Summary
Growing evidence suggests that cell signaling can occur through recognition of changes in extracellular pH. In the nematode C. elegans, defecation is a rhythmic behavior timed by oscillatory Ca2+ signaling in the intestine [1–5]. Here, using pH sensitive reporters, we show that the intracellular pH of the intestine also oscillates during defecation in a spatial and temporal pattern that resembles that of Ca2+ waves and that protons are an essential component of defecation signaling. Our results suggest that protons depart from the intestinal lumen, pass through the cell, and exit into the pseudocoelom during defecation. Integral to this process is NHX-7 (pbo-4), a Na+/H+ exchanger located on the basolateral membrane of posterior intestinal cells, which is necessary for robust proton efflux into the pseudocoelom following intracellular acidification. Transgene rescue of nhx-7 mutants suggests that these extruded protons are essential for strong contractions of the posterior body wall muscles during defecation. NHX-2 is a second Na+/H+ exchanger whose distribution is limited to the apical membranes facing the intestinal lumen. RNA interference of nhx-2 reduces the basal pH of the intestinal cells and also reduces the rate of proton movement between the lumen and the cytoplasm during defecation. These reductions extend the defecation period, suggesting that the cell may integrate both pH and calcium signals to regulate defecation timing. Overall, these results establish the defecation cycle as a model system for studying trans-epithelial proton flux in tissues that maintain systemic acid-base balance.
Keywords: calcium, H+ transport, oscillation, C. elegans, biosensor
Results and Discussion
Intracellular pH Oscillations in the Intestine Parallel Defecation
The C. elegans defecation cycle is an ultradian behavior that occurs rhythmically at ~45 s intervals [6] and is timed by oscillatory Ca2+ signaling in the intestine [1]. The timing of defecation relies critically on the inositol-1,4,5-trisphophate receptor (InsP3R), an intracellular Ca2+ release channel [1]. Mutations in the InsP3R itself or in upstream signaling processes that regulate InsP3 production can influence the defecation period [2, 7]. The three steps of the defecation motor program (DMP) consist of a posterior body wall muscle contraction (pBoc), anterior body wall muscle contraction (aBoc), and expulsion. These steps are temporally coordinated by an intestinal Ca2+ wave that initiates in the posterior-most ring of the twenty epithelial cells that comprise the nematode intestine and propagates forward [3–5]. Mutations in inx-16, a gap junction protein [5] and egl-8, coding for phospholipaseC-β [4], alter the site of Ca2+ wave initiation. When the waves in these mutants travel through the posterior intestine in reverse (ie. from anterior to posterior), focal contractions of the posterior body wall muscles have been noted, suggesting point-to-point secretion of a signaling molecule by the intestinal epithelia [4, 5].
A reduction in the expression level of the Na+/H+ exchanger gene nhx-7 by RNA interference (RNAi) resulted in a weak pBoc (data not shown). Na+/H+ exchangers play an important role in regulating pH and Na+ homeostasis by mediating the electroneutral counter-transport of extracellular Na+ and intracellular H+. To test the hypothesis that changes in pH may be involved in defecation and particularly in the execution of pBoc, we expressed a pH-sensitive reporter, pHluorin [8], in the twenty epithelial cells of the nematode intestine. Fluorescent imaging was then used to measure intestinal intracellular pH (pHi) in live behaving worms during defecation. The transgenic worms execute the DMP normally while being imaged and their defecation period is not significantly different than non-transgenic worms (Table S1).
The pHi of the intestinal cells begins to decrease immediately preceding contraction of the posterior body wall muscles, and the duration of the intracellular acidification persists over the entire period that the muscles are contracted (Figure 1A). The pHi oscillations occur with the same frequency as the DMP (Figure 1B; with a period of 44.3s versus 49.7s; not statistically different). Imaging of pHi oscillations in worms fed the vital pH-indicator dye BCECF confirms this observation (Figure 1C). On average, the pHi fluctuates by ~0.4 pH units during defecation, with pHi recovery quickly following acidification (Figure S1A). The recovery process results in an apparent “overshoot” and a slower rate of acidification that occurs between cycles, as well. Table S1 contains average values (± standard deviations) and statistical comparisons for the period, resting pH, and amplitude of these oscillations, as well as values obtained using the other biosensors and mutants described below.
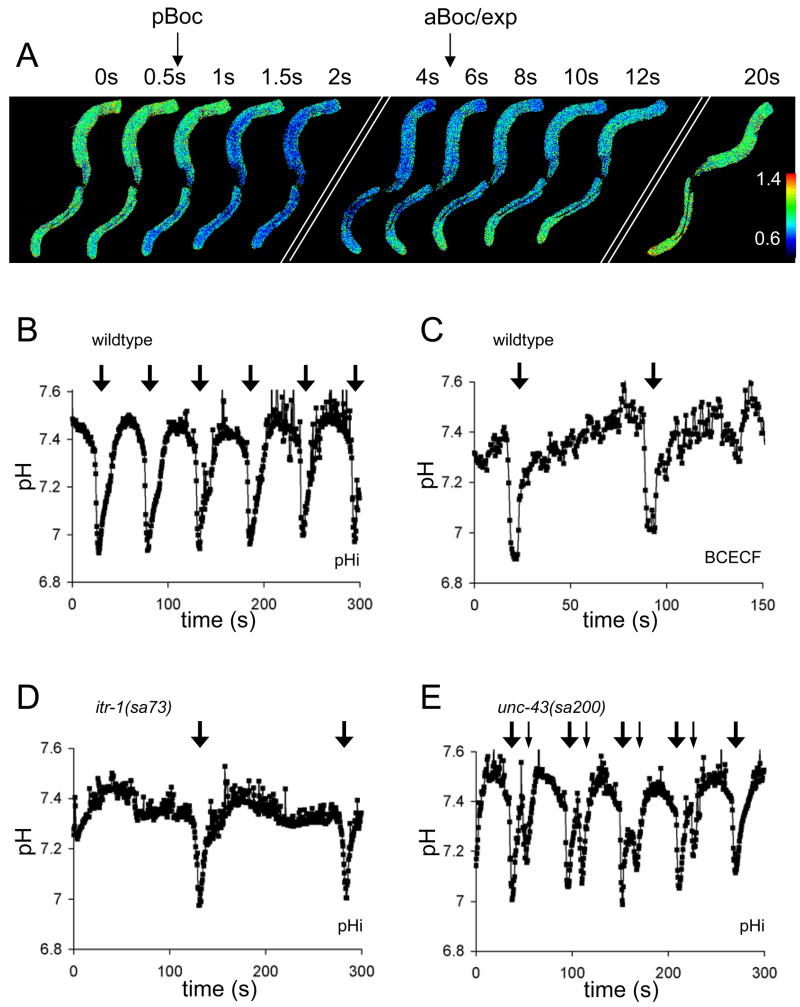
Figure 1. Intestinal pHi Dynamics in Wildtype, itr-1(sa73) and unc-43(sa200) Adult Animals.
(A) is a composite of individual frames extracted from a time-lapse acquisition of intestinal pHi in a transgenic worm expressing Pnhx-2::pHluorin during a single round of defecation. The frames are not consecutive and the times at which they were extracted are indicated (in seconds), as are the execution of pBoc and aBoc. The posterior end of the intestine is oriented toward the bottom of each frame and the anterior end toward the top. The fluorescent ratios obtained imaging live worms (410-nm/470-nm dual excitation, 535-nm emission) have been mapped to a rainbow palette, as shown to the right. Blue corresponds to a more acidic pHi (ratio of 0.6) and red to a more alkaline pHi (ratio of 1.4). (B–E) show representative traces of intestinal pHi oscillations derived from fluorescent imaging of live, behaving worms. The pHluorin emission ratio was converted to pH using a high K+/nigericin calibration technique [21]. The large arrows at the top of the traces represent execution of the DMP. (B) is from a control worm imaged over 300s. Table S1 contains the mean period, resting pHi, and amplitude of these oscillations from multiple worms. (C) is from a wildtype worm fed the pH-sensitive vital dye BCECF (490-nm/440-nm excitation, 535-nm emission), which was taken up mainly by the anterior cells of the intestine. Similar results were obtained from three separate worms. (D, E) are from itr-1(sa73) and unc-43(sa200) mutant worms, respectively. The small arrows in (E) denote where a reiteration of the principle defecation motor program occurs that result in tandem muscle contractions. All worms were imaged live and unrestrained on seeded agarose plates.
Spatially, the acidification initiates in the posterior intestinal cells, and propagates through the entire intestine in a posterior-to-anterior direction in less than one second (Figure 1A and S1B). In comparison, the Ca2+ wave rate is 340 μm/s and progresses through the intestine in less than a second [3–5]. The pHi oscillation period is extended in an InsP3R mutant with an extended defecation period (Figure 1D), while a Ca2+/calmodulin-dependent serine/threonine kinase type II (CamKII) mutant that can exhibit dual muscle contractions in a single cycle [9] also exhibits tandem pHi oscillations (Figure 1E). These observations suggest that the decline in pHi is controlled by a Ca2+-driven pacemaker.
H+ Extrusion via the Na+/H+ exchanger NHX-7 Signals pBoc
Na+/H+ exchange activity is enhanced by H+ binding to an allosteric regulatory site on cytoplasmic C-terminus of the antiporter and thus Na+/H+ exchangers are rapidly activated in response to cellular acidification [10]. The nhx-7 (pbo-4) gene codes for a Na+/H+ exchanger present at the basolateral membrane specifically in the posterior intestinal cells [11]. A deletion mutation in nhx-7 that is expected to result in a null allele reduces pBoc force (Figure S2A) without affecting rhythmic Ca2+ oscillations in the intestine, the defecation period (Table S1), or downstream motor components of the DMP. The posterior body wall muscle contraction (pbo) deficit can be rescued by an extra-chromosomal array expressing an NHX-7 translational GFP reporter (Figure S2). These observations led to the hypothesis that H+ efflux across the basolateral membrane via NHX-7 is necessary for pBoc.
Intestinal pHi oscillations in the nhx-7(ok583) deletion mutant resemble controls (Figure 2A and 2B; mutant provided courtesy of the C. elegans Gene Knockout Consortium). Recovery from intracellular acidification is indistinguishable, as well (Figure S3). Thus, NHX-7 does not play an important role in intestinal pHi homeostasis. To measure pseudocoelomic pH (pHo), pHluorin was targeted to the external face of the basolateral membrane in the posterior intestinal cells via a chimeric translational fusion to the PAT-3 integrin. The distribution of the pHo reporter was confirmed by confocal micrography (Figure S4). Moreover, when the cuticle of the worm was sliced gently to allow the intestine to protrude, the fluorescence of the pHo reporter was intrinsically sensitive to buffer pH, consistent with an extracellular, membrane-anchored distribution (Supplemental Methods).
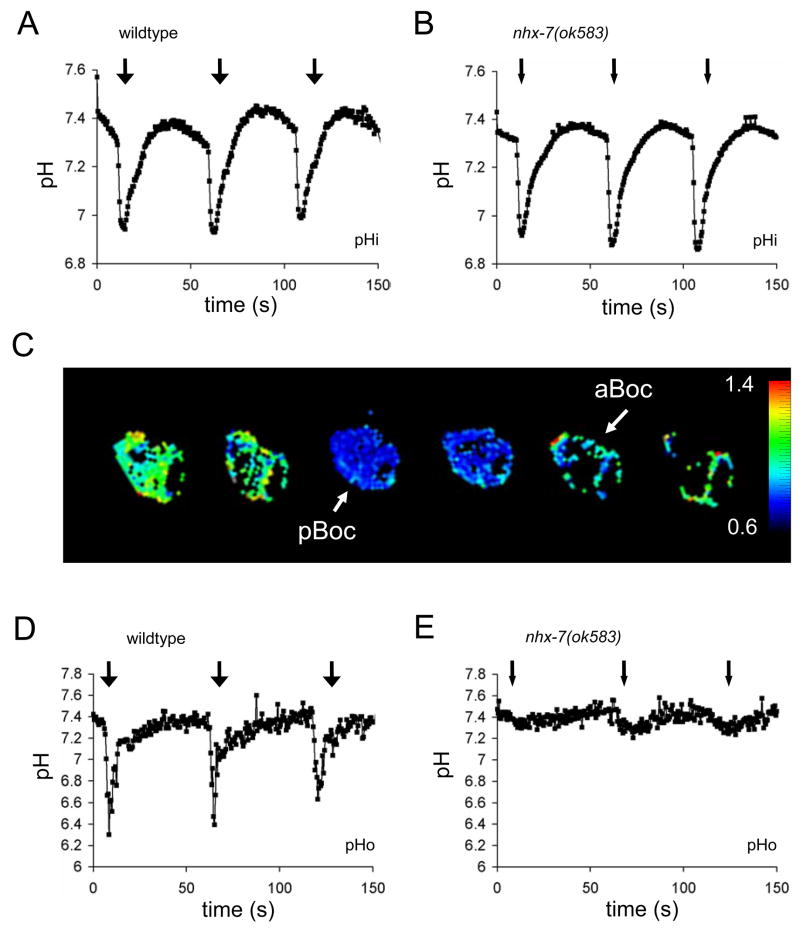
Figure 2. NHX-7 regulates Extracellular, but not Intracellular, pH dynamics.
(A, C, D) are wildtype and (B, E) are nhx-7(ok585) mutants. (A, B) show representative traces of intestinal pHi oscillations during defecation obtained using the Pnhx-2::pHluorin biosensor in live, behaving worms. (C) is a composite of individual frames extracted from an acquisition series of ratiometric images (non-confocal) obtained in live worms expressing an extracellular pHo Pnhx-7::PAT-3::pHluorin biosensor (410-nm/470-nm dual excitation, 535 emission, every 1.5s) in the posterior-most intestinal cells. The ratiometric images were pseudocolored using a rainbow palette, as shown, with blue representing low pH (ratio of 0.6) and red representing high pH (ratio of 1.4). The changes in color reflect pHo oscillations in the pseudocoelom surrounding the posterior-most intestinal ring. The arrow depicting pBoc is pointed from the posterior toward the anterior of the intestine. The arrow depicting aBoc is pointed from the anterior toward the posterior. These arrows reflect the movement of the contents of the intestinal lumen that occur during the respective muscle contractions. (D, E) show representative traces of pHo oscillations during defecation in control and nhx-7(ok583) mutants, respectively. Weak pBoc are denoted by small arrows, while normal pBoc are indicated by larger arrows above the traces. Please see Table S1 for average values and statistical significance.
Using the pHo biosensor, we found that the pseudocoelom surrounding the posterior intestinal cells is acutely acidified during contraction of the posterior body wall muscles (Figure 2C). The acidification persists for around three-seconds (Figure 2D), which is roughly the duration of pBoc (Figure S2). Acidification of the pseudocoelom is severely reduced in the nhx-7(ok583) mutant (Figure 2E, Table S1). These results suggest that NHX-7 causes an acute but temporally limited efflux of H+ from the intestine.
The weak residual pHo oscillations and contractions that remain in the nhx-7(ok583) mutant might reflect a response to cellular acidification by other nhx proteins residing in the intestinal basolateral membrane [11]. Rescue of these weak contractions in the nhx-7(ok583) background via transgene rescue strongly suggest that acute H+ extrusion across the basolateral membrane occurs predominantly via NHX-7 (Figure S2). However, it is possible that slower transport mechanisms at the basolateral membrane might contribute to pHi recovery in the cells, but that the H+ diffuse quickly through the pseudocoelom relative to their extrusion rate without inducing an acute or detectable pHo transient. These data demonstrate that H+ are a critical component of the signal secreted by the intestine that triggers contraction of the body wall muscles. It remains unclear whether Ca2+ signaling is required for NHX-7 activity during defecation, or whether acidification of the intestinal cells is sufficient to cause H+ efflux via an allosteric effect of H+ binding to NHX-7.
H+ enter the cell from the intestinal lumen
Since both the cytoplasm and pseudocoelom are acidified during defecation, we reasoned that the lumen may be the source of H+ driving this process. To test this hypothesis, we measured the pH of the lumen (pHl) by feeding worms dextran conjugated to the pH-sensitive vital dye Oregon Green-488 in S-basal media for one-hour followed by live imaging on plates (Figure 3A). The intestinal lumen rests at a pH value of ~4.1 in wildtype worms and, consistent with our hypothesis, rises quickly to ~6.0 prior to returning to an acidic resting pH in a rhythmic series of oscillations that coincide with defecation (Figure 3B; Table S1). So why does the luminal H+ concentration change by nearly two-thousand times that of the cytoplasm during defecation? One possibility is that the luminal volume may be much less than the volume of cytoplasm, reflecting the fact that pH is a function of H+ concentration. A second is that the cytoplasm may contain more buffering capacity than the lumen. A third possibility is that a uni-directional trans-epithelial flux of acid equivalents occurs, however, our data argues against this being strictly the case (Figure 2B).
Figure 3. Luminal Alkalinization During Defecation.
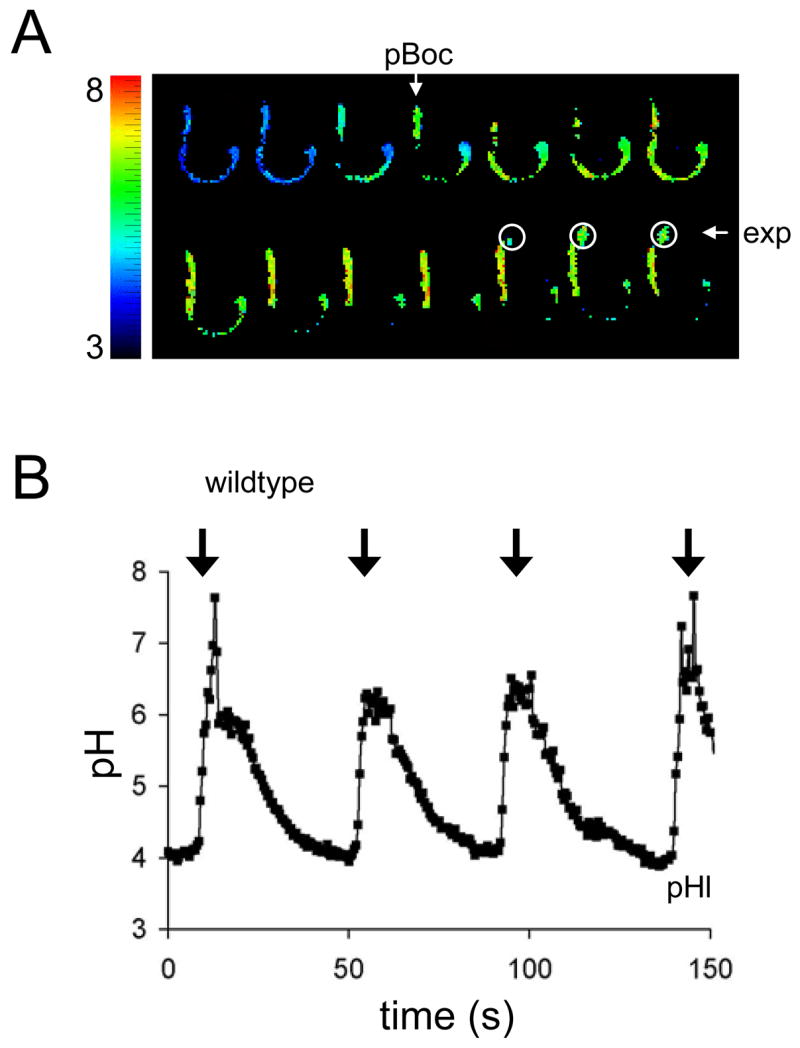
(A) Representative frames extracted from a time lapse acquisition of luminal pH (pHl) oscillations during defecation. Consecutive frames obtained at 2Hz are shown. Wildtype worms were fed dextran coupled to the pH sensitive vital dye Oregon Green-488. The fluorescent ratios (490-nm/440-nm dual excitation, 535-nm emission) obtained by imaging live worms during defecation were mapped to a rainbow palette, as indicated. The execution of pBoc and explusion are denoted. For orientation, the posterior end of the intestine is near the expelled luminal contents, encompassed by white circles. The plate is ~pH 6. In general, due to loading efficiency and retention of the dye, the worms used to obtain pHl measurements were larval rather than adult animals. (B) Representative trace of pHl oscillations during defecation in wildtype worms. The arrows denote pBoc. The ratios obtained from Oregon Green-488 imaging were converted to pH. Transient spikes in pHl above 6 are likely artifacts; as the ratio increases beyond the linear dynamic range of the calibration curve, small changes in ratio lead to progressively larger apparent changes in pH. A full description of this data can be found in Table S1.
In fact, these results suggest that the bulk of the intracellular H+ load both enters and exits the cell through the apical membrane, with only a limited efflux of H+ through NHX-7 being necessary to trigger pBoc (Figure 2B). Perhaps the direction of H+ transport through the apical membrane is determined by Ca2+ signaling? Regardless, we predict that H+ transport into the intestinal cells during defecation prevents their loss to the environment as the luminal contents are expulsed, and their return to the lumen preserves a gradient across the apical membrane that is used to drive H+-coupled nutrient uptake.
The Na+/H+ exchanger NHX-2 modulates defecation timing and H+ flux at the apical membrane
The Na+/H+ exchanger NHX-2 is targeted to the apical membrane facing the intestinal lumen [11]. Reducing the expression of nhx-2 by ~85–90% using RNAi (as determined by QPCR) has been shown to reduce the resting pHi of the intestinal cytoplasm [12], and doubles the average defecation period from ~44s to 86s (Table S1). Defecation timing is thought to be mainly a function of Ca2+ signaling, and regulation of defecation timing by an acid-base transporter may highlight a unique connection between pH and Ca2+ signaling processes.
The pH oscillation periods obtained from live, unrestrained worms following nhx-2 RNAi were calculated from fluorescent imaging experiments. These periods are ~81s in the transgenic pHi strain, ~95s in the transgenic pHo strain and ~71s in worms fed dextran-conjugated Oregon Green-488 (Table S1), which are comparable to the extension caused by nhx-2 RNAi in control nematodes. The fact that the defecation behavior occurs coincident with each pH oscillation in individual worms strongly suggests that the slight variations noted in oscillation periods are a function of either the biosensors or the heterogeneity inherent in RNAi.
The resting intestinal pHi is ~7.2 in the nhx-2(RNAi) worms (Figure 4A; Table S1), as noted previously [12], but the intestinal pHo lies ~4.1 (Figure 4B; Table S1), which is not significantly different from controls. However, the magnitude of both the pHi and pHl oscillations are reduced by loss of NHX-2 (Figure 4A and B, Figure S5, and Table S1). These data suggest that NHX-2 contributes to H+ movement through the apical membrane, either directly or indirectly.
Figure 4. pH Dynamics in Worms Treated with nhx-2 RNAi.
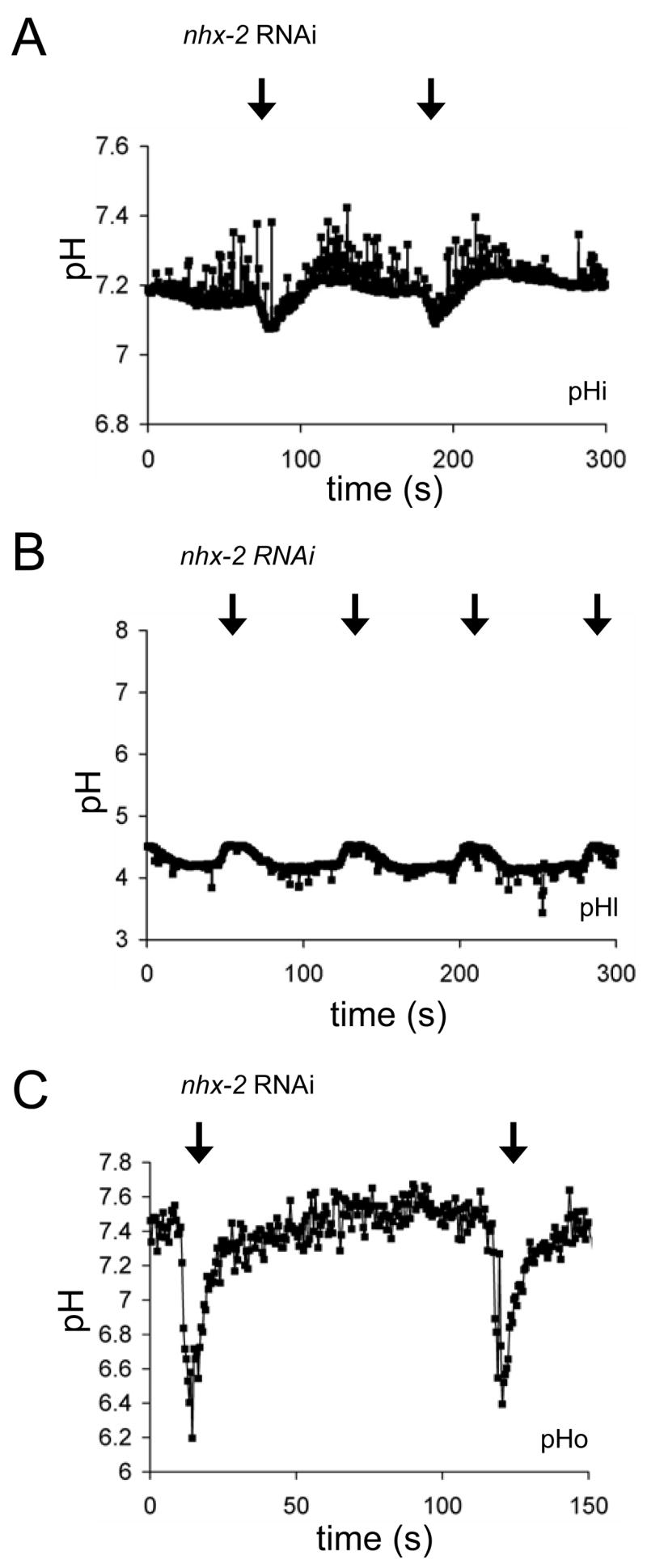
Representative traces following the loss of NHX-2 show (A) intestinal pHi oscillations in a live worm expressing a Pnhx-2::pHluorin biosensor, (B) pHl oscillations in a live worm fed Oregon Green-488, and (C) intestinal pHo oscillations in a live worm expressing a Pnhx-7::PAT-3::pHluorin biosensor. Table S1 contains average values and a statistical analysis of significance.
The apparently decreased rates of H+ flux across the apical membrane might be expected to translate into a decreased rate of H+ extrusion across the basolateral membrane, resulting in a pbo phenotype. But surprisingly we found that oscillations in pHo were of normal amplitude and were actually increased in duration (Figure 4C). These data were confirmed by visually observing luminal compression as a result of posterior body wall muscle contractions; nhx-2(RNAi) worms had significantly longer contractions than wildtype worms (Figure S2B). A reduced influx of H+ may be offset in part by the acidic resting pHi; because the pHi is more acidic to begin with, the nadir during pHi oscillations reaches 7.06, which is not significantly different from control worms (Table S1). This may be sufficient to elicit H+ efflux via NHX-7, resulting in strong body wall muscle contractions. However, since NHX-7 activity has little effect on pHi, it is unlikely that even the increased efflux observed here will result in significant cellular alkalinization.
Finally, we examined Ca2+ and pHi wave dynamics following loss of NHX-2 (Figure S6). Apart from the extended cycle period, the only significant difference appeared to be a slower rate of Ca2+ clearance in the anterior most intestinal cells. Although the pHi oscillations are reduced in amplitude, the anterior cells of the intestine begin to acidify within one second of the posterior cells, reflecting similar wave dynamics as in the controls (Figure S6D and S6F).
Several conclusions arise from these data. First, the H+ gradient between the cytoplasm and the lumen is not established solely as a function of NHX-2, which is consistent with theory (i.e., antiport activity is gradient driven). Given the magnitude of the pH change that occurs in the lumen and the observation that H+ extrusion from the cell into the lumen must eventually take place against a large pH gradient, energy dependent transporters such as V-ATPases are likely to be involved. Second, the normal flow of H+ between the lumen and the cytoplasm requires NHX-2. This could reflect direct transport by NHX-2 acting in reverse mode to allow H+ into the cell or alternatively through an indirect mechanism where the actual H+ conduit is inhibited by the reduced pHi that accompanies loss of NHX-2. Finally, both pBoc and basolateral H+ extrusion are enhanced following the loss of NHX-2, which, while counterintuitive, may reflect the reduced resting pHi in the cells. This observation may also highlight a role for Ca2+ oscillations in activating NHX-7, and our unpublished observations suggest that the carboxyl terminus of NHX-7 contains a high affinity calmodulin binding site.
Our work establishes the C. elegans defecation cycle as a model for cyclic trans-epithelial H+ secretion and systemic acid-base transport in secretory epithelia. We have identified H+ as a component of the signal secreted by the intestinal epithelia that is necessary for contraction of the posterior body wall muscles. Na+/H+ exchangers are ubiquitously expressed in both worms and mammals, and proton secretion via this class of protein may represent a novel mode of cell-cell communication. Recent work by Erik Jorgensen’s group (published while this manuscript was under review) used a genetic approach to identify nhx-7 and showed that pHo oscillations were both necessary and sufficient to induce pBoc [13]. Furthermore, their work identified a cys-loop type nicotinic acetylcholine receptor expressed in posterior body wall muscles that is activated by low pH and is necessary for pBoc, as well. The identification of this receptor, combined with a vertebrate family of H+-sensing G-protein coupled receptors (PSR) with widespread tissue distribution [14, 15], suggests that H+ signaling may be more commonplace than previously thought. Our data confirm that H+ can act as a transmitter for muscular contractions, and provide a mechanism for the secretion of H+ through cyclic intracellular acidification.
Our results also suggest a functional interplay between Ca2+ signaling and acid-base transport. There are many well established mechanisms through which pH can alter Ca2+ signaling. The binding of InsP3 to its receptor is pH-sensitive [16], as is the gating of TRPM type cation channels [17]; interestingly, the TRPM family members gtl-1 and gon-2 regulate electrolyte homeostasis in the intestine of worms [18]. pHi may also govern physiologic processes that occur downstream of oscillatory Ca2+ signaling such as Ca2+ waves. In fact, both pHi and Na+/H+ exchange activity in mammalian acinar cells modifies Ca2+ wave propagation [19]. Moreover, both Ca2+ and pH play a role in chemical gating of gap junction channels that allow the passage of small molecules between cells [20]. In the defecation model, H+ gating of gap junctions might prevent cellular metabolites from moving “backwards” through the intestine and help to shape the Ca2+ wave. In conclusion, our results suggest that interplay between two signaling molecules implement and condition a rhythmic motor program in an intact, behaving animal.
Supplementary Material
Acknowledgments
We would like to acknowledge the C. elegans Gene Knockout Consortium and C. elegans Genetic Center for strains. We also thank Teresa Sherman for expert technical assistance and Fred Hagen for critical comments on the manuscript. This work was supported in part by PHS grant R01 HL080810 (K.N.). David Johnson was supported by the PHS training grant T32 GM068411.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dal Santo P, Logan M, Chisholm A, Jorgensen E. The inositol triphosphate receptor regulates a 50-second behavioral rhythm in C. elegans. Cell. 1999;98:757–767. doi: 10.1016/s0092-8674(00)81510-x. [DOI] [PubMed] [Google Scholar]
- 2.Espelt M, Estevez A, Yin X, Strange K. Oscillatory Ca2+ Signaling in the Isolated Caenorhabditis elegans Intestine: Role of the Inositol-1,4,5-trisphosphate Receptor and Phospholipases C {beta} and {gamma} J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teramoto T, Iwasaki K. Intestinal calcium waves coordinate a behavioral motor program in C. elegans. Cell Calcium. 2006;40:319–327. doi: 10.1016/j.ceca.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Nehrke K, Denton J, Mowrey W. Intestinal Ca2+ wave dynamics in freely moving C. elegans coordinate execution of a rhythmic motor program. Am J Physiol Cell Physiol. 2007 doi: 10.1152/ajpcell.00303.02007. [DOI] [PubMed] [Google Scholar]
- 5.Peters MA, Teramoto T, White JQ, Iwasaki K, Jorgensen EM. A Gap-Junction-Mediated Calcium Wave Coordinates a Rhythmic Behavior in C. elegans. Current Biology . 2007 doi: 10.1016/j.cub.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Thomas J. Genetic analysis of defecation in Caenorhabditis elegans. Genetics. 1990;124:855–872. doi: 10.1093/genetics/124.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norman K, Fazzio R, Mellem J, Espelt M, Strange K, Beckerle M, Maricq A. The Rho\/Rac-family guanine nucleotide exchange factor VAV-1 regulates rhythmic behaviors in C. elegans. Cell. 2005;123:119–132. doi: 10.1016/j.cell.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Thomas J. Regulation of a periodic motor program in C. elegans. J Neurosci. 1994;14:1953–1962. doi: 10.1523/JNEUROSCI.14-04-01953.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson PS, Nee J, Suhm MA. Modifier role of internal H+ in activating the Na+/H+ exchanger in renal microvillus membrane vesicles. Nature. 1982;299:161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- 11.Nehrke K, Melvin JE. The NHX family of Na+/H+ exchangers in Caenorhabditis elegans. J Biol Chem. 2002;277:29036–29044. doi: 10.1074/jbc.M203200200. [DOI] [PubMed] [Google Scholar]
- 12.Nehrke K. A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J Biol Chem. 2003;278:44657–44666. doi: 10.1074/jbc.M307351200. [DOI] [PubMed] [Google Scholar]
- 13.Beg AAEG, Nix P, Davis MW, Jorgensen EM. Protons Act as a Transmitter for Muscle Contraction in C. elegans. Cell . 2008:149–160. doi: 10.1016/j.cell.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig MG, Vanek M, Guerini D, Gasser JA, Jones CE, Junker U, Hofstetter H, Wolf RM, Seuwen K. Proton-sensing G-protein-coupled receptors. Nature. 2003;425:93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 15.Ishii S, Kihara Y, Shimizu T. Identification of T cell death-associated gene 8 (TDAG8) as a novel acid sensing G-protein-coupled receptor. J Biol Chem. 2005;280:9083–9087. doi: 10.1074/jbc.M407832200. [DOI] [PubMed] [Google Scholar]
- 16.White AM, Varney MA, Watson SP, Rigby S, Liu CS, Ward JG, Reese CB, Graham HC, Williams RJ. Influence of Mg2+ and pH on n.m.r. spectra and radioligand binding of inositol 1,4,5-trisphosphate. Biochem J. 1991;278:759–764. doi: 10.1042/bj2780759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson DA, Chase HWN, Bevan S. TRPM8 Activation by Menthol, Icilin, and Cold Is Differentially Modulated by Intracellular pH. J Neurosci. 2004;24:5364–5369. doi: 10.1523/JNEUROSCI.0890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teramoto T, Lambie EJ, Iwasaki K. Differential regulation of TRPM channels governs electrolyte homeostasis in the C. elegans intestine. Cell Metab. 2005;1:343–354. doi: 10.1016/j.cmet.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez A, Pfeiffer F, Schmid A, Schulz I. Effect of intracellular pH on acetylcholine-induced Ca2+ waves in mouse pancreatic acinar cells. Am J Physiol Cell Physiol. 1998;275:C810–817. doi: 10.1152/ajpcell.1998.275.3.C810. [DOI] [PubMed] [Google Scholar]
- 20.Peracchia C. Chemical gating of gap junction channels: Roles of calcium, pH and calmodulin. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1662:61–80. doi: 10.1016/j.bbamem.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JA, Buchsbaum RN, Zimniak A, Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979;18:2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.