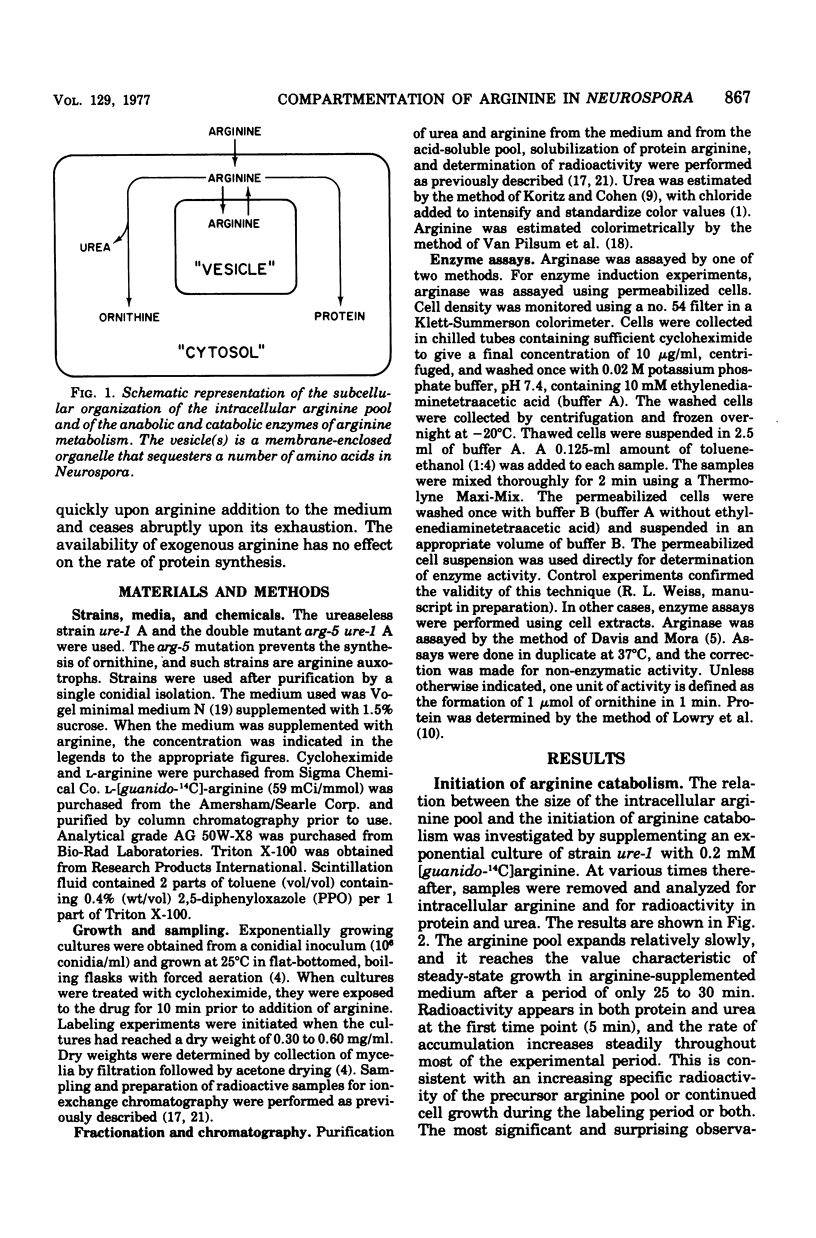
Abstract
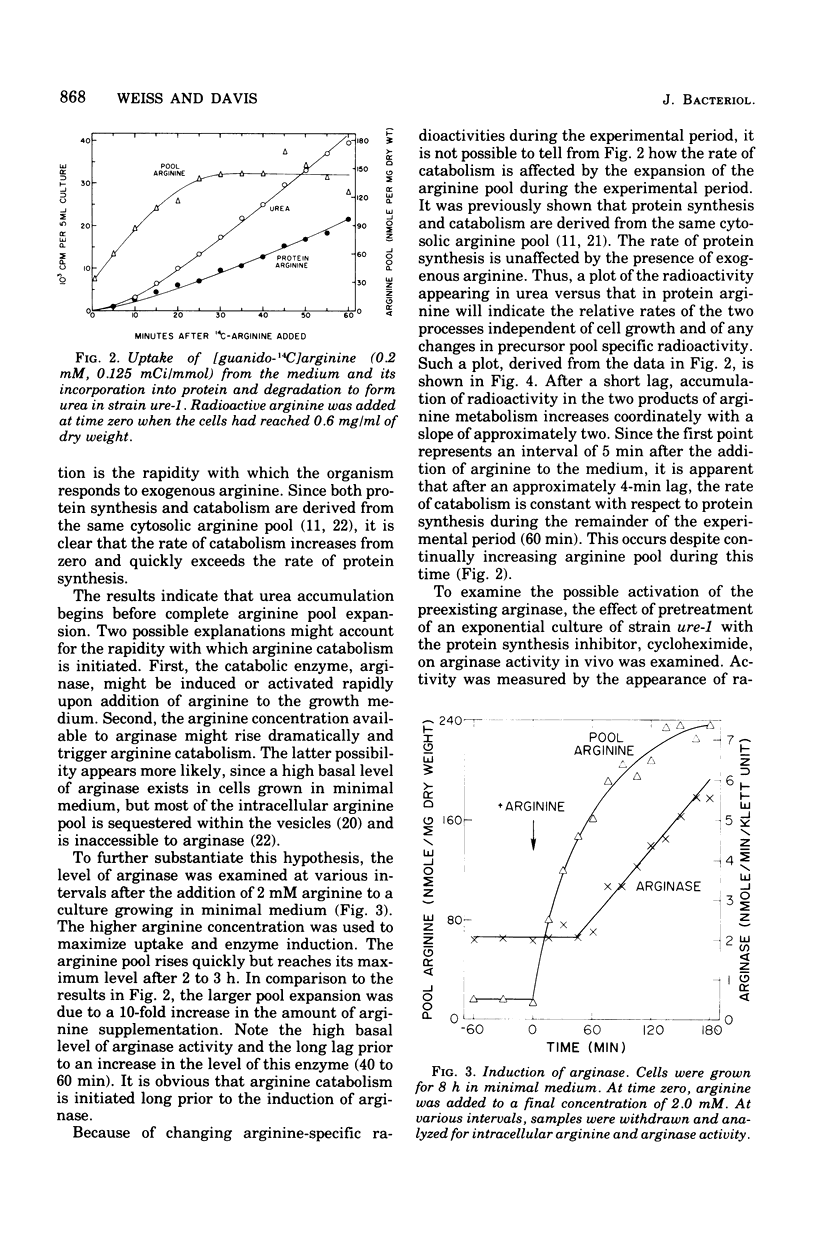
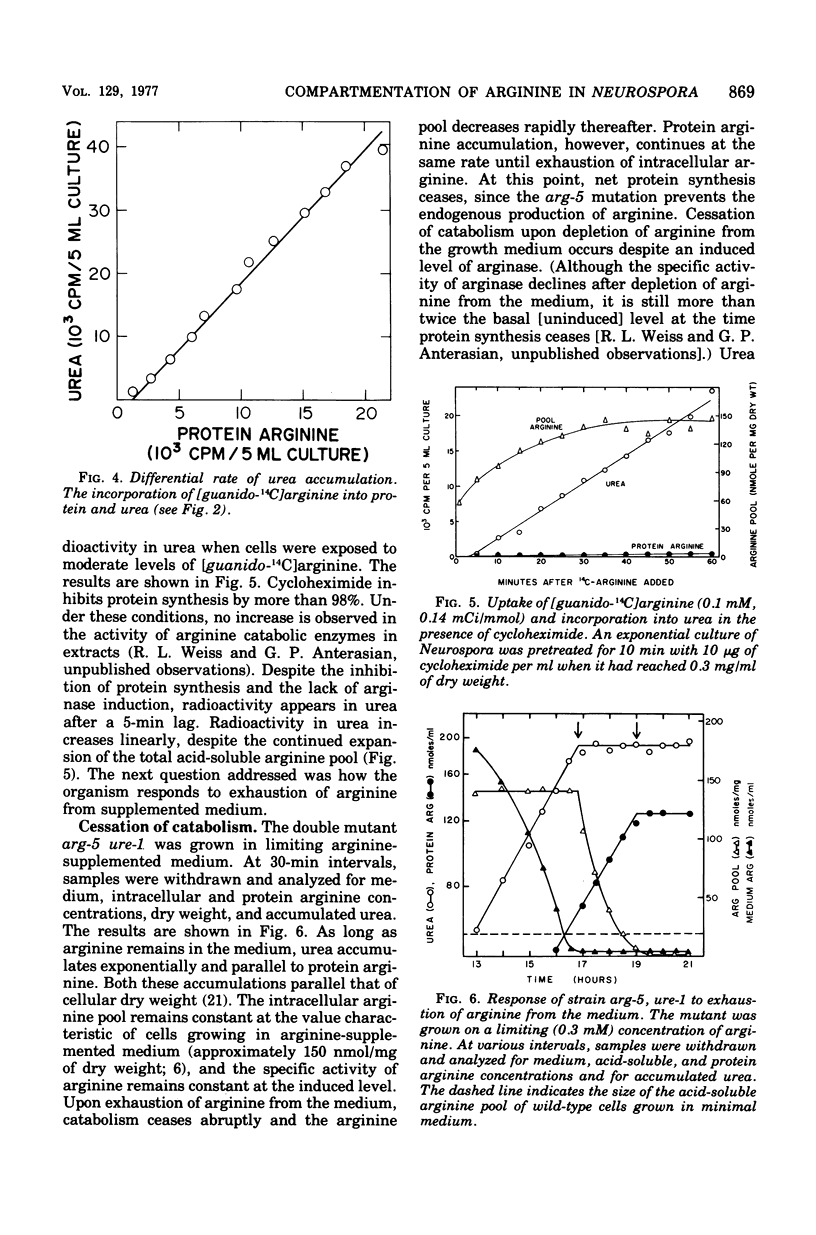
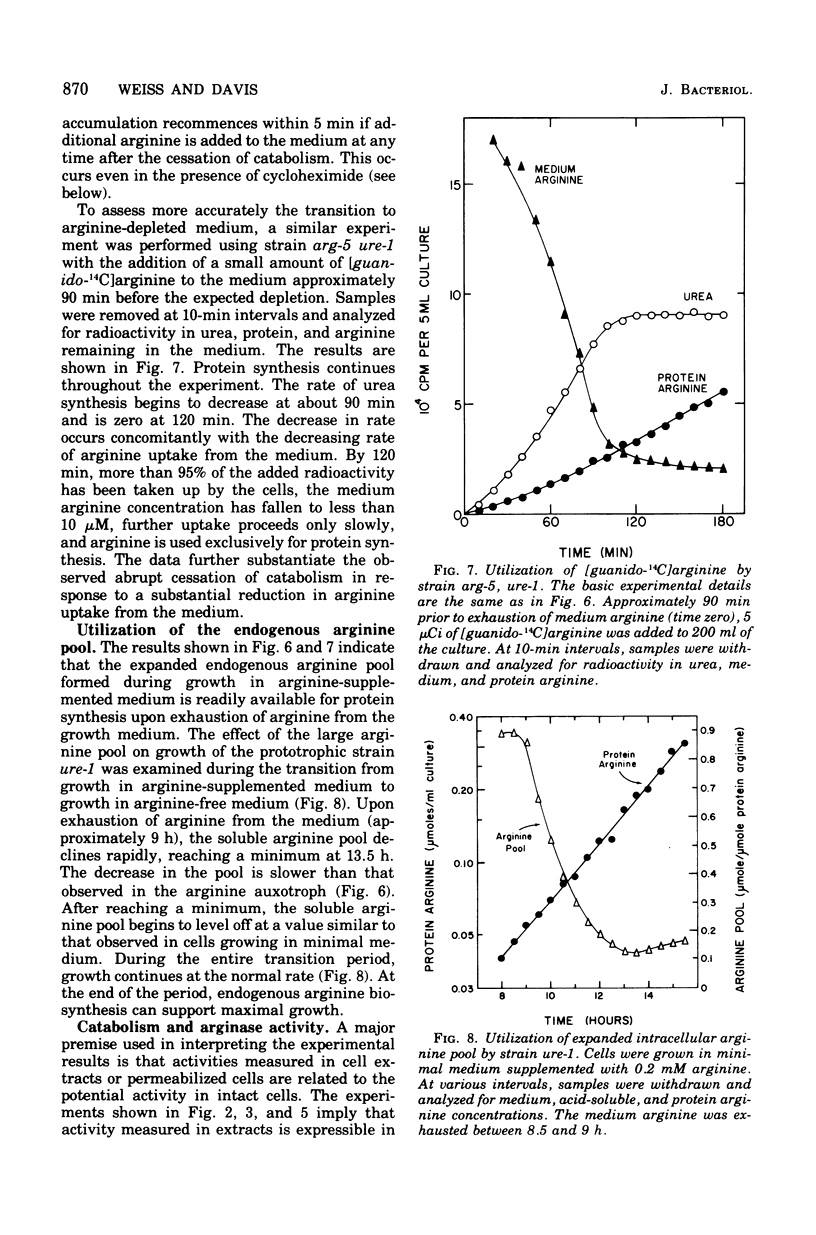
The response of Neurospora to changes in the availibility of exogenous arginine was investigated. Upon addition of arginine to the growth medium, catabolism is initiated within minutes. This occurs prior to expansion of the arginine pool or augmentation of catabolic enzyme levels. (Basal levels are approximately 25% of those found during growth in arginine-supplemented medium.) Catabolism of arginine is independent of protein synthesis, indicating that the catabolic enzymes are active but that arginine is not available for catabolism unless present in the medium. Upon exhaustion of the supply of exogenous arginine, catabolism ceases abruptly, despite an expanded arginine pool and induced levels of the catabolic enzymes. The arginine pool supports protein synthesis until the cells regain their normal capacity for endogenous arginine synthesis. These observations, combined with the known small level of induction of arginine catabolic enzymes, non-repressibility of most biosynthetic enzymes, and vesicular localization of the bulk of the arginine pool, suggest that compartmentation plays a significant role in controlling arginine metabolism in Neurospora.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROKAERT R., SCHRAM E. Dosage des N-carbamoyldérivés d'acides aminés par la diacétylmonoxime. Bull Soc Chim Biol (Paris) 1958;40(7-8):1093–1106. [PubMed] [Google Scholar]
- Cybis J. J., Davis R. H. Acetylglutamate kinase: a feedback-sensitive enzyme of arginine biosynthesis in Neurospora. Biochem Biophys Res Commun. 1974 Sep 23;60(2):629–634. doi: 10.1016/0006-291x(74)90287-3. [DOI] [PubMed] [Google Scholar]
- Cybis J., Davis R. H. Organization and control in the arginine biosynthetic pathway of Neurospora. J Bacteriol. 1975 Jul;123(1):196–202. doi: 10.1128/jb.123.1.196-202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Lawless M. B., Port L. A. Arginaseless Neurospora: genetics, physiology, and polyamine synthesis. J Bacteriol. 1970 May;102(2):299–305. doi: 10.1128/jb.102.2.299-305.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Mora J. Mutants of Neurospora crassa deficient in ornithine-delta-transmainase. J Bacteriol. 1968 Aug;96(2):383–388. doi: 10.1128/jb.96.2.383-388.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. O., COHEN G. N. Incorporation des amino-acides endogènes et exogènes dans les protéines de la levure. Ann Inst Pasteur (Paris) 1958 Jul;95(1):73–87. [PubMed] [Google Scholar]
- HENDLER R. W. A model for protein synthesis. Nature. 1962 Mar 3;193:821–823. doi: 10.1038/193821a0. [DOI] [PubMed] [Google Scholar]
- HESS J., KITO E., MARTIN R. P., VAN PILSUM J. F. Determination of creatine, creatinine, arginine, guanidinoacetic acid, guanidine, and methylguanidine in biological fluids. J Biol Chem. 1956 Sep;222(1):225–235. [PubMed] [Google Scholar]
- KORITZ S. B., COHEN P. P. Colorimetric determination of carbamylamino acids and related compounds. J Biol Chem. 1954 Jul;209(1):145–150. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mora J., Salceda R., Sanchez S. Regulation of arginase activity by intermediates of the arginine biosynthetic pathway in Neurospora crassa. J Bacteriol. 1972 Jun;110(3):870–877. doi: 10.1128/jb.110.3.870-877.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimore G. E., Woodside K. H., Henry J. E. Compartmentation of free valine and its relation to protein turnover in perfused rat liver. J Biol Chem. 1972 May 10;247(9):2776–2784. [PubMed] [Google Scholar]
- Nazario M. The accumulation of argininosuccinate in Neurospora crassa. II. Inhibition of arginyl-tRNA synthesis by argininosuccinate. Biochim Biophys Acta. 1967 Aug 22;145(1):146–152. doi: 10.1016/0005-2787(67)90663-6. [DOI] [PubMed] [Google Scholar]
- SIMS A. P., FOLKES B. F. A KINETIC STUDY OF THE ASSIMILATION OF (15N)-AMMONIA AND THE SYNTHESIS OF AMINO ACIDS IN AN EXPONENTIALLY GROWING CULTURE OF CANDIDA UTILIS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:479–502. doi: 10.1098/rspb.1964.0015. [DOI] [PubMed] [Google Scholar]
- Srere P. A., Mosbach K. Metabolic compartmentation: symbiotic, organellar, multienzymic, and microenvironmental. Annu Rev Microbiol. 1974;28(0):61–83. doi: 10.1146/annurev.mi.28.100174.000425. [DOI] [PubMed] [Google Scholar]
- Subramanian K. N., Weiss R. L., Davis R. H. Use of external, biosynthetic, and organellar arginine by Neurospora. J Bacteriol. 1973 Jul;115(1):284–290. doi: 10.1128/jb.115.1.284-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Compartmentation and control of arginine metabolism in Neurospora. J Bacteriol. 1976 Jun;126(3):1173–1179. doi: 10.1128/jb.126.3.1173-1179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L., Davis R. H. Intracellular localization of enzymes of arginine metabolism in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5403–5408. [PubMed] [Google Scholar]
- Weiss R. L. Intracellular localization of ornithine and arginine pools in Neurospora. J Biol Chem. 1973 Aug 10;248(15):5409–5413. [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G., Magasanik B. The induction of urea carboxylase and allophanate hydrolase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6203–6209. [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Wiemken A., Dürr M. Characterization of amino acid pools in the vacuolar compartment of Saccharomyces cerevisiae. Arch Microbiol. 1974;101(1):45–57. doi: 10.1007/BF00455924. [DOI] [PubMed] [Google Scholar]


