Abstract
Perinatal hypoxia-ischemia (HI) is the most common cause of various neurological disabilities in children with high societal cost. Hypoxic-ischemic brain damage is an evolving process and ample evidence suggests distinct difference between the immature and mature brain in the pathology and consequences of brain injury. Therefore, it is of utmost importance to better understand the mechanisms underlying the hypoxic-ischemic injury in neonatal brain to devise effective therapeutic strategies. Nonetheless, the mechanism(s) involved in this pathology in the developing brain remain inadequately understood. Effective neuroprotective strategies will include either inhibition of the death effector pathways or induction of their regulatory and survival promoting cellular proteins. Neuronal pentraxins (NPs) define a family of novel neuronal proteins “long pentraxins” that are exclusively expressed in the central neurons, and are homologous to the C-reactive and acute-phase proteins in the immune system. NPs have been shown to be involved in the excitatory synaptic remodeling. We found that the neuronal protein ‘neuronal pentraxin 1’ (NP1) is induced in neonatal rat brain following HI, and NP1 induction preceded the time of actual tissue loss in brain. In demonstrating this we also found that NP1 gene silencing is neuroprotective against hypoxia-induced neuronal death. This is the first evidence for a pathophysiological function of NP1 in central neurons. Our results suggest that NP1 is part of a death program triggered by HI. Most importantly, our findings of specific interactions of NP1 with the excitatory glutamate receptors AMPA GluR1 subunit and their co-localization suggest a role for this novel neuronal protein NP1 in the excitotoxic cascade. Blockade of AMPA-induced neuronal death following inhibition of NP1 expression further implicates a regulatory interaction between NP1 and AMPA glutamate receptors. Subsequent experiments using NP1 loss-of-function strategies, we have demonstrated specific requirements of NP1 induction in HI-induced neuronal death. Together our findings clearly identify a novel role for NP1 in the coupling between HI and cerebral cell death. Thus, NP1 could be a new molecular target in the central neurons for preventing hypoxic-ischemic neuronal death in developing brain. These very novel results could lead to more effective neuroprotective strategies against hypoxic-ischemic brain injury in neonates.
Keywords: Perinatal hypoxia-ischemia, Excitotoxicity, Glutamate receptors, Brain injury, Epilepsy, Apoptosis, Neuroprotection, Neonatal hypoxia-ischemia, Neuronal pentraxin 1, Gene silencing
Introduction
Hypoxic-ischemic brain damage in neonates is a major risk factor of a variety of serious human neurological disorders such as motor and learning disabilities, cerebral palsy, epilepsy and seizures or even death. Survivors of such injury can experience substantial and life long cognitive, sensory and motor disabilities, for which currently there is no promising therapy (Johnston 1997; Lorenz et al. 1998; Northington et al. 2001a,b). Hypoxia-ischemia (HI) damages selected regions of the immature brain at different ages, particularly that occurring during the prenatal/neonatal stages of critical cellular or tissue differentiation process have a serious impact on brain maturation. Thus the gestational and perinatal age of the infant is one of the main variables in determining the neuropathological picture of hypoxic-ischemic brain injury. For these reasons the prenatal/neonatal age is of great importance. Our understanding of the pathogenesis of HI brain injury in the fetus and neonates have increased considerably over the last two decades from the stand points of both clinical and laboratory observations (Johnston et al. 2001). Defective brain development or damage to motor areas in the brain disrupts brain's ability to adequately control movement and posture. For instance, cerebral palsy, characterized by an inability to fully control motor function, is one such disorder is triggered following hypoxic-ischemic brain damage in neonates. A great deal of laboratory work on cerebral blood flow and clinical data suggest that most hypoxic-ischemic injuries in fetus and infants reflect combination of hypoxia and ischemia (Vannucci 1990).
The brain is made of nerve cells known as neurons that form tracts throughout the brain. Neurons are generally viewed as amongst the most anoxia-sensitive of all cells within the central nervous system. Insufficient supply of oxygen (hypoxia) and/or poor blood flow (ischemia) reaching particular area of the fetal or newborn brain leads to damage to the brain via activation of various cytotoxic agents and death pathways that ultimately induce neuronal injury and death (McDonald et al. 1988; Choi and Rothman 1990; Barks and Silverstein 1992; Martin et al. 1997; Johnston 2001). It is known that ‘excitotoxicity’, a term referred to cell death due to overstimulation of the excitatory glutamate neurotransmitter receptors, plays a critical role in brain injury caused by HI (Choi and Rothman 1990; Barks and Silverstein 1992; Portera-Cailliau et al. 1997). However, the mechanism(s) leading to neuronal damage/death triggered by HI and the pathological consequences underlying various neurological disorders remain poorly understood. Clearly, the etiology involved is multi-factorial and complex. Therapeutic intervention to neuropathological disorders associated with HI brain damage will likely require a greater understanding of the discrete neurotoxic molecular mechanism(s) of the neuronal cell death program triggered in the developing brain. Our laboratory and collaborators have been interested in the problem of neuronal vulnerability and injury in the developing brain initiated by hypoxic-ischemic insult. Our major focus is to understand the underlying molecular and cellular signaling events contributing to the injury mechanism(s) using well-established neonatal rat model of HI and hypoxic-ischemic insult, modeled in vitro, using primary neuronal cultures.
Neonatal brain injury and neurological disorders
The brain can receive several different types of injuries and brain injury is unpredictable in its consequences. Hypoxic-ischemia is a common cause of damage to the fetal and neonatal brain, which occurs in 1 to 6 of every 1000 live term birth (Ferriero 2004). Neonatal brain injury is recognized on the basis of a unique encephalopathy that evolves from lethargy to hyperexcitability to stupor during the first few days of life (Sarnat and Sarnat 1976). Most form of hypoxic-ischemia in neonates causes injury to selected areas of the brain rather than to the entire brain (Johnston 2001; Johnston et al. 2001; Ferriero 2004). Ischemic brain injury from stroke is another major cause of neurological morbidity in infants and children. A stroke occurs when the blood supply to the brain is suddenly interrupted (ischemic stroke) or when a blood vessel in the brain bursts, spilling blood into the spaces surrounding the brain cells (hemorrhagic stroke). Perinatal strokes are often arterial origin and ischemic in nature, and may have residual motor and cognitive disabilities (Ferriero 2004; Nelson and Lynch 2004). In human brain damage begins from the moment the stroke starts and can become irreversible as little as within an hour that often continues for days afterward. Animal models of perinatal stroke have been developed to examine the nature and the time course occurring after the ischemic insult and the possible strategies useful in reducing ischemic damage (Levine 1960; Rice et al. 1981; Vannucci and Perlman 1997; Balduini et al. 2004). Traumatic brain injury is another leading cause of death in children. Traumatic brain injury is an insult to the brain, not of a degenerative or congenital nature but caused by an external force. Severity of traumatic brain injury depends on the amount and type of force that impact the head (McPeak et al. 2001). Studies in experimental models suggest that traumatic brain injury involves a primary injury that includes direct disruption of brain parenchyma and a secondary injury that is characterized by a cascade of biochemical, cellular and molecular events similar as those associated with ischemia, excitotoxicity, energy failure, and resultant cell death cascades (Kochanek et al. 2000).
Advanced methods of neuroimaging, such as magnetic resonance imaging (MRI) and diffusion-weighted MRI have supplemented postmortem studies that demonstrate patterns of selective vulnerability and neuronal loss in different brain areas at different stage and age during which the HI insult has occurred (Franck and Roberts 1990; Towfighi and Mauger 1998). Recent findings of Williams and co-workers (2004) on synaptic reorganization in the hippocampus in a perinatal rat model of HI, similar to that used in our study (Hossain et al. 2004), further support selective vulnerability and neuronal damage in different brain regions after HI (Williams et al. 2004). Magnetic resonance imaging has also revealed a special pattern of symmetric injury to the putamen, thalamus and cerebral cortex after severe or near-total asphyxia (Johnston and Hoon 2000; Johnston 2001). Although systemic and cerebrovascular factors may play an important role in the initial phases of hypoxic-ischemic injuries, more recent studies have uncovered important cellular and molecular aspects of injury and brain damage (Delivoria-Papadopoulos and Mishra 2000; Nakajima et al. 2000; Russell et al. 2006; Zhu et al. 2007). Understanding the mechanism of these complex molecular cascades of injury in the developing brain will provide much insight require to meet the challenges for HI brain damage and developing novel strategies to prevent or avert its long-term consequences of various neurological disorders such as epilepsy and cerebral palsy in neonates.
Spectrum of cell death
The developing brain is uniquely susceptible to injury after exposure to a hypoxicischemic environment (Volpe 2001). There is a spectrum of severity in hypoxic-ischemic injury in vivo depending on the model. Moreover, the intrinsic vulnerability of specific cell types and systems in the developing brain may be more important in determining the final pattern of damage and functional disabilities. If an ischemic insult occurs early in gestation, some developing oligodendrocytes and subpalte neurons are lost (Back et al. 2001; McQuillen et al. 2003). In term neonates with ischemic brain injury, certain neurons in the deep gray nuclei are most likely to be injured (Ferriero 2004). Neuropathology of brain injury after HI includes focal ischemic infarction, selective neuronal necrosis and apoptosis. It is evident that the intrinsic cellular suicide program is not only required for normal CNS development but also contributes to the pathology of neurodegenerative disorders associated with hypoxia and ischemic stroke in neonates (Rami et al. 2003; Saito et al. 2004). Global hypoxia and/or ischemia are always transient in nature and cell death takes place in two major phases: a fast rapid phase of necrotic cell death and a second delayed one that last from hours to days and produces cell death with more characteristics of the programmed cell death known as apoptosis (Balduini et al. 2004). Apoptosis is reported to be responsible for a significant proportion of the final cell loss in brain (Delivoria-Papadopoulos and Mishra 2000; Nakajima et al. 2000; Russell et al. 2006; Zhu et al. 2007). When global hypoxia/ischemia is prolonged, it may produce cell death that is predominantly necrotic type (Petito et al. 1997). Cell death in the core region of the infarction (ischemic core) in hypoxic-ischemic brain is often primarily necrotic in nature unless the duration of HI is very brief. In vivo model of HI as well as in vitro experiments using primary neuronal cultures indicate that HI triggers a cascade of biochemical and molecular events: energy failure, membrane depolarization, brain edema, increased neurotransmitter release and inhibition of neurotransmitter uptake, increased levels of intracellular calcium, production of oxygen-free radicals and lipid peroxidation that induce neuronal injury, neurodegeneration and cell death (McDonald et al. 1988; Choi and Rothman 1990; Barks and Silverstein 1992; Martin et al. 1997; Johnston 2001). Further studies of animal model and in vitro experiments have indicated that the cell death via apoptotic mechanism is one important aspect of these events (Martin et al. 1998; Hu et al. 2000; Nakajima et al. 2000; Northington et al. 2001b). However, larger studies are required to delineate other prognostic factor(s). The availability of animal model, which mimics more closely human neurological disabilities specially that associated with perinatal HI will facilitate efforts to characterize relevant mechanism(s) for effective therapeutic strategies.
Rodent model of perinatal hypoxia-ischemia
We used a well-established rodent model of unilateral cerebral hypoxia-ischemia in 7-dold (P7) rat pups, which is a neonatal adaptation of the Levine (1960) procedure (Levine 1960) modified by Rice (1981) and Vannucci (1997), commonly known as Rice-Vannucci model (Rice et al. 1981; Vannucci and Vannucci 1997). In this neonatal rat model one common carotid artery is permanently ligated followed by exposure to hypoxia (8% O2, balanced nitrogen) for 2 h as we have described previously (Hossain et al. 2004). This procedure causes injury to one side of the brain remarkably similar to that found in human babies (Kuban and Leviton 1994; Menkes and Curran 1994). When examined following HI brains from these animals show neuronal loss and glial scarring in the caudate-putamen, resembling the injury in human postmortem brain specimens of patients sustained hypoxic-ischemic insult (Barkovich 1992). In our experiments using the P7 neonatal rat model of HI, we found that the hemisphere ipsilateral to the ligation appeared edematous within 12 to 24 h and liquefaction was apparent by 48 h after HI. Triphenyltetrazolium chloride (TTC)-stained brain sections showed clear-cut infarction of the ipsilateral cerebral cortex and hippocampal CA1 and CA3 areas. We found significant infarction of the brain occurred during the 24−48 h period of post-HI. Quantitative morphometry of brain injury showed a significant loss in volume of the ipsilateral striatal, cortical and hippocampal regions one week after HI (Hossain et al. 2004). Our laboratory group has been interested in the problem of selective neuronal vulnerability in the developing brain to elucidate the molecular basis of this differential sensitivity to hypoxic-ischemic neuronal injury/death using the neonatal animal model of HI and hypoxic-ischemic injury, modeled in vitro, following oxygen and glucose deprivation of cultured primary neurons (Hossain et al. 2004; Russell et al. 2006). For instance, we found that hippocampal CA3 and CA1 areas sustained severe injury, but not the dentate gyrus (DG) region, following HI. Similarly, excitotoxic cell death in neonatal brain was found to preferentially decrease the number and density of the nNOS+ neurons in the CA3 hippocampal region relative to other brain areas (Hossain et al. 1998). This differential sensitivity to injury is possibly due to the distinct function of different neuronal populations and subpopulations. Further studies of in vivo animal model and in vitro experiments using cultured primary neurons indicate that neuronal injuries in the CNS is caused by a cascade of biochemical events and induction of novel molecules within selective central neurons triggered by the original insult (Hossain et al. 2004; Russell et al. 2006).
Excitatory neurotransmission and hypoxic-ischemic brain injury
One of the earliest events in the excitatory synapse formation is the clustering of postsynaptic glutamate receptors, and nearly all-excitatory synapses are associated with postsynaptic receptor clusters (O'Brien et al. 1997). In the CNS, ionotrophic glutamate receptors are the major excitatory neurotransmitter receptors that mediate almost all-excitatory synaptic transmission in the brain (Hollman and Heinemann 1994). Glutamate receptors are divided into three broad classes, termed α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), N-methyl-D-aspartate (NMDA) and kainite type receptors, on the basis of their molecular and pharmacological criteria (Hollman and Heinemann 1994). Subsequent studies have shown that the targeting and cell surface clustering of the AMPA-and NMDA-type of glutamate receptors to the synapses are essential for efficient excitatory synaptic transmission (O'Brien et al. 1998a). These studies revealed that glutamate receptors are highly concentrated in neurons at excitatory synapses on dendritic spines and shafts (O'Brien et al. 2002). Neuronal injuries occurring with cerebral HI has been attributed to over-stimulation of the NMDA and AMPA subtypes of glutamate receptors (Choi and Rothman 1990; Barks and Silverstein 1992; Portera-Cailliau et al. 1997). The AMPA subtype of glutamate receptors is the predominant charge carrier during routine fast excitatory synaptic transmission, while NMDA receptors contribute significant calcium current and modulate second messengers and kinases (O'Brien et al. 1998b). Functional AMPA receptors are multimeric complex of homologous subunits GluR1-GluR4 (Mano and Teichberg 1998). Recent studies have shown that members of the “long pentraxins” family of proteins such as neuronal activity regulated pentraxin (Narp) are linked to glutamate receptors at synaptic sites (O'Brien et al. 1998a; O'Brien et al. 1999), and functions as an extracellular aggregating factor for AMPA receptors clustering at the synapse (O'Brien et al. 2002; Xu et al. 2003). Thus, it has been proposed that changes in the expression or function of long pentraxin proteins may contribute to changes in the excitatory synaptic transmission induced by neuronal stimulation (Reti and Baraban 2000).
Neuronal pentraxin proteins
The pentraxin family of proteins are divided into two structural classes (subfamilies) based on size, the classical “short” and the novel “long” proteins, are originally named for their distinct structural organization of five identical subunits arranged non-covalently in a pentameric radial fashion (Osmand et al. 1977). The “long pentraxins”, a newly recognized subfamily of proteins, have several structural and functional characteristics that might play a role in promoting excitatory synapse formation and synaptic remodeling (Schlimgen et al. 1995; Kirkpatrick et al. 2000). Included among these are the ability to form side-to-side and head-to-head multimeric aggregates (Gewurz et al. 1995; Goodman et al. 1996; Bottazzi et al. 1997) and the ability to bind other proteins via a lectin-like domain. Members of this subfamily include neuronal pentraxin 1 (NP1), neuronal activity regulated pentraxin (Narp; also called NP2) and neuronal pentraxin receptor (NPR) (Schlimgen et al. 1995; Goodman et al. 1996; Dodds et al. 1997; Kirkpatrick et al. 2000). Narp encodes an unique N-terminal coiled-coil domain that is likely to mediate self aggregation and a single C-terminal pentraxin domain that may be required for axonal transport and secretion (Fig. 1) (Goodman et al. 1996; Tsui et al. 1996; Dodds et al. 1997; O'Brien et al. 2002).
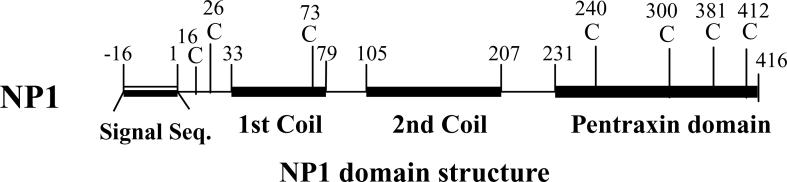
Figure 1.
Schematic diagram of the NP1 protein domain structure. Cysteine rich N terminus contributes to polymerization. NP1 cDNA encodes a 432 amino acid protein with an N-terminal signal sequence, followed by two coiled-coil domains (33−79 and 105−207) and a C-terminal pentraxin domain corresponding to a molecular mass of 47 kDa (Tsui et al. 1996; Xu et al. 2003).
The pentraxin domain on neuronal pentraxins is similar to the mammalian C-reactive protein (CRP) and serum amyloid P protein (SAMP) (Tsui et al. 1996) -members of the “classical pentraxin” family. The long pentraxins are approximately twice the size of classical pentraxin proteins. Among the long pentraxin family of proteins, Narp and NP1 are secreted glycoproteins and are expressed exclusively in the CNS (Schlimgen et al. 1995; Goodman et al. 1996; Omeis et al. 1996; Dodds et al. 1997). Exclusive expression of NP1 in the CNS, its characteristic feature as secretory protein and its large molecular size relative to the classical pentraxins (>50 vs. 30 kDa), suggest that NP1 may have novel, yet unknown, neuronal functions.
Neuronal pentraxins interact with the AMPA-type glutamate receptor
The long pentraxin NP1 is closely related to Narp (NP2) based on similar structural features, sequence homology and functional domains (Schlimgen et al. 1995; Goodman et al. 1996; Tsui et al. 1996; O'Brien et al. 2002). We found that NP1 is induced in response to HI in the hippocampal pyramidal layers of CA3 and CA1 in contrast to that reported for Narp that is expressed primarily in the hippocampal dentate gyrus in response to various pathologic insults (O'Brien et al. 1999; Reti et al. 2002). This differential expression suggests that NP1 and Narp serve distinct functions in different neuronal populations/subpopulations. It is possible that selective induction of NP1 in certain subpopulations of neurons reflects their particularly vulnerability to injury. Based on their structural and sequence similarities, we asked if NP1 is similar to Narp is interacting with glutamate receptors (O'Brien et al. 1998a; O'Brien et al. 1999; O'Brien et al. 2002). We found that NP1 co-localizes and physically associates with the fast excitatory GluR1 AMPA receptors, and that hypoxia induces a time-dependent increase in the NP1-GluR1 interactions (Hossain et al. 2004). It appears from our study that hypoxia recruits NP1 protein to GluR1 subunits concurrent with the hypoxic excitotoxic cascade. Furthermore, the disappearance of NP1 specific immunostaining without a concurrent loss of GluR1-specific clusters in NP1 knockdown neurons suggests that, in contrast to Narp, NP1 may not play a substantial role in the formation or stabilization of GluR1 clusters. Based on our findings, we propose that NP1 modulates the function of AMPA receptors through interactions with GluR1 clusters in a manner that sensitizes neurons to hypoxia-induced excitotoxic death. This is further evidenced by our findings that knockdown of NP1 protein in cortical neurons were protected against AMPA-induced death. Our findings demonstrate a direct link between NP1 and AMPA receptor (GluR1)-mediated neuronal death. It is likely that NP1 may also interact with other subunits of excitatory AMPA and NMDA subtypes of glutamate receptors that are activated during hypoxia in addition to GluR1, which is a subject of our ongoing investigations.
Hypoxic-ischemic injury induces NP1 in neonatal rat brain
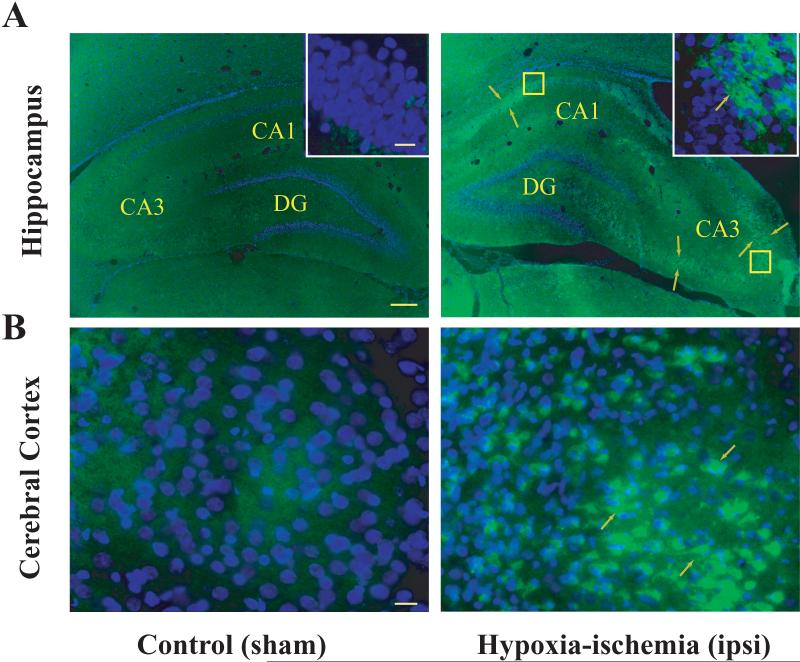
The physiological functions of neuronal pentraxins remain elusive. It is now known that Narp regulates AMPA receptor clustering at synaptic sites (O'Brien et al. 1998a; O'Brien et al. 1999; O'Brien et al. 2002). In contrast to Narp, a function for NP1 in central neurons has not yet been characterized. Based on available literatures on neuronal pentraxins, their associations with the excitatory glutamate receptor subtype AMPA GluR1 (O'Brien et al. 1998a; O'Brien et al. 1999; O'Brien et al. 2002; Xu et al. 2003), and the reported involvement of glutamate-mediated excitotoxicity in hypoxic-ischemic brain injury (McDonald et al. 1988; Choi and Rothman 1990; Barks and Silverstein 1992; Portera-Cailliau et al. 1997; Johnston 2001; Johnston et al. 2001), we have examined specifically the role of NP1 in hypoxic-ischemic brain injury. We have used the in vivo neonatal rat model of HI and in vitro model of hypoxic-ischemic injury using cultured primary cortical neurons. We found a temporal relationship between NP1 induction and cell injury/death initiated after HI by immunofluorescence analyses of brain sections obtained at 6, 12, 24 48 h and 7 d following HI and from sham controls. Fluorescence microscopy revealed that HI induced NP1-specific intense immunoreactivity in the ipsilateral frontal and parietal cortex from 6 h following HI onset and in the pyramidal layer of hippocampal CA3 and CA1 regions, but not in the dentate gyrus (DG), from 24 h HI onset (Hossain et al. 2004). NP1-specific immunofluorescence persisted for at least 7 d after HI was examined (Fig. 2).
Figure 2.
Induction of NP1 in the ipsilateral hippocampus and cerebral cortex of neonatal rat brain following HI. Neonatal rats were sacrificed at indicated time after HI exposure. Representative coronal brain sections (20 μm) from sham controls and HI brains were immuno-stained using mouse anti-rat NP1 monoclonal primary antibody (1:500) (BD Transduction laboratories, CA) and FITC-conjugated secondary antibody (1:500) (Jackson ImmunoResearch) as described previously (Hossain et al. 2004). A high level of NP1 immunoreactivity was observed in the pyramidal layer of CA3 and CA1 but not in the dentate gyrus of the ipsilateral hippocampus and in the frontal and parietal cortex relative to sham controls. Immunofluorescence analyses were performed using a fluorescence microscope (Carl Zeiss Axioplan 1 microscope fitted with AxioVision 3.0 software). NP1 specific immunofluorescence (green) from representative brain sections is shown (arrows) at low (5 ×) and high (100 ×) magnifications. Scale bar: A, 200 μm; B and inset 20 μm.
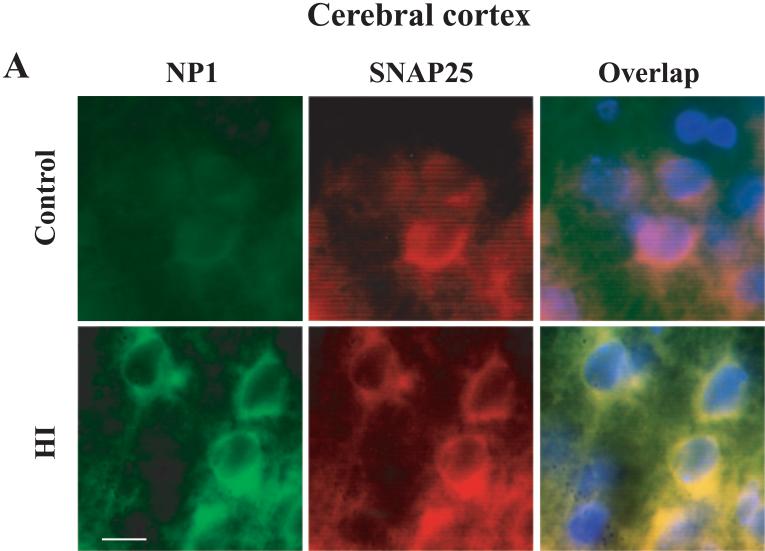
Next, we examined cell specificity of NP1 induction in brain. Fluorescence microscopic analyses of brain sections from sham controls and HI animals immunohistochemically stained with antibodies specific for NP1, neuronal specific marker SNAP25 or glial cell marker GFAP as described earlier (Hossain et al. 2004). We found increased NP1-specific fluorescence (green) in the cytoplasm of cells that exhibited a neuronal morphology as evident by double immunostaining with the neuronal-specific marker SNAP25 (red). Overlapping of digitized images showed colocalization of NP1 with neuronal marker SNAP25 (Fig. 3), but no colocalization of NP1 was found with glial marker GFAP (not shown), suggesting induction of NP1 expression is exclusively in the neurons of hypoxic-ischemic brain. The specificity of NP1 and SNAP25 immunofluorescence was confirmed by incubating brain sections with nonimmune IgG (negative control).
Figure 3.
NP1 induction in neonatal brain following HI is neuronal specific. Brain sections (15 μm) collected from sham controls and HI animals sacrificed at 24 h post-HI. Immunofluorescence staining was performed with respective primary antibody for NP1 (1:500), SNAP25 (1:500) or GFAP (1:1000) followed by incubation with appropriate FITC- (green) and Texas red- (red) conjugated secondary antibodies. Brain sections were coverslipped with Prolong Gold (Invitrogen) antifed mounting medium containing 4,6diamino-2-phenylindole (DAPI) that stains nuclei (blue). Immunofluorescence analyses were performed using a fluorescence microscope (Carl Zeiss Axioplan 1 microscope fitted with AxioVision 3.0 software). Representative images are shown taken from cerebral cortex ischemic penumbra and viewed at 100 × magnifications. Bar = 10 μm.
Comparing the post-HI time of NP1 induction relative to the appearance of brain injury we found that NP1 induction occurs prior to actual tissue loss. Similarly, NP1 was also induced in the primary cortical neurons cultured for 8−10 days in vitro and exposed to hypoxia (humidified 95 % N2 /5% CO2) under the conditions we found significant cell death compared to normoxia control cultures (Hossain et al. 2004). Together these results confirm NP1 expression in cortical and hippocampal brain areas and its potential association with HI brain injury.
Accumulating evidence indicates that hypoxic-ischemic cell death occurs either through necrosis and/or apoptosis. Early HI-induced neuronal death occurs through necrosis (primary damage) (Northington et al. 2001c). The delayed neuronal death (secondary damage) that occurs hours or days later through a series of complex and highly regulated biochemical and molecular events leading to apoptosis (Puka-Sundvall et al. 1997; Leist and Nocotera 1998; Northington et al. 2001b). Apoptosis plays a prominent role in the evolution of hypoxic-ischemic injury in the neonatal brain and may be more important than necrosis after injury (Hu et al. 2000). During neonatal brain injury, excitotoxicity, oxidative stress and inflammation all contribute to accelerated cell death by means of either apoptosis or necrosis, depending on the region of the brain affected and the severity of the insult (Martin et al. 1998). Further examination of the nature of cell death in our model and the potential role of NP1, we used terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) histochemistry as a tool for detection of degenerated neurons in brain sections. TUNEL- (+) cells were detected from 24 h onset of HI and examination brain areas revealed intensely stained TUNEL- (+) cells found in the ipsilateral frontal and parietal cortex and pyramidal layer of CA1 and CA3, brain areas that showed NP1 induction at post-HI periods. A similar temporal pattern of caspase-3, an important neuronal death effector in HI models, activation was found in the same brain areas following HI (Chen et al. 1998; Namura et al. 1998; Russell et al. 2006). These TUNEL-positive cells featured characteristics of both apoptotic and necrotic morphologies, which are consistent with previous findings in a similar neonatal rat model (Nakajima et al. 2000). Our study demonstrates that induction of NP1 in the ipsilateral hemisphere of cortical and hippocampal CA1 and CA3 areas occurred at earlier period of post-HI before the appearance of degenerated cells. This is consistent with a role for NP1 in the injury cascade.
NP1 gene silencing is neuroprotective
If neuronal pentraxin NP1 is involved in neuronal injury program triggered by HI, then inhibition of NP1 induction or blockade of NP1 function will prevent neuronal death caused by HI. To address this issue we used NP1 loss-of-function strategies. NP1 gene silencing was achieved by anti-sense knockdown strategy to demonstrate mechanistically the role of NP1 in hypoxic-ischemic neuronal injury. We found cortical neurons transfected with a 21-base long phosphorothioated antisense oligonucleotides (ODNs) directed against NP1 mRNA (NP1AS) (DeGregorio-Rocasolano et al. 2001) showed neuroprotection against hypoxia-induced neuronal death under conditions that completely inhibited the expression of NP1 protein in response to hypoxia (Hossain et al. 2004). Our findings further support the previous report of NP1AS incubated with cerebellar granule neurons blocked overexpression of NP1 in cerebellar granule cells undergoing apoptosis (DeGregorio-Rocasolano et al. 2001). These results directly demonstrate that NP1 gene silencing by NP1AS rescues primary cortical neurons from hypoxia-induced death. Using NP1-specific dominant negative inhibitor we further demonstrated the specific involvement of NP1 induction in hypoxia-induced neuronal death (unpublished data). Subsequent experiments using cultured cortical neurons showed that inhibition of NP1 by dominant negative inhibitor effectively blocked NMDA and AMPA-induced excitotoxic cell death, but failed to block H2O2 and tumor necrosis factor-related apoptosis inducing ligand (TRAIL)-induced neuronal death (unpublished data). It appears form our results that induction of NP1 expression is specifically associated with hypoxic-ischemic neuronal death.
NP1 co-localization with the AMPA GluR1 clusters, physical association between NP1 and GluR1 and hypoxia time-dependent increase in NP1 protein levels in GluR1 immunoprecipitates further suggest that hypoxia leads to the recruitment of NP1 to GluR1 subunits and a role for NP1 in the excitotoxic cascade. Particularly interesting is the interactions between NP1 and GluR1, and observed neuroprotection against AMPA-induced excitotoxicity following the inhibition of NP1 function. Collectively, our findings suggest a novel mechanism by which NP1 induction during HI accentuates excitotoxicity through interactions with GluR1 and potentially other glutamate receptor subtypes, thereby contributing to neuronal injury and death. Our study identifies a new molecular target in the central neurons for preventing hypoxic-ischemic injury, which could lead to more effective neuroprotective strategies in neonates suffering from pathological consequences of hypoxic-ischemic insult.
Summary
Brain injury from HI is not immediate but evolves over a period of times after initial insult. When a brain injury occurs, the functions of the neurons, nerve tracts or certain sections of the brain are affected. Various studies on experimental HI in immature animal models demonstrate that delayed neuronal damage is triggered initially by overstimulation of excitatory glutamate receptors and executed by a cascade of events. The neonatal brain is far more electrically excitable and prone to excitotoxicity than the adult brain, and the excitatory cascade rapidly becomes self-perpetuating causing ultimately to neuronal injury and degeneration. These studies indicate that therapy for hypoxic-ischemic injury in developing brain and associated neurological disorders of infants and children will be feasible in the future. Studies in laboratory animals have shown that the immature brain responds differently to treatment than does the mature brain (Volpe 2001). Furthermore, therapy designed to ameliorate brain injury in adults may worsen outcomes in neonates, possibly by accentuating apoptotic cell death cascade. Control of neuronal injury involves a balance between expression of numerous apoptotic and anti-apoptotic proteins after injury, providing many potential approaches to modifying outcome (Cheng et al. 1997; Cheng et al. 1998; Schulz et al. 1999). It is noteworthy that identification of novel neuronal target molecules such as NP1, which is possibly acting upstream in regulating the glutamate receptor mediated excitotoxicity and/or the onset of the cascade of delayed events in the cell death pathway will have the better potential to prevent the serious neuropathological effects of perinatal brain injury. Recently, we found that transgenic expression of FGF-1 in neonatal brain protects against hypoxic-ischemic injury by blocking the activation of the death cascade components (Russell et al. 2006). Most importantly, our identification of the novel neuronal protein NP1 induction in neonatal brain following HI and further characterization of its specific involvement in HI-induced neuronal death positions NP1 as a potential new molecular target in central neurons for preventing neuronal injury. This is the first evidence for a pathophysiologic role of NP1 in central neurons and our findings point to a mechanism by which NP1 modulate s hypoxic-ischemic injury at the level of excitatory cascade (Hossain et al. 2004). These very novel results could lead to more effective neuroprotective strategies against hypoxic-ischemic brain injury. However, developing novel strategies to treat and avert the consequences of hypoxic-ischemic brain injury in children will require greater understanding of the unique mechanism(s) of injury initiation and propagation in the immature brain, and it remains for future research to uncover new and effective strategies for clinical management of neurologically compromised infants.
Acknowledgements
This work was supported by grants from the American Heart Association (National, 0030004N) and the National Institute of Neurological Disorders and Stroke (NINDS RO1 NS046030).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini W, Carloni S, Mazzoni E, Cimino M. New therapeutic strategies in perinatal stroke. Curr Drug Targets CNS Neurol Disord. 2004;3:315–323. doi: 10.2174/1568007043337247. [DOI] [PubMed] [Google Scholar]
- Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. AJNR Am J Neuroradiol. 1992;13:959–972. discussion 973−955. [PMC free article] [PubMed] [Google Scholar]
- Barks JD, Silverstein FS. Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain Pathol. 1992;2:235–243. doi: 10.1111/j.1750-3639.1992.tb00697.x. [DOI] [PubMed] [Google Scholar]
- Bottazzi B, Vouret-Craviari V, Bastone A, De Gioia L, Matteucci C, Peri G, Spreafico F, Pausa M, D'Ettorre C, Gianazza E, Tagliabue A, Salmona M, Tedesco F, Introna M, Mantovani A. Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component. J Biol Chem. 1997;272:32817–32823. doi: 10.1074/jbc.272.52.32817. [DOI] [PubMed] [Google Scholar]
- Chen J, Nagayama T, Jin K, Stetler RA, Zhu RL, Graham SH, Simon RP. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Gidday JM, Yan Q, Shah AR, Holtzman DM. Marked age-dependent neuroprotection by brain-derived neurotrophic factor against neonatal hypoxic-ischemic brain injury. Ann Neurol. 1997;41:521–529. doi: 10.1002/ana.410410416. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Deshmukh M, D'Costa A, Demaro JA, Gidday JM, Shah A, Sun Y, Jacquin MF, Johnson EM, Holtzman DM. Caspase inhibitor affords neuroprotection with delayed administration in a rat model of neonatal hypoxic-ischemic brain injury. J Clin Invest. 1998;101:1992–1999. doi: 10.1172/JCI2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Rothman SW. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Ann Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- DeGregorio-Rocasolano N, Gasull T, Trullas R. Overexpression of neuronal pentraxin 1 is involved in neuronal death evoked by low K(+) in cerebellar granule cells. J Biol Chem. 2001;276:796–803. doi: 10.1074/jbc.M007967200. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M, Mishra OP. Mechanisms of perinatal cerebral injury in fetus and newborn. Ann N Y Acad Sci. 2000;900:159–168. doi: 10.1111/j.1749-6632.2000.tb06226.x. [DOI] [PubMed] [Google Scholar]
- Dodds DC, Omeis IA, Cushman SJ, Helms JA, Perin MS. Neuronal pentraxin receptor, a novel putative integral membrane pentraxin that interacts with neuronal pentraxin 1 and 2 and taipoxin-associated calcium-binding protein 49. J Biol Chem. 1997;272:21488–21494. doi: 10.1074/jbc.272.34.21488. [DOI] [PubMed] [Google Scholar]
- Ferriero DM. Neonatal brain injury. N Engl J Med. 2004;351:1985–1995. doi: 10.1056/NEJMra041996. [DOI] [PubMed] [Google Scholar]
- Franck JE, Roberts DL. Combined kainate and ischemia produces ‘mesial temporal sclerosis’. Neurosci Lett. 1990;118:159–163. doi: 10.1016/0304-3940(90)90616-h. [DOI] [PubMed] [Google Scholar]
- Gewurz H, Zhang XH, Lint TF. Structure and function of the pentraxins. Curr Opin Immunol. 1995;7:54–64. doi: 10.1016/0952-7915(95)80029-8. [DOI] [PubMed] [Google Scholar]
- Goodman AR, Cardozo T, Abagyan R, Altmeyer A, Wisniewski HG, Vilcek J. Long pentraxins: an emerging group of proteins with diverse functions. Cytokine Growth Factor Rev. 1996;7:191–202. doi: 10.1016/1359-6101(96)00019-6. [DOI] [PubMed] [Google Scholar]
- Hollman M, Heinemann S. Cloned glutamate receptors. Ann Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Russell JC, O'Brien R, Laterra J. Neuronal pentraxin 1: a novel mediator of hypoxic-ischemic injury in neonatal brain. J Neurosci. 2004;24:4187–4196. doi: 10.1523/JNEUROSCI.0347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Fielding KE, Trescher WH, Ho T, Wilson MA, Laterra J. Human FGF-1 gene delivery protects against quinolinate-induced striatal and hippocampal injury in neonatal rats. Eur J Neurosci. 1998;10:2490–2499. [PubMed] [Google Scholar]
- Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjo BK. Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab. 2000;20:1294–1300. doi: 10.1097/00004647-200009000-00003. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Hypoxic and ischemic disorders of infants and children. Lecture for 38th meeting of Japanese Society of Child Neurology, Tokyo, Japan, July 1996. Brain Dev. 1997;19:235–239. doi: 10.1016/s0387-7604(96)00561-x. [DOI] [PubMed] [Google Scholar]
- Johnston MV. Excitotoxicity in neonatal hypoxia. Ment Retard Dev Disabil Res Rev. 2001;7:229–234. doi: 10.1002/mrdd.1032. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Hoon AH,, Jr. Possible mechanisms in infants for selective basal ganglia damage from asphyxia, kernicterus, or mitochondrial encephalopathies. J Child Neurol. 2000;15:588–591. doi: 10.1177/088307380001500904. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins. Neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–17792. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Clark RS, Ruppel RA, Adelson PD, Bell MJ, Whalen MJ, Robertson CL, Satchell MA, Seidberg NA, Marion DW, Jenkins LW. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr Crit Care Med. 2000;1:4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–195. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- Leist M, Nocotera P. Apoptosis, excitotoxicity and neuropathology. Exp Cell Res. 1998;239:183–201. doi: 10.1006/excr.1997.4026. [DOI] [PubMed] [Google Scholar]
- Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- Lorenz JM, Wooliever DE, Jetton JR, Paneth N. A quantitative review of mortality and developmental disability in extremely premature newborns. Arch Pediatr Adolesc Med. 1998;152:425–435. doi: 10.1001/archpedi.152.5.425. [DOI] [PubMed] [Google Scholar]
- Mano I, Teichberg VI. A tetrameric subunit stoichiometry for a glutamate receptor-channel complex. Neuroreport. 1998;9:327–331. doi: 10.1097/00001756-199801260-00027. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Brambrink A, Koehler RC, Traystman RJ. Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia-ischemia. J Comp Neurol. 1997;377:262–285. doi: 10.1002/(sici)1096-9861(19970113)377:2<262::aid-cne8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Al-Abdulla NA, Brambrink AM, Kirsch JR, Sieber FE, Portera-Cailliau C. Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis. Brain Res Bull. 1998;46:281–309. doi: 10.1016/s0361-9230(98)00024-0. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Silverstein FS, Johnston MV. Neurotoxicity of N-methyl-D-aspartate is markedly enhanced in developing rat central nervous system. Brain Res. 1988;459:200–203. doi: 10.1016/0006-8993(88)90306-x. [DOI] [PubMed] [Google Scholar]
- McPeak LA, Stiers WM, Cope DN. Disability evaluation following traumatic brain injury. Phys Med Rehabil Clin N Am. 2001;12:587–601. [PubMed] [Google Scholar]
- McQuillen PS, Sheldon RA, Shatz CJ, Ferriero DM. Selective vulnerability of subplate neurons after early neonatal hypoxia-ischemia. J Neurosci. 2003;23:3308–3315. doi: 10.1523/JNEUROSCI.23-08-03308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkes JH, Curran J. Clinical and MR correlates in children with extrapyramidal cerebral palsy. AJNR Am J Neuroradiol. 1994;15:451–457. [PMC free article] [PubMed] [Google Scholar]
- Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA. Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Martin LJ. Neurodegeneration in the thalamus following neonatal hypoxia-ischemia is programmed cell death. Dev Neurosci. 2001a;23:186–191. doi: 10.1159/000046141. [DOI] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Flock DL, Martin LJ. Delayed neurodegeneration in neonatal rat thalamus after hypoxia- ischemia is apoptosis. J Neurosci. 2001b;21:1931–1938. doi: 10.1523/JNEUROSCI.21-06-01931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early Neurodegeneration after Hypoxia-Ischemia in Neonatal Rat Is Necrosis while Delayed Neuronal Death Is Apoptosis. Neurobiol Dis. 2001c;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- O'Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Lau LF, Huganir RL. Molecular mechanisms of glutamate receptor clustering at excitatory synapses. Curr Opin Neurobiol. 1998a;8:364–369. doi: 10.1016/s0959-4388(98)80062-7. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Mammen AL, Blackshaw S, Ehlers MD, Rothstein JD, Huganir RL. The development of excitatory synapses in cultured spinal neurons. J Neurosci. 1997;17:7339–7350. doi: 10.1523/JNEUROSCI.17-19-07339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998b;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Omeis IA, Hsu YC, Perin MS. Mouse and human neuronal pentraxin 1 (NPTX1): conservation, genomic structure, and chromosomal localization. Genomics. 1996;36:543–545. doi: 10.1006/geno.1996.0503. [DOI] [PubMed] [Google Scholar]
- Osmand AP, Friedenson B, Gewurz H, Painter RH, Hofmann T, Shelton E. Characterization of C-reactive protein and the complement subcomponent C1t as homologous proteins displaying cyclic pentameric symmetry (pentraxins). Proc Natl Acad Sci U S A. 1977;74:739–743. doi: 10.1073/pnas.74.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Torres-Munoz J, Roberts B, Olarte JP, Nowak TS,, Jr., Pulsinelli WA. DNA fragmentation follows delayed neuronal death in CA1 neurons exposed to transient global ischemia in the rat. J Cereb Blood Flow Metab. 1997;17:967–976. doi: 10.1097/00004647-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Price DL, Martin LJ. Non-NMDA and NMDA receptor mediated excitotoxic neuronal deaths in adult brain are morphologically distinct: Further evidence for an apoptosis-necrosis continuum. J Comp Neurol. 1997;378:88–104. [PubMed] [Google Scholar]
- Puka-Sundvall M, Sandberg M, Hagberg H. Brain injury after hypoxia-ischemia in newborn rats: relationship to extracellular levels of excitatory amino acids and cysteine. Brain Res. 1997;750:325–328. doi: 10.1016/s0006-8993(97)00024-3. [DOI] [PubMed] [Google Scholar]
- Rami A, Jansen S, Giesser I, Winckler J. Post-ischemic activation of caspase-3 in the rat hippocampus: evidence of an axonal and dendritic localisation. Neurochem Int. 2003;43:211–223. doi: 10.1016/s0197-0186(03)00002-0. [DOI] [PubMed] [Google Scholar]
- Reti IM, Baraban JM. Sustained increase in Narp protein expression following repeated electroconvulsive seizure. Neuropsychopharmacology. 2000;23:439–443. doi: 10.1016/S0893-133X(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Prominent Narp expression in projection pathways and terminal fields. J Neurochem. 2002;82:935–944. doi: 10.1046/j.1471-4159.2002.01051.x. [DOI] [PubMed] [Google Scholar]
- Rice JE,, 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141. doi: 10.1002/ana.410090206. [DOI] [PubMed] [Google Scholar]
- Russell JC, Szuflita N, Khatri R, Laterra J, Hossain MA. Transgenic expression of human FGF-1 protects against hypoxic-ischemic injury in perinatal brain by intervening at caspase-XIAP signaling cascades. Neurobiol Dis. 2006;22:677–690. doi: 10.1016/j.nbd.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Saito A, Hayashi T, Okuno S, Nishi T, Chan PH. Oxidative stress affects the integrin-linked kinase signaling pathway after transient focal cerebral ischemia. Stroke. 2004;35:2560–2565. doi: 10.1161/01.STR.0000144653.32853.ed. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- Schlimgen AK, Helms JA, Vogel H, Perin MS. Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron. 1995;14:519–526. doi: 10.1016/0896-6273(95)90308-9. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Weller M, Moskowitz MA. Caspases as treatment targets in stroke and neurodegenerative diseases. Ann Neurol. 1999;45:421–429. doi: 10.1002/1531-8249(199904)45:4<421::aid-ana2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Towfighi J, Mauger D. Temporal evolution of neuronal changes in cerebral hypoxia-ischemia in developing rats: a quantitative light microscopic study. Brain Res Dev Brain Res. 1998;109:169–177. doi: 10.1016/s0165-3806(98)00077-7. [DOI] [PubMed] [Google Scholar]
- Tsui CC, Copeland NG, Gilbert DJ, Jenkins NA, Barnes C, Worley PF. Narp, a novel member of the pentraxin family, promotes neurite outgrowth and is dynamically regulated by neuronal activity. J Neurosci. 1996;16:2463–2478. doi: 10.1523/JNEUROSCI.16-08-02463.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC. Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res. 1990;27:317–326. doi: 10.1203/00006450-199004000-00001. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics. 1997;100:1004–1014. doi: 10.1542/peds.100.6.1004. [DOI] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. A model of perinatal hypoxic-ischemic brain damage. Ann N Y Acad Sci. 1997;835:234–249. doi: 10.1111/j.1749-6632.1997.tb48634.x. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Perinatal brain injury: from pathogenesis to neuroprotection. Ment Retard Dev Disabil Res Rev. 2001;7:56–64. doi: 10.1002/1098-2779(200102)7:1<56::AID-MRDD1008>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Williams PA, Dou P, Dudek FE. Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia. Epilepsia. 2004;45:1210–1218. doi: 10.1111/j.0013-9580.2004.60403.x. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O'Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
- Zhu C, Wang X, Huang Z, Qiu L, Xu F, Vahsen N, Nilsson M, Eriksson PS, Hagberg H, Culmsee C, Plesnila N, Kroemer G, Blomgren K. Apoptosis-inducing factor is a major contributor to neuronal loss induced by neonatal cerebral hypoxia-ischemia. Cell Death Differ. 2007;14:775–784. doi: 10.1038/sj.cdd.4402053. [DOI] [PubMed] [Google Scholar]