Abstract
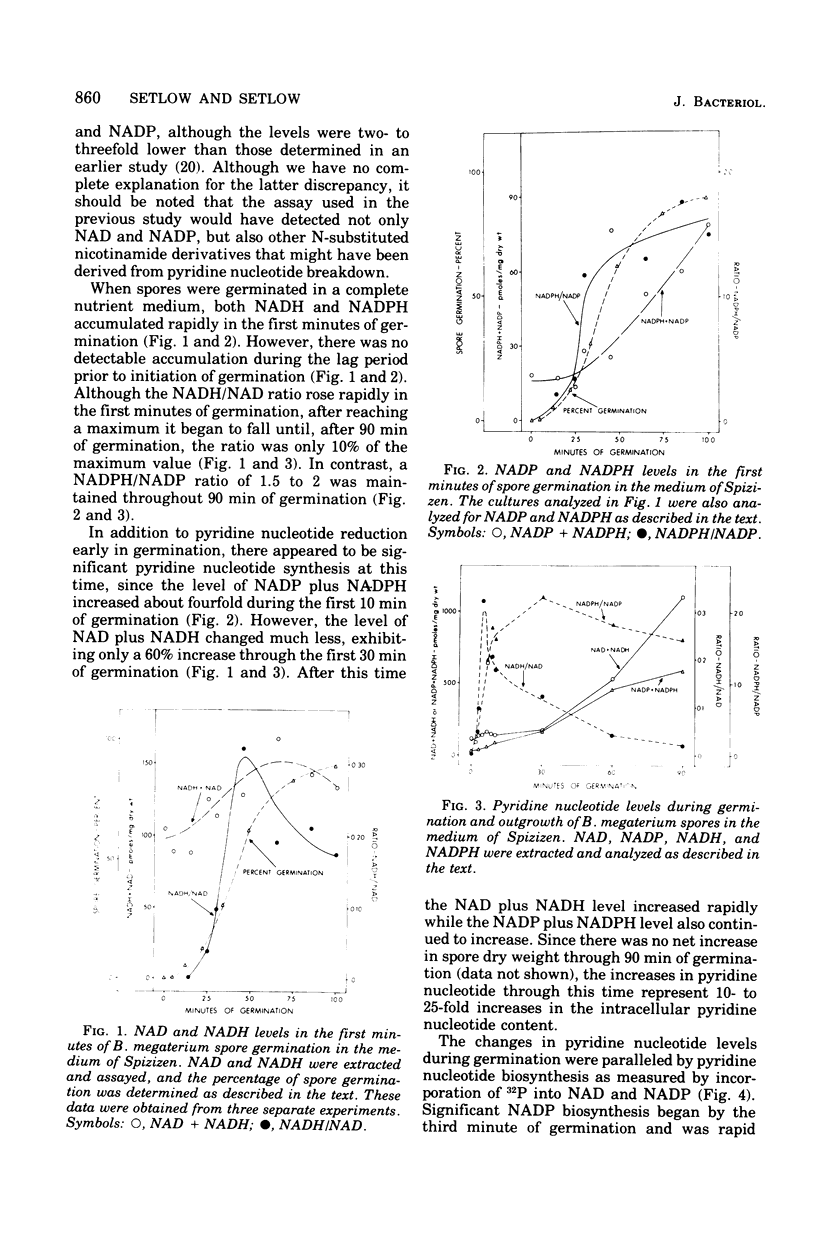
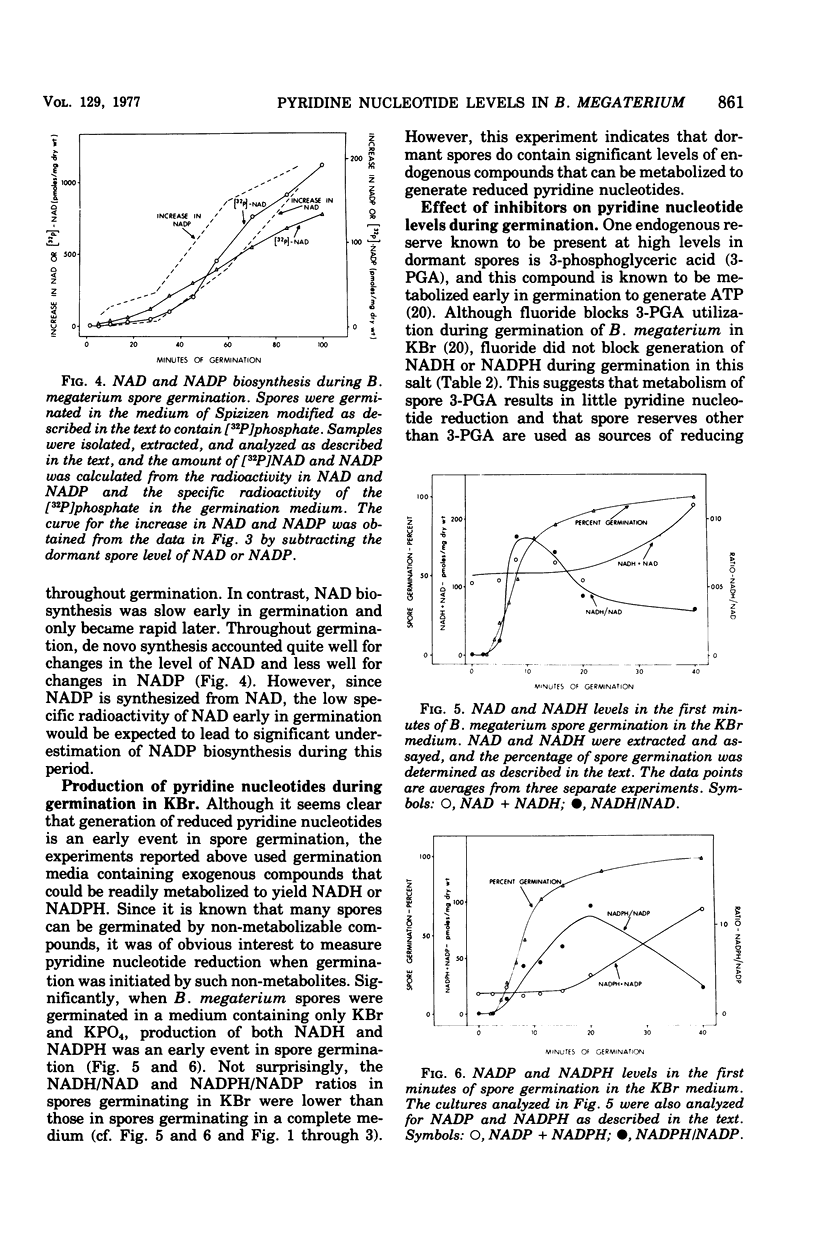
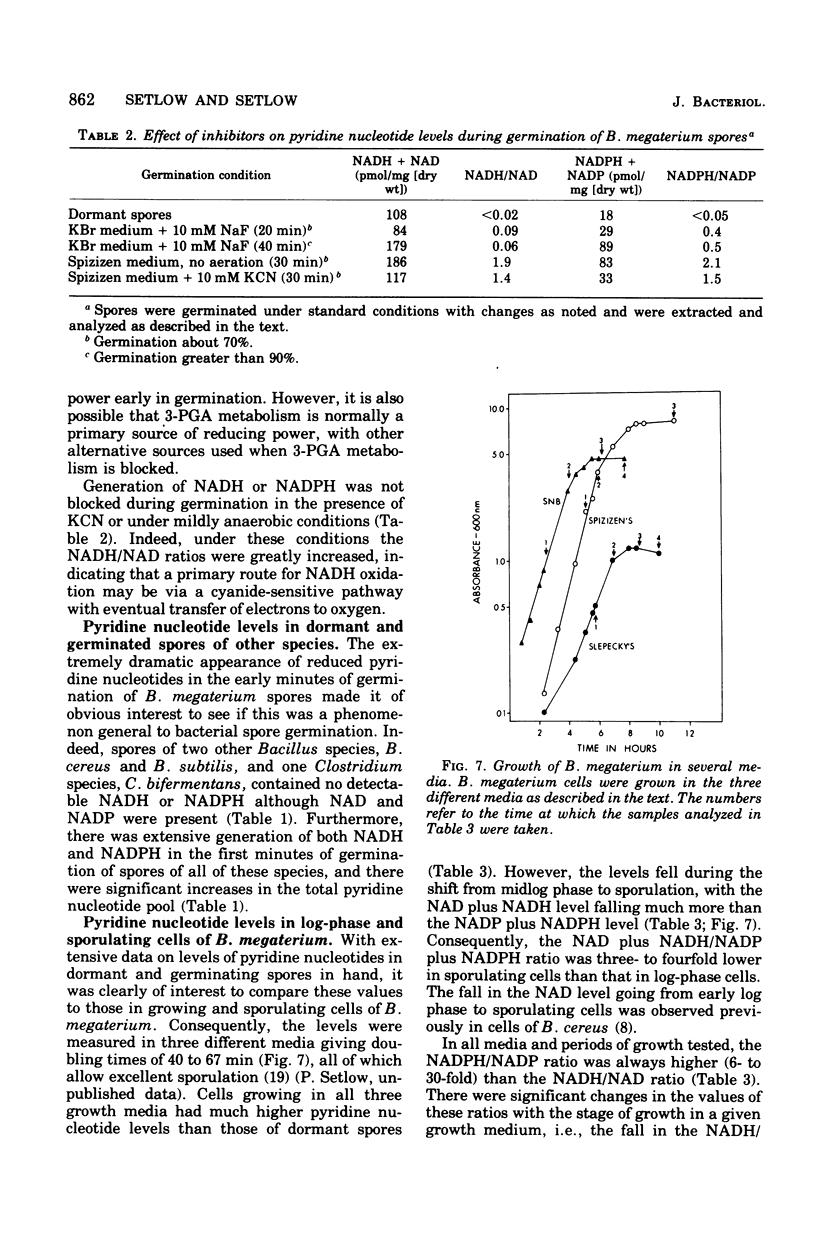
Dormant spores of Bacillus megaterium contained no detectable reduced nicotinamide adenine dinucleotide (NADH) or reduced nicotinamide adenine dinucleotide phosphate (NADPH) despite significant levels of the oxidized forms of these nucleotides (NAD and NADP). During the first minutes of spore germination there was rapid accumulation of NADH and NADPH. However, this accumulation followed the fall in optical density that is characteristic of the initiation of spore germination. Accumulation of NADH and NADPH early in germination was not blocked by fluoride or cyanide, and it occurred even when germination was carried out in the absence of an exogenous source of reducing power. In addition to pyridine nucleotide reduction, de novo synthesis also began early in germination as the pyridine nucleotide levels increased to those found in growing cells. Midlog-phase cells grown in several different media had 20 to 35 times as much total pyridine nucleotide as did dormant spores. However, as growth and sporulation proceeded, the NADH plus NAD level fell four- to fivefold whereas the NADPH plus NADP level fell by a lesser amount. From min 10 of spore germination until midway through sporulation the value for the ratio of NADH/NAD is about 0.1 (0.03 to 0.18) while the ratio of NADPH/ANDP is about 1.4 (0.3 to 2.4). Comparison of these ratios in log-phase versus stationary phase (sporulation) growth in all three growth media tested did not reveal any common pattern of changes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blankenship L. C., Pallansch M. J. Differential analysis of sulfhydryl and disulfide groups of intact spores. J Bacteriol. 1966 Dec;92(6):1615–1617. doi: 10.1128/jb.92.6.1615-1617.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHURCH B. D., HALVORSON H. Intermediate metabolism of aerobic spores. I. Activation of glucose oxidation in spores of Bacillus cereus var terminalis. J Bacteriol. 1957 Apr;73(4):470–476. doi: 10.1128/jb.73.4.470-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey R. C., Brody S., Mikolajczyk S. D. Changes in the glutathione thiol-disulfide status of Neurospora crassa conidia during germination and aging. J Bacteriol. 1975 Jan;121(1):144–151. doi: 10.1128/jb.121.1.144-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick-Silversmith L., Torriani A. Macromolecular syntheses during germination and outgrowth of Bacillus subtilis spores. J Bacteriol. 1973 May;114(2):507–516. doi: 10.1128/jb.114.2.507-516.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould G. W., Hitchins A. D., King W. L. Function and location of a "germination enzyme" in spores of Bacillus cereus. J Gen Microbiol. 1966 Aug;44(2):293–502. doi: 10.1099/00221287-44-2-293. [DOI] [PubMed] [Google Scholar]
- Kato T., Berger S. J., Carter J. A., Lowry O. H. An enzymatic cycling method for nicotinamide-adenine dinucleotide with malic and alcohol dehydrogenases. Anal Biochem. 1973 May;53(1):86–97. doi: 10.1016/0003-2697(73)90409-0. [DOI] [PubMed] [Google Scholar]
- London J. Changes in nicotinamide adenine dinucleotide concentration of Bacillus cereus during growth. Biochim Biophys Acta. 1966 Aug 24;124(2):241–245. doi: 10.1016/0304-4165(66)90185-1. [DOI] [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. 18. Free amino acids in spores. J Biol Chem. 1970 Mar 10;245(5):1128–1136. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem. 1970 Mar 10;245(5):1137–1145. [PubMed] [Google Scholar]
- Prasad C., Diesterhaft M., Freese E. Initiation of spore germination in glycolytic mutants of Bacillus subtilis. J Bacteriol. 1972 Apr;110(1):321–328. doi: 10.1128/jb.110.1.321-328.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmit J. C., Brody S. Biochemical genetics of Neurospora crassa conidial germination. Bacteriol Rev. 1976 Mar;40(1):1–41. doi: 10.1128/br.40.1.1-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Identification and localization of the major proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8159–8167. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 23. Nucleotide metabolism during spore germination. J Biol Chem. 1970 Jul 25;245(14):3645–3652. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Setlow P. Percent charging of transfer ribonucleic acid and levels of ppGpp and pppGpp in dormant and germinated spores of Bacillus megaterium. J Bacteriol. 1974 Jun;118(3):1067–1074. doi: 10.1128/jb.118.3.1067-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Primus G. Protein metabolism during germination of Bacillus megaterium spores. I. Protein synthesis and amino acid metabolism. J Biol Chem. 1975 Jan 25;250(2):623–630. [PubMed] [Google Scholar]
- Setlow P., Waites W. M. Identification of several unique, low-molecular-weight basic proteins in dormant spores of clastridium bifermentans and their degradation during spore germination. J Bacteriol. 1976 Aug;127(2):1015–1017. doi: 10.1128/jb.127.2.1015-1017.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites W. M., Wyatt L. R. Germination of spores of Clostridium bifermentans by certain amino acids, lactate and pyruvate in the presence of sodium or potassium ions. J Gen Microbiol. 1971 Aug;67(2):215–222. doi: 10.1099/00221287-67-2-215. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Firth A. Levels of nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide in facultative bacteria and the effect of oxygen. J Bacteriol. 1972 Jul;111(1):24–32. doi: 10.1128/jb.111.1.24-32.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



