Abstract
Objectives
Safe and effective device closure of ventricular septal defects (VSD) remains a challenge. We have developed a per-cardiac approach to close VSDs using a patch delivery and fixation system that can be secured under real-time 3D echo (RT3DE) guidance.
Methods
In Yorkshire pigs (n = 8), through a purse-string suture placed on the left ventricle (LV) apex, a coring device was introduced into the LV and a muscular VSD was created. Through another purse-string on the LV apex, the patch deployment device containing a 20 mm polyester patch was advanced toward the VSD and the patch was deployed under RT3DE guidance. The anchor delivery device was then introduced into the LV through the first purse string. Nitinol anchors to attach the patch around the VSD were deployed under RT3DE guidance. After patch attachment, residual shunts were sought by 2D and 3D Color Doppler echocardiography. The heart was then excised, and the septum with patch was inspected.
Results
A VSD was created in the midventricular (n = 4), anterior (n = 2) and apical (n = 2) septum. The mean size was 9.8 mm (8.2–12.0 mm) as determined by 2D Color Doppler. The VSDs were completely closed in 7 animals. In one, a 2.4 mm residual shunt was identified. No anatomical structures were compromised.
Conclusions
Beating-heart perventricular muscular VSD closure without cardiopulmonary bypass can be successfully achieved using a catheter-based patch delivery and fixation system under RT3DE guidance. This approach may be a better alternative to open-heart surgery or transcatheter device closure.
Ultramini-Abstract
Muscular ventricular septal defects were created and successfully closed under real-time 3D echo guidance on the beating heart using an original patch delivery and fixation system in a pig model. This approach may be a better alternative to open-heart surgery or transcatheter closure.
Introduction
Although pediatric cardiopulmonary bypass and open-heart repair of intracardiac defects have matured over the last decades, becoming relatively safe, they remain an invasive procedure, associated with a potential, albeit rare, of neurologic amongst other complications [1]. At the same time, there is an increasing amount of basic, translational and clinical research currently being conducted in the area of beating-heart repairs, including transapical aortic valve and perventricular pulmonary valve implantation [2–4], trans-cardiac ventricular septal defect (VSD) closure [5,6], as well as mitral valvuloplasty and atrial septal defect (ASD) closure [7–9]. A key component enabling such procedures is the advance in imaging technology, which allow real-time visualization of heart structures and instruments while the heart is beating. Three-dimensional ultrasound (3DUS) imaging has made significant progress in the past few years with improvements in spatial and temporal resolution. The ease of data acquisition, real-time three-dimensional (3D) volume rendering, the ability to focus on a specific anatomic structure, as well as variety of additional quantification tools have enabled virtually routine application of 3DUS in cardiology practice [10, 11]. Real-time 3D echocardiography (RT3DE) is an ideal imaging modality for septal defect closure because it provides precise anatomic images of complex anatomical structures such as the atrial or ventricular septum and margins of septal defects. Obstacles to application of 3DUS, such as acoustic interference from metallic instruments, due to shadowing and side-lobe artifacts, in the field of view can be minimized with instrument modifications such as the application of surface coating, and by varying the angle of instrument navigation with respect to the ultrasound (US) probe [12]. The high frame rates currently achieved with RT3DE obviate the need for electrographic or respiratory gating for image acquisition. We have recently demonstrated that epicardial RT3DE provides adequate anatomic detail for surgical task-performance in an in vitro model [7] and in vivo model of beating-heart patch ASD closure [8, 9].
The aim of the present animal study was to investigate the feasibility and efficacy of beating-heart trans-cardiac closure of muscular VSD using our patch delivery and mini anchor deployment system under RT3DE guidance.
Materials and Methods
The experimental protocol was approved by the Children’s Hospital Boston Institutional Animal Care and Use Committee. All animals received humane care in accordance with the 1996 Guide for the Care and Use of Laboratory Animals recommended by the U.S. National Institute of Health.
Yorkshire pigs (70–80 kg, n = 8) were anesthetized by intramuscular injection of tiletamine/zolazepam (7 mg/kg) and xylazine (4 mg/kg). The animals were intubated with a cuffed endotracheal tube and ventilated with a pressure control ventilator (Healthdyne 105; Healthdyne Technologies, Marietta, GA); anesthesia was maintained with 1–2% isoflurane. The electrocardiogram and arterial blood pressure were continuously monitored. A left anterolateral thoracotomy in the fourth intercostal space was performed, and stay sutures were placed on the pericardium to optimize access to the left ventricle (LV) free wall and the apex. RT3DE was performed using the X4 matrix probe on a SONOS 7500 system (Philips Medical Systems, Andover, MA). The US probe was inserted into a sleeve (CIVCO Medical Instruments, Kalona, IA) filled out with an ultrasound gel (Parker Laboratories, Inc., Fairfield, NJ) providing approximately 2 cm of stand-off. The outer surface of the sleeve was lubricated with 0.9% Sodium Chloride solution and applied to the surface of the LV. After intravenous heparin administration (100 U/kg) a VSD was created solely under RT3DE guidance. Through a 3-0 polypropylene felt pledget-reinforced purse-string suture placed on the apical portion of the LV free wall, an original 10 mm coring device was introduced into the LV and advanced through the ventricular septum. This created a muscular VSD, which was confirmed by 2D and 3D Color Doppler echocardiography.
The VSD was then closed with a polyester patch using our patch delivery and fixation system previously described [9]. Briefly, the system includes two parts: the catheter-based patch delivery device and the patch fixation device. The patch delivery device consists of a self-expanding Nitinol frame and a grip. A polyester patch is appropriately trimmed, and then attached to the frame by 0.1 mm Nitinol release wire (Figure 1A, B). The device is delivered through a 9 Fr sheath, and the frame with patch is deployed through the end of the sheath and allowed to expand. The polyester patch is attached to the septum by Nitinol mini-anchors (Figure 1C), deployed using a pistol-type anchor delivery device.
Figure 1.
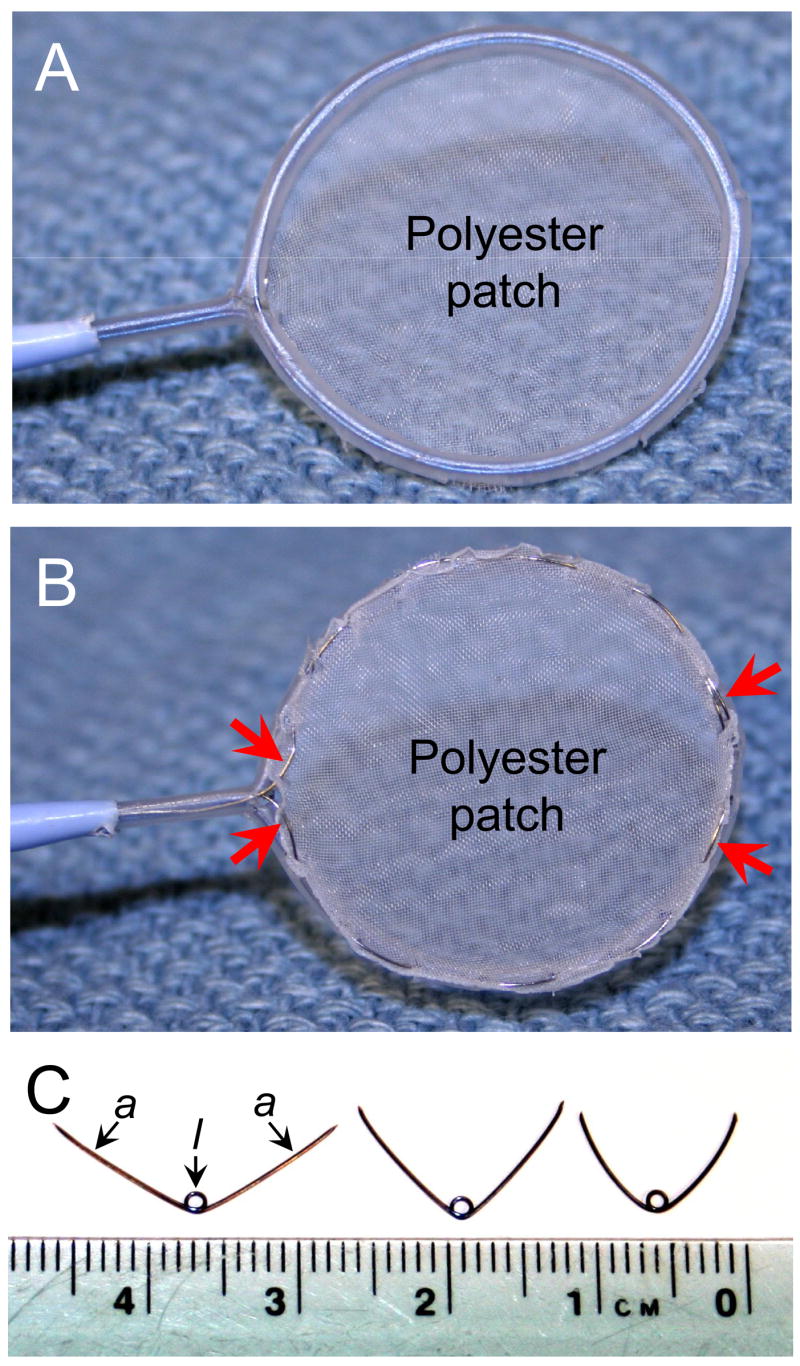
Patch deployment device, front view (A), rear view (B), and the mini-anchors (C). Polyester patch material is attached to the Nitinol frame by 0.1 mm release Nitinol wire (red arrows). During deployment the arms of the anchor (a) penetrate patch and attach it to the septum tissue, while the loop (l) stays on the front side of the patch.
The patch deployment device was introduced through a second 3-0 polypropylene purse-string suture in the LV, and advanced toward the VSD under RT3DE guidance, staying parallel to the ventricular septum and away from the papillary muscles and the mitral valve chordae and leaflets. Once the frame with the patch was deployed and placed over the VSD, the fixation device was introduced into the LV and advanced towards the patch (Figure 2). The anchors were deployed using a fixation device, one by one around the circumference of the patch. This latter maneuver was also performed under RT3DE guidance and in two steps. First, the arms of the anchor were deployed, and successful penetration of both the patch material and the septum was confirmed with tactile sensation and applying of gentle traction force on the deployed anchor. Next, the anchor loop was fully deployed, and the anchor was released from the fixation device. After the patch was fixed to the septum, the release wire was removed allowing separation of the patch from the frame, and the frame was withdrawn into the sheath. This technique leaves only the polyester patch and Nitinol anchors inside the heart. After VSD closure, 2D and 3D Color Doppler echocardiography was performed to look for any residual shunts. Finally, the animal was sacrificed; the heart was excised, and the efficacy of closure and patch fixation was determined by direct inspection.
Figure 2.
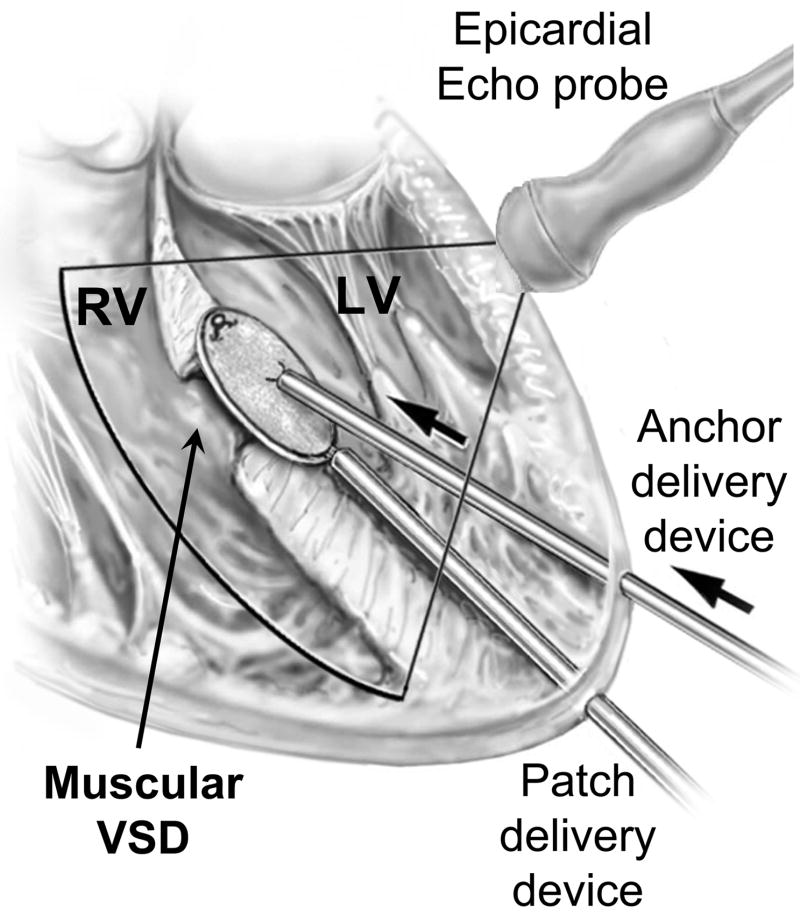
Scheme of the procedure. Explanations in the text. LV, left ventricle; RV, right ventricle; VSD, ventricular septal defect.
Results
In order to safely perform the entire procedure solely under RT3DE guidance, the optimal orientation of the epicardial US probe and different insertion points for the devices was determined in the first 2 animals. The transducer was positioned at approximately 45 degrees to the surface of the LV free wall, close to the atrioventricular groove, aiming towards the apex. With this orientation, nearly the entire LV chamber was able to be visualized, and the devices could be navigated from the insertion points toward the ventricular septum under constant 3DUS monitoring. The devices were introduced coaxially into the LV from the apical region. This orientation provided optimal ergonomics for device manipulation, adequate visualization for safe device navigation inside the LV and minimized trauma of the LV wall, avoiding the coronary arteries (Figure 2).
VSD creation
The VSDs were created in the midventricular (n = 4), anterior (n = 2) and apical (n = 2) muscular septum. The mean size of the VSD was 9.8 mm (8.2–12.0 mm) as determined by 2D Color Doppler echocardiography (Figure 3A). Successful creation of the defects was also confirmed by 3D Color Doppler echocardiography (Figure 3B, C). The size of the patch used was 20 mm in all the cases.
Figure 3.
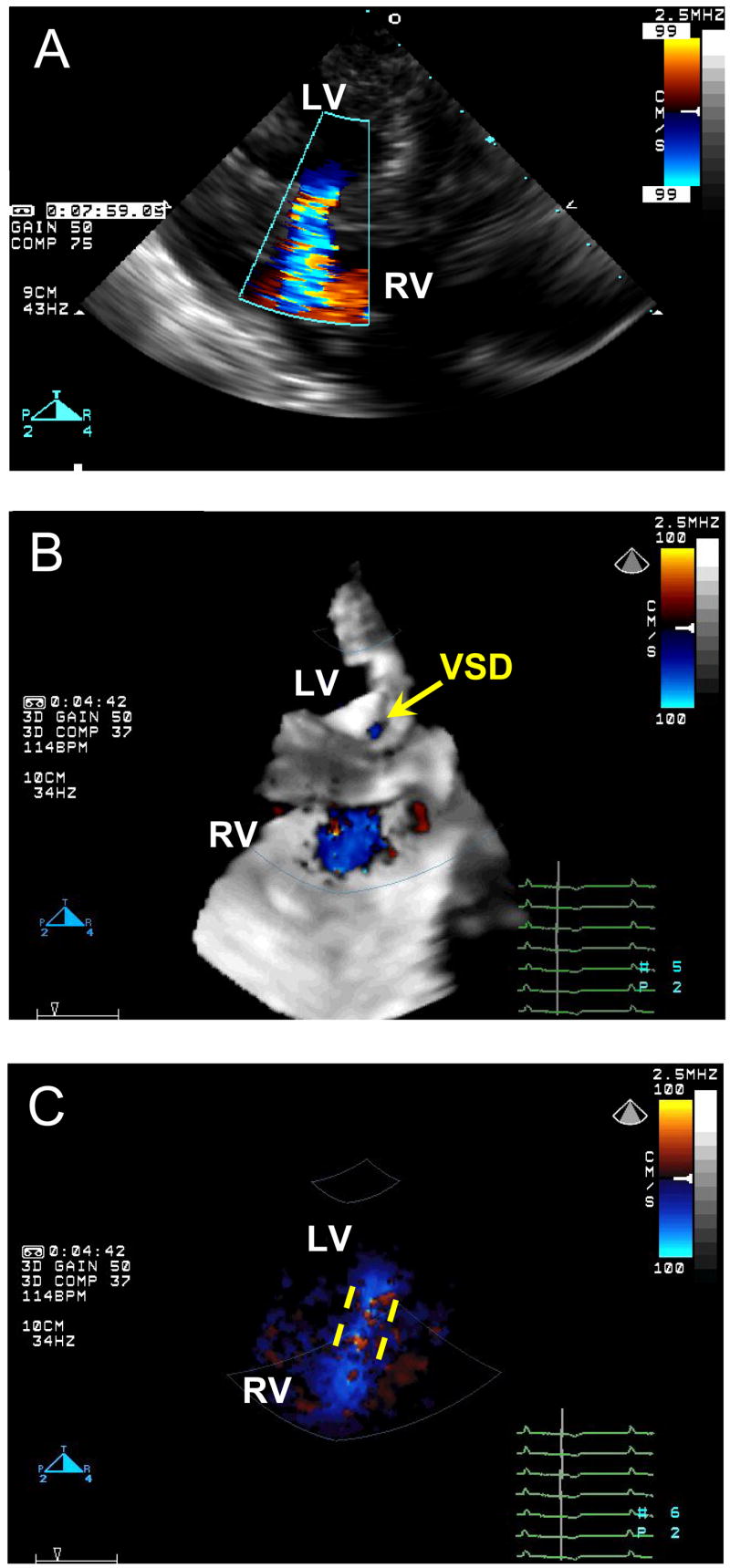
Color Doppler images of the created VSD. A, 2D Color Doppler. B, 3D Color Doppler, yellow arrow points to the orifice of the VSD on the left side of the ventricular septum. C, the same 3D Color Doppler image with the black-and-white suppress, yellow dashed lines demonstrate the VSD tunnel inside the ventricular septum. LV, left ventricle; RV, right ventricle; VSD, ventricular septal defect.
VSD closure
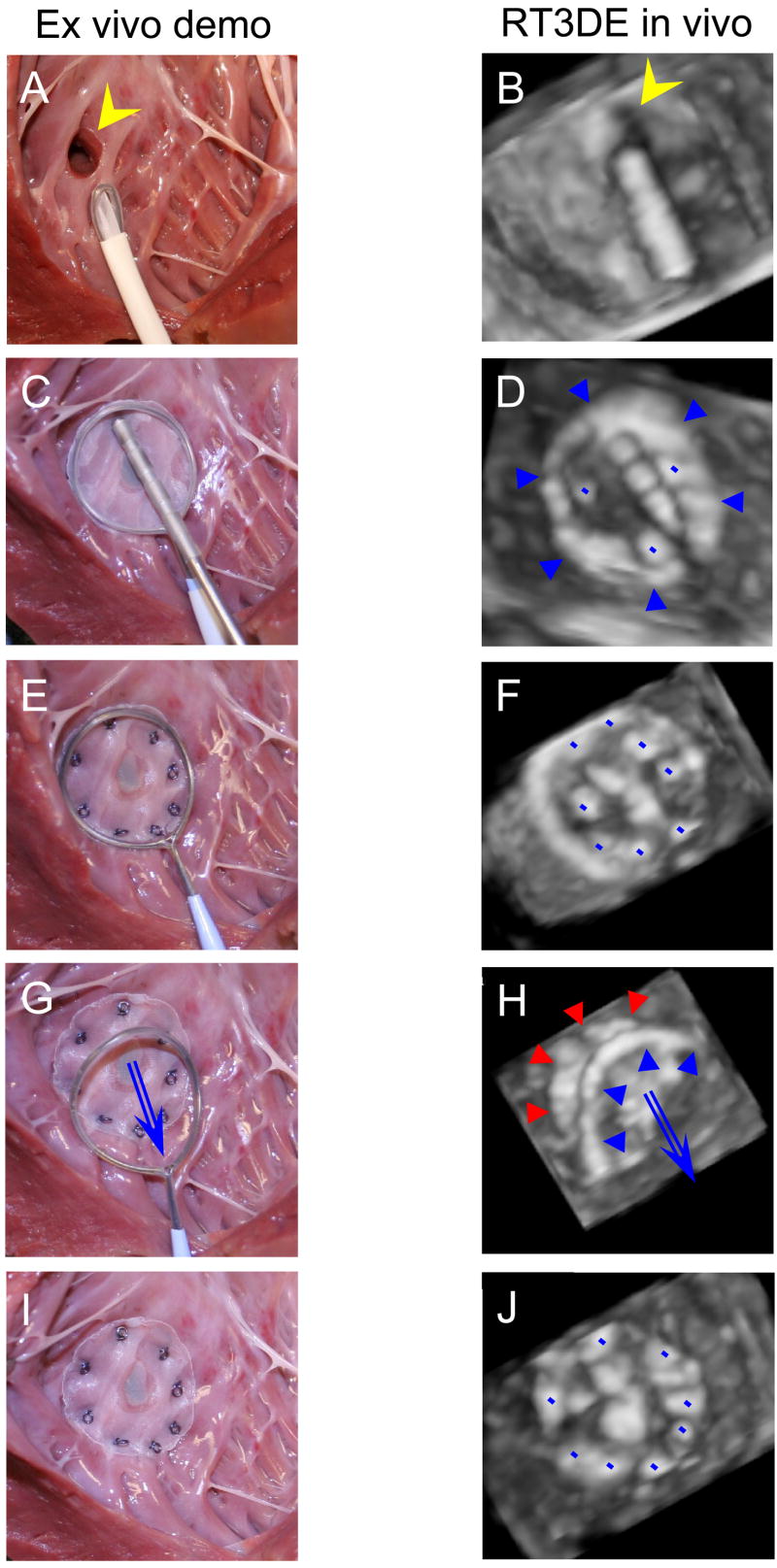
Representative sequential RT3DE images obtained during VSD closure are shown in Figure 4 (right column); left column shows ex vivo demonstration of the procedure for better comprehension. The VSDs were closed completely in 7 animals with no residual shunts detected by Color Doppler (Figure 5A). A mean of 8 (range 7–10) anchors was used per case. In 1 animal a 2.4 mm residual defect was identified around the edge of the patch. That animal had the largest VSD (12 mm) of the series, with the VSD having been created in the midventricular septum by repeated coring of the muscular tissue. As a result, the tissue around the VSD was porous and friable, and it was difficult to transfix the patch to the septum with the anchors.
Figure 4.
VSD patch closure demonstration ex vivo (left column) and sequence of the RT3DE images acquired during the procedure (right column), view from the left ventricle. A, B, The patch is delivered through an introducer sheath towards the VSD (yellow arrowhead). C, D, After the frame expands (blue arrowheads) and the patch covers the VSD, it is attached to the ventricular septum by the Nitinol mini-anchors (blue dots). Note segmented polymer coating of the anchor deployment device for optimizing the instrument visualization under ultrasound. E, F, All the anchors are deployed, and the patch is completely attached to septum closing the VSD. G, H. The release wire is removed, and the frame is withdrawn into the introducer sheath. The patch (red arrowheads) is left attached to septum. I, J, Final view, the patch and the anchors (blue dots). RT3DE, real-time three-dimensional echocardiography; VSD, ventricular septal defect.
Figure 5.
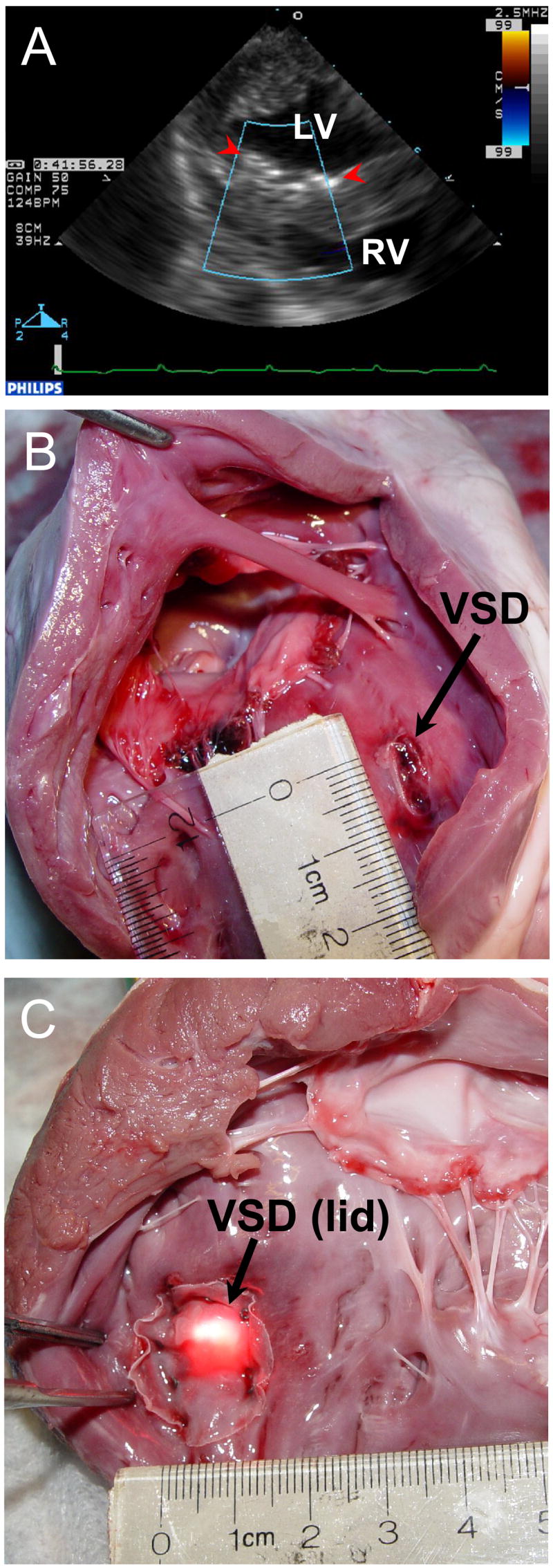
A, 2D Color Doppler of the closed VSD. The patch (red arrowheads) completely sealed the VSD. Post-mortem photographs, view from the RV (B), view from the LV (C). The patch is properly fixed around the 10 mm VSD. No residual defect is present. LV, left ventricle; RV, right ventricle; VSD, ventricular septal defect.
Direct inspection of the septum after excision of the heart confirmed the intraoperative echocardiography findings (Figure 5B, C). The anchors consistently penetrated the patch material and the ventricular septal myocardium. The VSDs were sealed completely in 7 cases. In 1 animal the residual communication of 2 mm was found. No other anatomical structures in the LV and right ventricle (RV) were compromised in any of the cases.
Discussion
The present study demonstrates that 1. muscular VSDs can be successfully created in pigs using a perventricular coring device, and, 2. muscular VSDs can be closed under RT3DE guidance on the beating-heart, via a left perventricular approach using a patch anchored to the left side of the septum.
Closure of an isolated VSD is one of the oldest open-heart procedures, beginning with the first successful closure in 1955 by C. Walton Lillehei [13]. The procedure currently enjoys a low complication and mortality rate, with surgical repair remaining the mainstay of therapy. Percutaneous device closure techniques have recently emerged, and have had mixed success [14]. While device closure has been successfully applied in cases of multiple muscular VSDs or mid-muscular isolated VSDs for example, it has so far not proven to be a superior to open heart surgery when it comes to complete closure of the defects. For membranous VSDs, development of complete heart block, sometimes occurring late after device implantation, has been a source of concern [15]. Furthermore, the presence of an often bulky device in close proximity to the aortic valve and left ventricular outflow tract has the potential for resulting in other late complications. Furthermore, the chordae of the subvalvar apparatus of the mitral and tricuspid valves, and integrity of the aortic valve cusps are at risk during repeated passages of wires and sheaths into the heart. The perventricular techniques, developed clinically for muscular VSDs [5] and in animal experiments for membranous VSDs [6] avoid some of these hazards, such as damage to the valves, because the VSD is approached directly at the ventricular level. No valves are crossed, and the angle of approach is close to 90 degrees to the plane of the septum, providing much more favorable conditions for crossing of the VSD and device deployment. However, perventricular device-based procedures still had significant drawbacks in terms of device bulkiness (expansion in a hypertrophied RV apex for example), or residual VSD from undersizing or malpositioning of the device. Devices themselves, as demonstrated from recent reports [16], can erode through cardiac structures such as the aortic root. Endocarditis and thromboembolic episodes are other feared complications [17].
The present procedure circumvents many of these complications. There is no need to first cross the VSD with a guide wire, one of the major initial difficulties in any device-based VSD closure. The catheter-mounted patch delivery device allows for a left-sided approach, and is easily positioned and deployed over the VSD using RT3DE. Since the patch is anchored on the left side of the septum, it is subjected to 4–5 fold higher pressure in systole, compressing it against the septal tissue, likely rendering small residual VSDs inconsequential in the long term. Unlike with open surgical repairs, where sutures have to be closely aligned to prevent residual VSDs, anchors have to secure the patch in place, but do not have to be very closely spaced. In our studies, the anchors were secure, and completely penetrated the muscular septum tissue. By virtue of its ventricular approach, this procedure retains the advantages of (right-sided) perventricular VSD closure, avoiding the crossing of valves. In addition, as opposed to the tricuspid valve which has many septal attachments, there are no mitral valve papillary muscles based on the ventricular septum. This, therefore, provides more favorable conditions to operate in the beating-heart RT3DE-guided environment, because there is no nearby subvalvular apparatus.
Limitations of the study and remaining challenges
Compared with our previous experience of RT3DE-guided ASD patch closure [9] we encountered few important technical differences between these two procedures. High velocity and contractile force of the ventricular septum, unlike atrial septal motion, makes free-hand deployment of the first anchors crucial, which in inexperienced hands may lead to torn myocardium and dislodged anchors. To address the problems with collision with the rapidly moving septum, and in order to simplify anchor fixation to a moving target we are developing a motion compensation tool that tracks the motion of the septum in real-time and may provide an easier method of patch fixation [18]. Initial visualization and, more importantly, tracking of the muscular VSD with 3DUS imaging throughout the procedure is challenging. To address this problem, our group has developed an image processing algorithm for block flow ASD detection and tracking [19]. This technique has to be further optimized for real-time VSD tracking in a cluttered operative field in the LV cavity. Finally, since there are no naturally occurring large animal models of muscular VSDs, we developed this model using an original coring device to create the defects. The muscular VSDs were easily created using the same entry point as was later used for the anchoring device. A final potential disadvantage is the possibility of pseudo-aneurysm formation at the LV free wall puncture site.
In summary, this study demonstrates that muscular VSDs can be successfully created and closed using our patch delivery device under RT3DE guidance on the beating heart in a large animal. In future work, we aim to test a similar technique for membranous VSD closure using Yucatan pigs [6].
Supplementary Material
Acknowledgments
We acknowledge Mr. Randal S. McKenzie for the illustrations.
This work was supported in part by National Institute of Health Grants No. HL-073647 and HL-71128 (PJdN).
Abbreviations and Acronyms
- 2D
two-dimensional
- 3D
three-dimensional
- 3DUS
three-dimensional ultrasound
- ASD
atrial septal defect
- LV
left ventricle
- RT3DE
real-time three-dimensional echocardiography
- RV
right ventricle
- VSD
ventricular septal defect
- US
ultrasound
Footnotes
Boston, Massachusetts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Menache CC, du Plessis AJ, Wessel DL, Jonas RA, Newburger JW. Current incidence of acute neurologic complications after open-heart operations in children. Ann Thorac Surg. 2002 Jun;73(6):1752–8. doi: 10.1016/s0003-4975(02)03534-8. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein SV, Cheung A, Ye J, Thompson CR, Carere RG, Pasupati S, Webb JG. Transapical transcatheter aortic valve implantation in humans: initial clinical experience. Circulation. 2006 Aug 8;114(6):591–6. doi: 10.1161/CIRCULATIONAHA.106.632927. [DOI] [PubMed] [Google Scholar]
- 3.Walther T, Falk V, Borger MA, Dewey T, Wimmer-Greinecker G, Schuler G, et al. Minimally invasive transapical beating heart aortic valve implantation – proof of concept. Eur J Cardiothorac Surg. 2007 Jan;31(1):9–15. doi: 10.1016/j.ejcts.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber C, Horer J, Vogt M, Fratz S, Kunze M, Galm C, et al. A new treatment option for pulmonary valvar insufficiency: first experiences with implantation of a self-expanding stented valve without use of cardiopulmonary bypass. Eur J Cardiothorac Surg. 2007 Jan;31(1):26–30. doi: 10.1016/j.ejcts.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Bacha EA, Cao QL, Starr JP, Waight D, Ebeid MR, Hijazi ZM. Perventricular device closure of muscular ventricular septal defects on the beating heart: technique and results. J Thorac Cardiovasc Surg. 2003 Dec;126(6):1718–23. doi: 10.1016/s0022-5223(03)01043-2. [DOI] [PubMed] [Google Scholar]
- 6.Amin Z, Danford DA, Lof J, Duncan KF, Froemming S. Intraoperative device closure of perimembranous ventricular septal defects without cardiopulmonary bypass: preliminary results with the perventricular technique. J Thorac Cardiovasc Surg. 2004 Jan;127(1):234–41. doi: 10.1016/j.jtcvs.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Suematsu Y, Takamot S, Kaneko Y, Ohtsuka T, Takayama H, Kotsuka Y, et al. Beating atrial septal defect closure monitored by epicardial real-time three-dimensional echocardiography without cardiopulmonary bypass. Circulation. 2003;107:785–90. doi: 10.1161/01.cir.0000047210.07839.7b. [DOI] [PubMed] [Google Scholar]
- 8.Suematsu Y, Martinez JF, Wolf BK, Marx GR, Stoll JA, DuPont PE, et al. Three-dimensional echo-guided beating-heart surgery without cardiopulmonary bypass: Atrial septal defect closure in a swine model. J Thor Cardiovasc Surg. 2005;130:1348–57. doi: 10.1016/j.jtcvs.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 9.Vasilyev NV, Martinez JF, Freudenthal FP, Suematsu Y, Marx GR, del Nido PJ. Three-dimensional echo and videocardioscospy guided atrial septal defect closure. Ann Thorac Surg. 2006;86:1322–6. doi: 10.1016/j.athoracsur.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Mor-Avi V, Sugeng L, Nieman PS, Sahn DJ. Three-dimensional echocardiography: the benefits of the additional dimension. J Am Coll Cardiol. 2006 Nov 21;48(10):2053–69. doi: 10.1016/j.jacc.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Ryan LP, Salgo IS, Gorman RC, Gorman JH. The emerging role of three-dimensional echocardiography in mitral valve repair. Semin Thorac Cardiovasc Surg. 2006;18(2):126–34. doi: 10.1053/j.semtcvs.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Triedman JK, Vasilyev NV, Suematsu Y, Cleveland RO, Dupont PE. Imaging artifacts of medical instruments in ultrasound guided interventions. J Ultrasound Med. 2007 doi: 10.7863/jum.2007.26.10.1303. In Press. [DOI] [PubMed] [Google Scholar]
- 13.Lillehei CW, Cohen M, Warden HE, Ziegler NR, Varco RL. The results of direct vision closure of ventricular septal defects in eight patients by means of controlled cross circulation. Surg Gynecol Obstet. 1955 Oct;101(4):446–466. [PubMed] [Google Scholar]
- 14.Holzer R, Balzer D, Cao QL, Lock K, Hijazi ZM Amplatzer Muscular Ventricular Septal Defect Investigators. Device closure of muscular ventricular septal defects using the Amplatzer muscular ventricular septal defect occluder: immediate and mid-term results of a U.S. registry. J Am Coll Cardiol. 2004 Apr 7;43(7):1257–63. doi: 10.1016/j.jacc.2003.10.047. [DOI] [PubMed] [Google Scholar]
- 15.Holzer R, de Giovanni J, Walsh KP, Tometzki A, Goh T, Hakim F, et al. Transcatheter closure of perimembranous ventricular septal defects using the amplatzer membranous VSD occluder: immediate and midterm results of an international registry. Catheter Cardiovasc Interv. 2006 Oct;68(4):620–8. doi: 10.1002/ccd.20659. [DOI] [PubMed] [Google Scholar]
- 16.Mello M, Fahey J, Kopf GS. Repair of aortic–left atrial fistula following the transcatheter closure of an atrial septal defect. Ann Thorac Surg. 2005;80:1495–1498. doi: 10.1016/j.athoracsur.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 17.Scheuerman O, Bruckheimer E, Marcus N, Hoffer V, Garty BZ. Endocarditis after closure of ventricular septal defect by transcatheter device. Pediatrics. 2006;117(6):e1256–8. doi: 10.1542/peds.2005-2498. [DOI] [PubMed] [Google Scholar]
- 18.Kettler DT, Plowes RD, Novotny PM, Vasilyev NV, del Nido PJ, Howe RD. An active motion compensation instrument for beating heart mitral valve surgery. Proc IEEE Intelligent Robots and Systems – IROS. 2007 In press. [Google Scholar]
- 19.Linguraru MG, Vasilyev NV, del Nido PJ, Howe RD. Atrial septal defect tracking in 3D cardiac ultrasound. Med Image Comput Comput Assist Interv. 2006;9(Pt 1):596–603. doi: 10.1007/11866565_73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.