Abstract
Objective
To determine the proportion of women with primary ovarian insufficiency who achieve normal serum LH levels on transdermal estradiol therapy.
Design
Prospective
Setting
National Institutes of Health Clinical Center
Patient(s)
Women with spontaneous primary ovarian insufficiency (N=137) and 70 regularly menstruating control women (N=70).
Main Outcome Measure(s)
Serum LH
Intervention(s)
Blood sampled from controls in the mid-follicular phase and from patients while off estradiol for 2 weeks, then again 3 months later during the estradiol-only phase of replacement (100 micrograms/day estradiol patch, oral medroxyprogesterone acetate 10 mg for 12 days each month).
Result(s)
While on transdermal estradiol therapy significantly more women (51.1%, 70/137, 95% confidence interval 42%, 60%) had serum LH levels in the normal range (5/137, 3.9% at baseline). Mean (SD) serum estradiol level significantly increased on therapy to 95.4 (84.9) pg/mL.
Conclusion(s)
A regimen of 100 microgram per day of transdermal estradiol replacement achieves normal serum LH levels in approximately one-half of women with spontaneous primary ovarian insufficiency. Theoretically, by avoiding inappropriate luteinization, physiologic estradiol replacement therapy might improve follicle function in these women. Controlled studies to assess the effect of transdermal estradiol replacement on follicle function in these women are warranted.
Keywords: transdermal estradiol therapy, premature ovarian failure, premature menopause, primary ovarian insufficiency, primary hypogonadism, hypergonadotropic hypogonadism, LH, FSH, hormone replacement therapy
Introduction
It has been well established that many young women with spontaneous premature ovarian failure have remaining ovarian follicles that may function intermittently even years after the diagnosis (1-3). There are many reports in the literature of pregnancy developing after the diagnosis has been clearly established. Many of these pregnancies occurred during or soon after estrogen therapy, suggesting that this might be a method to improve fertility in these women (4-6).
Premature ovarian failure, previously known as premature menopause, has been defined as the development of hypergonadotropic hypogonadism before the age of 40 years, which is two standard deviations below the mean age of natural menopause (7-9). This condition is associated with amenorrhea, symptoms of estrogen deficiency, and high gonadotropin levels. The incidence is approximately 1 in 250 by age 35 and 1 in 100 by age 40 (10). In reality the term “premature ovarian failure” is problematic because it implies the permanent cessation of ovarian function. In fact, many women with this condition experience intermittent ovarian function that may last for decades after the diagnosis. Pregnancy may occur in some women many years after the diagnosis (11). Our preferred term for the condition is “primary ovarian insufficiency” (POI) as first introduced by Fuller Albright in 1942 (12).
Normally ovarian follicles grow in response to FSH stimulation then the mid-cycle LH surge induces follicle rupture, ovulation, terminal differentiation of granulosa cells into luteal cells, and formation of the corpus luteum (13). In POI the normal process of ovulation usually fails to occur despite the presence of antral follicles in up to 78% of women with this disorder (2,3). Many of these follicles fail to function normally because they become lutienized prematurely due to the associated chronically elevated serum LH levels (2,3). This process is akin to what has been termed the “lutienized unruptured follicle syndrome.” (14-16).
Theoretically estrogen replacement therapy might improve ovulation rates in women with spontaneous POI by reducing the associated chronically elevated serum LH levels to normal. One randomized controlled trial of oral estradiol replacement therapy (2 mg per day) assessing this found no significant improvement (3). However, the specific agent and route of estrogen administration might differentially affect gonadotropin levels and ovulation rates. The purpose of this study was to define the percentage of women with spontaneous POI who achieve normal mid-follicular serum LH levels on a standard regimen of continuous transdermal estradiol therapy.
Subjects and Methods
Subjects
Between January 2000 and November 2004, we recruited 137 women with spontaneous POI and 70 control women of similar age. The study was approved by the Institutional Review Board of the National Institute of Child Health and Human Development, National Institutes of Health (Bethesda, MD). All women provided written informed consent.
Women with primary ovarian insufficiency
Study patients had to meet the following inclusion criteria: (1) diagnosis of spontaneous POI before the age of 40 years (i.e., at least 4 months of oligo-amenorrhea and two FSH levels in the menopausal range, confirmed on two separate occasions, at least 1 month apart); (2) age between 18 and 42; (3) no iatrogenic cause or known chromosomal abnormality; and (4) no contraindication for hormone therapy. Screening baseline evaluation was previously described (17). Due to an administrative error baseline values for hormone measurements on 11 patients were missing.
Controls
Control women were healthy, non-pregnant, and menstruating regularly (cycles between 21 and 35 days) using no chronic medication or hormonal contraception. They were between the ages of 18 and 42 years.
Protocol
Women with POI were seen for two visits: (1) a baseline screening evaluation at which time they had been off any estrogen/progestin hormone therapy for at least two weeks, and (2) after receiving a standardized hormone regimen for at least 3 months consisting of a 100 mcg estradiol patch (Vivelle Dot, Novartis, East Hanover, NJ) and cyclic oral medroxyprogesterone acetate (10 mg for 12 days/month) (Provera, Pharmacia and Upjohn, Kalamazoo, MI).
For women with spontaneous POI, blood samples were drawn at baseline (off any estrogen/progestin therapy for at least two weeks) and after at least 3 months on the estradiol/progestin hormone therapy (during the estradiol-only phase of the hormone cycle; not while on progestin). For control women, blood samples were drawn during the mid-follicular phase (days 5-12).
Hormone Assays
FSH and LH were measured by microparticle enzyme immunoassay (AxSYM System, Abbott Diagnostics, Abbott Park, IL). FSH intra-assay CV was 4.9% and the inter-assay CV was 6.5%; for LH these were 5.8% and 6.4%, respectively. Estradiol was measured by competitive chemiluminescence immunoassay (Immulite 2000 analyzer, Diagnostic Products Corporation, Los Angeles, California); intra-assay and inter-assay CV were <11.0%) (To convert from the conventional unit (pg/mL) to the SI unit (pmol/L), multiply by 3.671.)
Statistical Analysis
Results are presented as mean±SD. All p-values are two-tailed and were considered significant when <0.05. SAS version 6.12 (SAS Institute, Cary, NC) was used for all analyses. Paired comparisons of clinical characteristics as well as hormone levels were made using the signed rank test. Comparison of hormone levels between POI and control groups was by rank sum test. Multiple regression was used to evaluate the relative influence of several factors on outcome measures. Correlations were assessed through the Spearman rank correlation.
Results
The normal mid-follicular LH and FSH levels in our assays were 3 to 14 IU/L and 3 to 10 IU/L, respectively, based on the 5th and 95th percentiles from 67 control samples. (We excluded 3 control samples that had predefined mid-cycle LH levels of greater than 20 IU/L.)
The POI group (N=137) did not differ significantly from the control group (N=67) with regard to mean±SD age (30.5±5.6 vs. 28.2±7.2 years), body mass index (23±3.2 vs. 22.3 ±2.8 kg/m2), or age of menarche (12.6±1.5 vs. 12.4±1.3 years). In the POI group, the mean age of onset of menstrual abnormalities was 24.6±8.1 years, age of diagnosis was 28.2±6.9 years, and mean time since diagnosis at presentation to our study was 40.4±45.5 months.
In women with POI the mean (SD) serum estradiol level on therapy increased to 95.4 (84.9) pg/mL [350.21(311.66) pmol/mL] from 40.0 (42.7) pg/mL [146.84(156.75) pmol/mL] at baseline (p<0.001, Figure 1A). Estradiol therapy significantly reduced serum LH levels from 52.2 (26.2) IU/L while off therapy to 15.2(15.7) IU/L while on therapy (p<0.001). Similarly, FSH levels reduced from 88.5(39.3) IU/L to 23.9(23.7) IU/L (p<0.001) while on transdermal estradiol therapy.
Figure 1A.
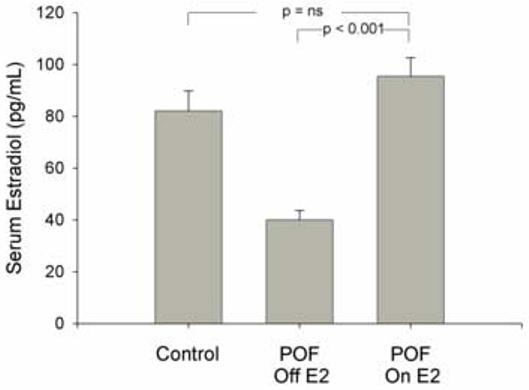
Mean serum estradiol levels in normal control women sampled on day 5 to 12 of the menstrual cycle (N=67) and women with primary ovarian insufficiency (POI) (N=137) before and again at least 3 months later during the estradiol-only phase of replacement therapy (100 mcg/day estradiol patch, medroxyprogesterone acetate 10 mg for 12 days each month). To convert estradiol measurement to SI unit, multiply by 3.671.
The proportion of women who had FSH and LH levels in the normal range while receiving transdermal estradiol therapy are shown in Table 1 and Figure 1B. While on transdermal estradiol therapy 70/137 (51.1%) (95% confidence interval 42%, 60%) of these women had serum LH levels in the normal range, as compared to 5/126(4.0%) at baseline (p<0.001). With regard to FSH levels, 38/137(27.7%) patients had FSH in the normal range as compared to 2/126 (1.6%) at baseline (p<0.001). Only 2/137(1.5 %) of these women had FSH levels suppressed below normal, whereas18/137 (13.1%) had LH levels suppressed. On this replacement regimen, LH was significantly more likely to be in the normal range than FSH (p<0.001) (Figure 1B).
Table 1.
Number (%) of women with primary ovarian insufficiency (POI) segregated by category of low, normal, or high gonadotropin levels before and after therapy a
| LH Off E2 (n=126) | LH On E2 (n=137) | P | FSH Off E2 (n=126) | FSH On E2 (n=137) | P | |
|---|---|---|---|---|---|---|
| Low | 1 (0.8) | 18 (13.0) | <0.001 | 0 (0.0) | 2 (1.5) | NS |
| Normal | 5 (3.9) | 70 (51.1) | <0.001 | 2 (1.5) | 38 (28.4) | <0.001 |
| High | 120 (95.3) | 49 (38.3) | <0.001 | 124 (98.5) | 97 (70.1) | <0.001 |
Replacement therapy with100 mcg/day estradiol patch, medroxyprogesterone acetate 10 mg for 12 days each month for at least 3 months.
Figure 1 B.
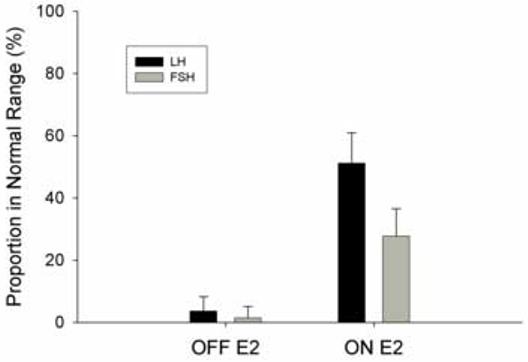
Proportion (95% confidence interval) of women with primary ovarian insufficiency (POI) (N=137) who have serum LH or FSH in the normal range before and while on at least 3 months of 100 mcg per day of transdermal estradiol patch therapy (***, p<0.001).
In women with POI serum LH levels correlated significantly with estradiol levels both while on estradiol therapy (r=-0.30, p=0.0004,) and while off (r=-0.23, p=0.01). In the control group only a trend was seen for this association (r=-0.23, p=0.06). In women with POI serum FSH levels also correlated significantly with estradiol levels while on replacement (r=-0.37, p<0.0001) and off (r=-0.29, p=0.001). In the control group FSH levels correlated significantly with estradiol levels (r=-0.43, P=0.0003)
Time since diagnosis was negatively correlated with LH levels while on estradiol replacement (r=-0.25, p= 0.003). Regression analysis indicated that for each year since diagnosis, LH level was lowered by 1 IU/L. This trend remained significant (p=0.045) after adjusting for age of onset of menstrual abnormality, age, BMI, serologic evidence of autoimmunity, and whether or not the patient received prior hormone replacement therapy. Time since diagnosis was not significantly correlated with LH levels while patients were off hormone replacement. Time since diagnosis was also negatively correlated with FSH levels while on estradiol replacement (r=-0.20, p= 0.017) and also while off estradiol replacement (r=-0.18, p=0.040).
Discussion
We have shown that while off estrogen replacement therapy approximately 95% of women with spontaneous POI have serum LH levels above normal for the mid-follicular phase. A properly timed LH surge luteinizes the mature follicle, reduces granulosa cell mitogenic activity (18), and induces changes in the steady state level of messenger ribonucleic acids for enzymes involved in progesterone production (19). Inappropriate premature luteinization of a growing follicle would thus be expected to impair follicle growth, reduce estradiol production, and impair ovulation (2).
At present there are no proven therapies to improve ovulatory function in women with spontaneous POI. There is no question that many of these women have a few potentially functional follicles remaining in the ovary (1-3). However, these follicles develop in a milieu of chronically elevated serum LH levels. The normal menopausal elevation of serum LH is known to result from inadequate negative feedback due to a reduced number of follicles (20). It has been demonstrated by ovarian biopsy that most women with POI have a scarcity of follicles (2,21). In women with normal ovarian function the large cohort of atresia-destined follicles may play a more important role than has been recognized. By providing negative feedback this cohort of follicles maintains a proper gonadotropin environment, and thus prevents premature luteinization (2,22,23).
A strategy to provide appropriate physiological negative feedback and maintain LH levels in the normal range might avoid inappropriate luteinization of the few remaining follicles that women with POI have in their ovaries. To our knowledge this is the first report to define the percentage of women with spontaneous POI who have serum LH levels in the normal range on a specific form of estrogen replacement therapy. We have shown that this regimen can achieve normal mid-follicular phase serum LH levels in approximately one-half of these women. On average the 100 mcg estradiol patch restores estradiol levels to the normal mid-follicular phase range of 95 pg/mL [348.7 pmol/mL], which suggests this as a true physiological replacement dose.
The fact that while on transdermal replacement serum LH and estradiol levels are significantly correlated raises an interesting theoretical possibility. Possibly titration of the estradiol dose in a manner to specifically normalize serum LH levels could be used as a therapeutic measure to improve ovulation rates in women with POI. Interestingly, we found that while on transdermal estradiol replacement the longer the time from diagnosis the lower the serum LH level, even after adjusting for age, BMI, prior HRT and age of menstrual abnormality in a multivariate model. The mechanism for this is unclear, but this observation is reassuring in that the ability to titrate serum LH levels by estradiol administration appears to remain as the time since diagnosis increases.
We conclude that it is possible to achieve normal serum LH levels on a standard regimen of 100 microgram transdermal estradiol replacement in approximately one-half of women with spontaneous POI. However, at this point there is no evidence that transdermal estradiol therapy improves ovulation rates. An NICHD protocol has been approved to investigate this question. Theoretically this may reduce the chance of inappropriate follicle luteinization and improve ovulatory rates. Very few women had FSH levels suppressed below the normal range on this regimen. Controlled studies to assess the effect of estradiol replacement regimens on ovulatory rates in these women are warranted.
Acknowledgments
Supported by the Intramural Research Program, National Institute of Child Health and Human Development, National Institutes of Health. VHV and LMN are Commissioned Officers in the United States Public Health Service.
Footnotes
Capsule: A regimen of 100 microgram/day transdermal estradiol replacement achieved normal serum LH levels in approximately one-half of 137 women with spontaneous primary ovarian insufficiency.
Where the work was done:
National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rebar RW, Erickson GF, Yen SSC. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil Steril. 1982;37:35–41. [PubMed] [Google Scholar]
- 2.Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized Graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–1475. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 3.Taylor AE, Adams JM, Mulder JE, Martin KA, Sluss PM, Crowley WFJ. A randomized, controlled trial of estradiol replacement therapy in women with hypergonadotropic amenorrhea. J Clin Endocrinol Metab. 1996;81:3615–3621. doi: 10.1210/jcem.81.10.8855811. [DOI] [PubMed] [Google Scholar]
- 4.Wright CSW, Jacobs HS. Spontaneous pregnancy in a patient with hypergonadotrophic ovarian failure. Br J Obstet Gynaecol. 1979;86:389–392. doi: 10.1111/j.1471-0528.1979.tb10617.x. [DOI] [PubMed] [Google Scholar]
- 5.Alper MM, Jolly EE, Garner PR. Pregnancies after premature ovarian failure. Obstet Gynecol. 1986;67:59s–62s. doi: 10.1097/00006250-198603001-00018. [DOI] [PubMed] [Google Scholar]
- 6.Kreiner D, Droesch K, Navot D, Scott R, Rosenwaks Z. Spontaneous and pharmacologically induced remissions in patients with premature ovarian failure. Obstet Gynecol. 1988;72:926–928. doi: 10.1097/00006250-198812000-00024. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon B, Worcester J. Age at menopause. United States--1960-1962. Vital Health Stat. 1966;11:1–20. [PubMed] [Google Scholar]
- 8.Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139:64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- 9.van Noord PA, Dubas JS, Dorland M, Boersma H, te Velde E. Age at natural menopause in a population-based screening cohort: the role of menarche, fecundity, and lifestyle factors. Fertil Steril. 1997;68:95–102. doi: 10.1016/s0015-0282(97)81482-3. [DOI] [PubMed] [Google Scholar]
- 10.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 11.Nelson LM, Covington SN, Rebar RW. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril. 2005;83:1327–1332. doi: 10.1016/j.fertnstert.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. American Journal of the Medical Sciences. 1942;204:625–648. [Google Scholar]
- 13.Adashi EY. Endocrinology of the ovary. Hum Reprod. 1994;9:815–827. doi: 10.1093/oxfordjournals.humrep.a138602. [DOI] [PubMed] [Google Scholar]
- 14.Marik J, Hulka J. Luteinized unruptured follicle syndrome: a subtle cause of infertility. Fertil Steril. 1978;29:270–274. doi: 10.1016/s0015-0282(16)43151-1. [DOI] [PubMed] [Google Scholar]
- 15.LeMaire GS. The luteinized unruptured follicle syndrome: anovulation in disguise. J Obstet Gynecol Neonatal Nurs. 1987;16:116–120. doi: 10.1111/j.1552-6909.1987.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 16.Qublan H, Amarin Z, Nawasreh M, Diab F, Malkawi S, Al-Ahmad N, et al. Luteinized unruptured follicle syndrome: incidence and recurrence rate in infertile women with unexplained infertility undergoing intrauterine insemination. Hum Reprod. 2006 doi: 10.1093/humrep/del113. [DOI] [PubMed] [Google Scholar]
- 17.Kim TJ, Anasti JN, Flack MR, Kimzey LM, Defensor RA, Nelson LM. Routine endocrine screening for patients with karyotypically normal spontaneous premature ovarian failure. Obstet Gynecol. 1997;89:777–779. doi: 10.1016/s0029-7844(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 18.Yong EL, Baird DT, Yates R, Reichert LE, Jr., Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74:842–849. doi: 10.1210/jcem.74.4.1548349. [DOI] [PubMed] [Google Scholar]
- 19.Doody KJ, Lorence MC, Mason JI, Simpson ER. Expression of messenger ribonucleic acid species encoding steroidogenic enzymes in human follicles and corpora lutea throughout the menstrual cycle. J Clin Endocrinol Metab. 1990;70:1041–1045. doi: 10.1210/jcem-70-4-1041. [DOI] [PubMed] [Google Scholar]
- 20.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55:699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coulam CB. Premature gonadal failure. Fertil Steril. 1982;38:645–655. doi: 10.1016/s0015-0282(16)46688-4. [DOI] [PubMed] [Google Scholar]
- 22.Lacker HM, Ethan A. How do the ovaries count? Mathematical Biosciences. 1988;90:305–332. [Google Scholar]
- 23.Duncan M, Cummings L, Chada K. Germ cell deficient (gcd) mouse as a model of premature ovarian failure. Biol Reprod. 1993;49:221–227. doi: 10.1095/biolreprod49.2.221. [DOI] [PubMed] [Google Scholar]


