was reversed by prazosin in several studies (27, 28, 29). REM sleep disruption induced by increasing CNS adrenergic activity with the NE reuptake inhibitor desipramine was reversed by prazosin but not by the beta adrenoreceptor antagonist propranolol (30).
The current study is the only placebo-controlled PTSD drug treatment trial to have evaluated effects on objective sleep measures. The 94 minute increase in total sleep time during prazosin treatment in the current study is substantially greater than that demonstrated for any sedative hypnotic in a placebo-controlled trial for primary insomnia (31, 32). Although inferences from such comparisons are limited by likely differences between the physiology of sleep disturbance in PTSD and in primary insomnia, there are no published sleep physiology data from trials of sedative hypnotics in a PTSD sample.
Study limitations include the small sample size and the heterogeneity of the subjects’ trauma stresses, age of trauma occurrence, and duration of PTSD symptoms. Determination of blood pressure responses to postural change may have revealed greater prazosin effects on blood pressure. Although subjects’ maintenance psychotropic medications were held constant during the trial, differences among these drugs may have had differential effects on baseline sleep and noradrenergic function. For example, subjects taking the norepinephrine reuptake inhibitor duloxetine may have increased their baseline noradrenergic activity. Despite these limitations, home REMView monitoring revealed large effects of prazosin on sleep physiology that were consistent with subjects’ ratings of their PTSD symptoms and global clinical status. Studies of longer duration in larger samples are necessary to confirm these preliminary findings.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support of the VA Clinical Science Research and Development Service, Barry Anton, Dale Howard, Susan Martin, and the study participants. This work was supported by the Department of Veterans Affairs and the National Institute of Mental Health grant number MH069867.
The authors reported no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman MJ. Future pharmacotherapy for post-traumatic stress disorder: prevention and treatment. Psychiatr Clin North Am. 2002;25:427–441. doi: 10.1016/s0193-953x(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 2.van Liempt S, Vermetten E, Geuze E, Westenberg HGM. Pharmacotherapy for disordered sleep in posttraumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21:193–202. doi: 10.1097/00004850-200607000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 4.Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, et al. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- 5.Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, et al. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- 6.Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- 7.Chu NS, Bloom FE. Activity patterns of catecholamine-containing pontine neurons in the dorso-lateral tegmentum of unrestrained cats. J Neurobiol. 1974;5:527–544. doi: 10.1002/neu.480050605. [DOI] [PubMed] [Google Scholar]
- 8.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Motor dysfunction during sleep in posttraumatic stress disorder. Sleep. 1994;17:723–732. doi: 10.1093/sleep/17.8.723. [DOI] [PubMed] [Google Scholar]
- 9.Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T. Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry. 2004;61:508–516. doi: 10.1001/archpsyc.61.5.508. [DOI] [PubMed] [Google Scholar]
- 10.Mellman TA, Kulick-Bell R, Ashlock L, Nolan B. Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:110–115. doi: 10.1176/ajp.152.1.110. [DOI] [PubMed] [Google Scholar]
- 11.Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B. REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry. 2002;159:1696–1701. doi: 10.1176/appi.ajp.159.10.1696. [DOI] [PubMed] [Google Scholar]
- 12.Stickgold R. Of Sleep, Memories and Trauma. Nat Neurosci. 2007;10(5):540–542. doi: 10.1038/nn0507-540. [DOI] [PubMed] [Google Scholar]
- 13.Menkes DB, Baraban JM, Aghajanian GK. Prazosin selectively antagonizes neuronal responses mediated by alpha1-adrenoceptors in brain. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:273–5. doi: 10.1007/BF00503830. [DOI] [PubMed] [Google Scholar]
- 14.Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 15.Taylor F, Raskind MA. The α1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacology. 2002;22:82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbances in combat veterans with posttraumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Ajilore O, Stickgold R, Rittenhouse CD, Hobson JA. Nightcap: laboratory and home-based evaluation of a portable sleep monitor. Psychophysiology. 1995;32:92–98. doi: 10.1111/j.1469-8986.1995.tb03410.x. [DOI] [PubMed] [Google Scholar]
- 18.Edinger JD, Means MK, Stechuchak KM, Olsen MK. A pilot study of inexpensive sleep-assessment devices. Beh Sleep Med. 2004;2:41–49. doi: 10.1207/s15402010bsm0201_4. [DOI] [PubMed] [Google Scholar]
- 19.Weathers FW, Litz BT, Herman JA, Huska JA, Keane TM. The PTSD Checklist: reliability, validity, and diagnostic utility. Presented at the annual meeting of the International Society for Traumatic Stress Studies; San Antonio, Texas. October 24–27, 1993. [Google Scholar]
- 20.Blake DD, Weathers FW, Nagy LM, Kaloupeck DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–80. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 21.Physician’s Desk Reference. 55. Medical Economics Company; 2001. pp. 2501–02. [Google Scholar]
- 22.Laska EM, Klein DF, Laveri DW, Levine J, Robinson DS. Design issues for the clinical evaluation of psychotropic drugs. In: Prien RF, Robinson DS, editors. Clinical Evaluation of Psychotropic Drugs: Principles and Guidelines. New York: Raven Press; 1994. pp. 29–68. [Google Scholar]
- 23.Thompson C, Taylor FB, Jennings D, McFall M, Peskind ER, Raskind MA. A comparison of severely distressed nocturnal awakenings with and without trauma-relevant nightmares in male veterans with combat PTSD and the efficacy of prazosin in reducing both: a chart review. Presented at the International Society for Traumatic Stress Studied Annual Scientific Meeting; Toronto, Canada. November 2005. [Google Scholar]
- 24.Lehmann E. Practicable and valid approach to evaluate the efficacy of nootropic drugs by means of rating scales. Pharmacopsychiatry. 1984;17:71–85. doi: 10.1055/s-2007-1017411. [DOI] [PubMed] [Google Scholar]
- 25.Esposito K, Benitez A, Barza L, Mellman TA. Evaluation of dream content in combat-related PTSD. J Trauma Stress. 1999;12:681–687. doi: 10.1023/A:1024725319777. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson AF, Goldstein BJ, Dominquez RA, Steinbook RM. Interrater agreement and reliability measures of SAFTEE: general inquiry vs. systematic inquiry. Psychopharmacol Bull. 1987;23:97–101. [PubMed] [Google Scholar]
- 27.Hilakivi I, Leppavuori A. Effects of methoxamine, an alpha-1 adrenoreceptor agonist, and prazosin, an alpha-1 antagonist, on the stages of the sleep-waking cycle in the cat. Acta Physiol Scand. 1984;120:363–372. doi: 10.1111/j.1748-1716.1984.tb07396.x. [DOI] [PubMed] [Google Scholar]
- 28.Pellejero T, Monti JM, Baglietto J, Jantos H, Pazos S, Cichevski V, et al. Effects of methoxamine and alpha-adrenoceptor antagonists, prazosin and yohimbine, on the sleep-wake cycle of the rat. Sleep. 1984;7:365–372. doi: 10.1093/sleep/7.4.365. [DOI] [PubMed] [Google Scholar]
- 29.Cirelli C, Tononi G, Pompeiano M, Pompeiano O, Gennari A. Modulation of desynchronized sleep through microinjection of alpha 1-adrenergic agonists and antagonists in the dorsal pontine tegmentum of the cat. Pflugers Arch. 1992;422:273–279. doi: 10.1007/BF00376213. [DOI] [PubMed] [Google Scholar]
- 30.Ross RJ, Gresch PJ, Ball WA, Sanford LD, Morrison AR. REM sleep inhibition by desipramine: evidence for an alpha-1 adrenergic mechanism. Brain Res. 1995;701:129–34. doi: 10.1016/0006-8993(95)00984-x. [DOI] [PubMed] [Google Scholar]
- 31.Nowell PD, Mazumdar S, Buysse DJ, Dew MA, Reynolds CF, Kupfer DJ. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–2177. [PubMed] [Google Scholar]
- 32.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia. Arch Intern Med. 2004;164:1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.