Abstract
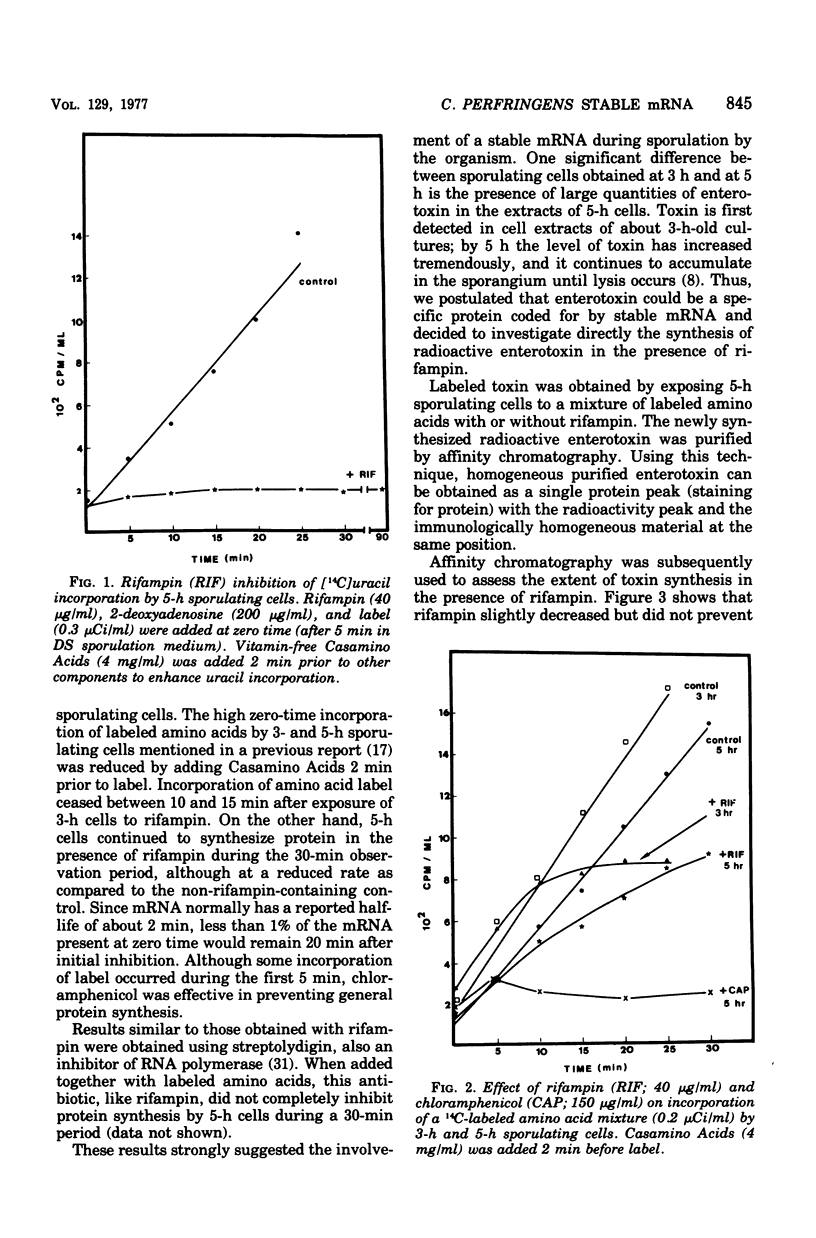
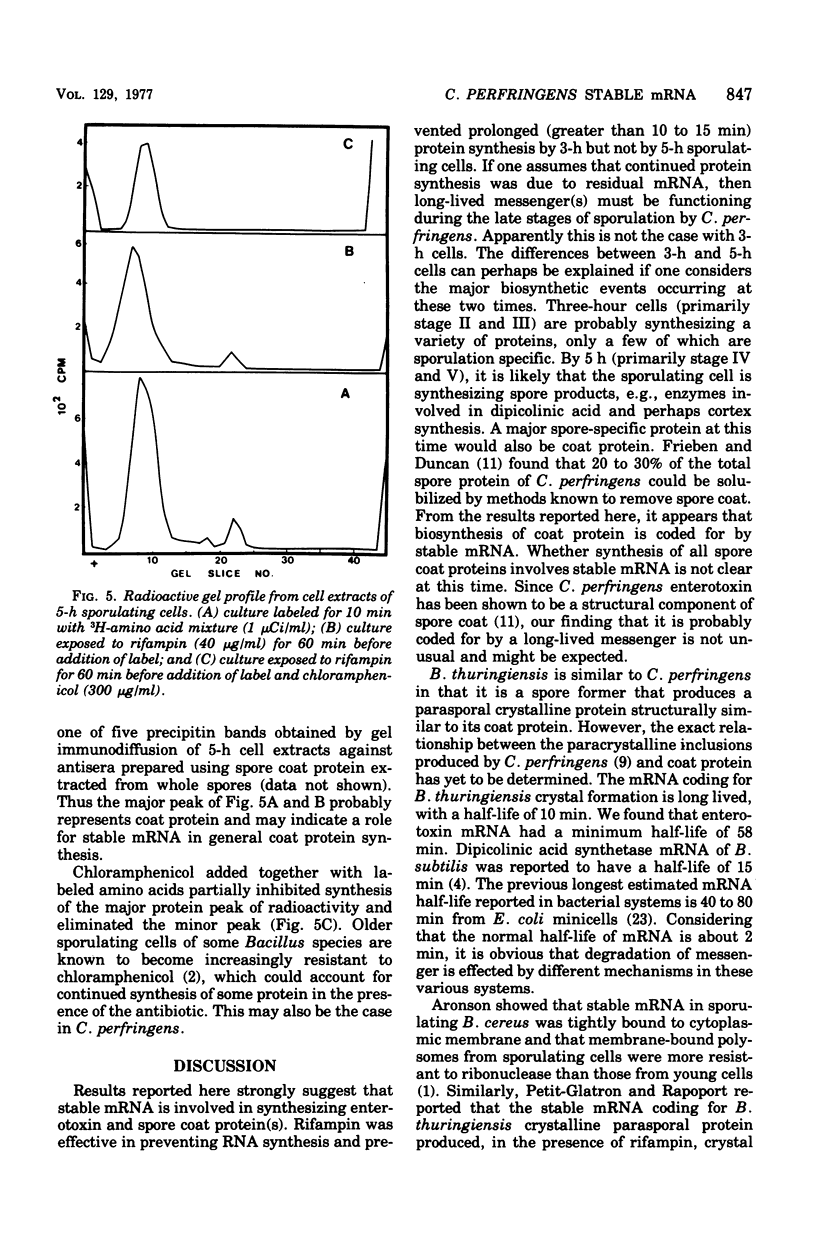
Stable messenger ribonucleic acid (mRNA) was shown to be involved in both enterotoxin synthesis and synthesis of other spore coat proteins in Clostridium perfringens. When used at a concentration that inhibited [14C]uracil incorporation, rifampin, a specific inhibitor of deoxyribonucleic acid-dependent RNA polymerase, prevented incorporation of a mixture of labeled amnoo acids by 3-h sporulating cells. At that time, enterotoxin protein was first detectable and cells were primarily at stage II or III of sporulation. When rifampin or streptolydigin was added to 5-h sporulating cells, which were primarily at stage IV or V and had significant toxin levels, incorporation of labeled amino acids continued through 30 min despite its presence. Rifampin also failed to prevent the specific synthesis of enterotoxin, a structural protein of the spore coat. The half-life of enterotoxin RNA was estimated to be at least 58 min. When cell extracts from 5-h sporulating cells that had been exposed to 3H-labeled amino acids for 10 min were subjected to electrophoresis on polyacrylamide gels and the gels were subsequently analyzed for radioactivity, two major peaks of radioactivity were obtained. The two peaks corresponded to enterotoxin and another spore coat protein(s). Similar results were obtained when the cells had been preincubated for 60 min with rifampin before label addition, indicating the functioning of stable mRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON A. I., ROSASDELVALLE M. RNA AND PROTEIN SYNTHESIS REQUIRED FOR BACTERIAL SPORE FORMATION. Biochim Biophys Acta. 1964 Jun 22;87:267–276. doi: 10.1016/0926-6550(64)90222-1. [DOI] [PubMed] [Google Scholar]
- Aronson A. Membrane-bound messenger RNA and polysomes in sporulating bacteria. J Mol Biol. 1965 Aug;13(1):92–104. doi: 10.1016/s0022-2836(65)80082-1. [DOI] [PubMed] [Google Scholar]
- Balassa G. Synthèse et fonction des ARN messagers au cours de la sporulation de Bacillus subtilis. Ann Inst Pasteur (Paris) 1966 Feb;110(2):175–191. [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DEL VALLE M. R., ARONSON A. I. Evidence for the synthesis of stable informational RNA required for bacterial spore formation. Biochem Biophys Res Commun. 1962 Nov 27;9:421–425. doi: 10.1016/0006-291x(62)90027-x. [DOI] [PubMed] [Google Scholar]
- Duncan C. L., King G. J., Frieben W. R. A paracrystalline inclusion formed during sporulation of enterotoxin-producing strains of Clostridium perfringens type A. J Bacteriol. 1973 May;114(2):845–859. doi: 10.1128/jb.114.2.845-859.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Strong D. H. Improved medium for sporulation of Clostridium perfringens. Appl Microbiol. 1968 Jan;16(1):82–89. doi: 10.1128/am.16.1.82-89.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L. Time of enterotoxin formation and release during sporulation of Clostridium perfringens type A. J Bacteriol. 1973 Feb;113(2):932–936. doi: 10.1128/jb.113.2.932-936.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieben W. R., Duncan C. L. Heterogeneity of enterotoxin-like protein extracted from spores fo Clostridium perfringens type A. Eur J Biochem. 1975 Jul 1;55(2):455–463. doi: 10.1111/j.1432-1033.1975.tb02182.x. [DOI] [PubMed] [Google Scholar]
- Frieben W. R., Duncan C. L. Homology between enterotoxin protein and spore structural protein in Clostridium perfringens type A. Eur J Biochem. 1973 Nov 15;39(2):393–401. doi: 10.1111/j.1432-1033.1973.tb03137.x. [DOI] [PubMed] [Google Scholar]
- HARRIS H., SABATH L. D. INDUCED ENZYME SYNTHESIS IN THE ABSENCE OF CONCOMITANT RIBONUCLEIC ACID SYNTHESIS. Nature. 1964 Jun 13;202:1078–1080. doi: 10.1038/2021078a0. [DOI] [PubMed] [Google Scholar]
- Horn D., Aronson A. I., Golub E. S. Development of a quantitative immunological assay for the study of spore coat synthesis and morphogenesis. J Bacteriol. 1973 Jan;113(1):313–321. doi: 10.1128/jb.113.1.313-321.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINTHAL C., KEYNAN A., HIGA A. Messenger RNA turnover and protein synthesis in B. subtilis inhibited by actinomycin D. Proc Natl Acad Sci U S A. 1962 Sep 15;48:1631–1638. doi: 10.1073/pnas.48.9.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Sporulation and enterotoxin production by Clostridium perfringens type A under conditions of controlled pH and temperature. Can J Microbiol. 1974 Nov;20(11):1493–1501. doi: 10.1139/m74-233. [DOI] [PubMed] [Google Scholar]
- Labbe R. G., Duncan C. L. Synthesis of deoxyribonucleic acid, ribonucleic acid, and protein during sporulation of Clostridium perfringens. J Bacteriol. 1976 Feb;125(2):444–452. doi: 10.1128/jb.125.2.444-452.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Leighton T. Further studies on the stability of sporulation messenger ribonucleic acid in Bacillus subtilis. J Biol Chem. 1974 Dec 25;249(24):7808–7812. [PubMed] [Google Scholar]
- Lester W. Rifampin: a semisynthetic derivative of rifamycin--a prototype for the future. Annu Rev Microbiol. 1972;26:85–102. doi: 10.1146/annurev.mi.26.100172.000505. [DOI] [PubMed] [Google Scholar]
- Martinez R. J. The formation of bacterial flagella. II. The relative stability of messenger RNA for flagellin biosynthesis. J Mol Biol. 1966 May;17(1):10–17. doi: 10.1016/s0022-2836(66)80090-6. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R. THE DIFFERENTIAL EFFECT OF ACTINOMYCIN D ON THE BIOSYNTHESIS OF ENZYMES IN BACILLUS SUBTILIS AND BACILLUS CEREUS. Biochim Biophys Acta. 1963 Sep 17;76:80–93. [PubMed] [Google Scholar]
- Pearce S. M., Fitz-James P. C. Spore refractility in variants of Bacillus cereus treated with actinomycin D. J Bacteriol. 1971 Jul;107(1):337–344. doi: 10.1128/jb.107.1.337-344.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., PREVIC E. P., GILLESPIE M. E. MESSENGER RNA AND POLYRIBOSOMES IN BACILLUS MEGATERIUM. J Mol Biol. 1965 May;12:119–129. doi: 10.1016/s0022-2836(65)80286-8. [DOI] [PubMed] [Google Scholar]
- SZULMAJSTER J., CANFIELD R. E., BLICHARSKA J. [Action of actinomycin D on the sporulation of Bacillus subtilis]. C R Hebd Seances Acad Sci. 1963 Feb 25;256:2057–2060. [PubMed] [Google Scholar]
- Scott V. N., Duncan C. L. Affinity chromatography purification of Clostridium perfringens enterotoxin. Infect Immun. 1975 Sep;12(3):536–543. doi: 10.1128/iai.12.3.536-543.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhikol C., Erbstoeszer J. W., Weisblum B. Mode of action of streptolydigin. J Bacteriol. 1969 Jul;99(1):151–155. doi: 10.1128/jb.99.1.151-155.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R. L., Duncan C. L. Biological characteristics of Clostridium perfringens type A enterotoxin. Infect Immun. 1971 Aug;4(2):89–96. doi: 10.1128/iai.4.2.89-96.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


