Abstract
Accumulation of GABAA receptors (GABAARs) at GABAergic synapses requires the cytoplasmic loop region and C-terminal transmembrane domain of the receptor γ2 subunit. We here report a novel interaction of γ2 with Calcium-Modulating cyclophilin Ligand (CAML), an integral membrane protein that regulates this mechanism. Interaction of GABAARs with CAML depends on both the cytoplasmic region and fourth transmembrane domain of the γ2 subunit, CAML immunoprecipitates with GABAARs from transfected cells and brain lysates and colocalizes with γ2 in ER vesicles in soma and dendrites of neurons. CAML shRNA treatment results in reduced expression of postsynaptic GABAARs, along with significant reductions in GABA-evoked whole-cell currents and GABAergic synaptic function, while glutamatergic transmission is unaffected. Reduced surface expression of GABAARs in CAML mutant neurons is associated with selective deficits in recycling of endocytosed GABAARs to the cell surface. Our results indicate a specific role of CAML in functional expression and endocytic recycling of postsynaptic GABAARs.
Keywords: inhibitory synapses, protein trafficking, endocytic recycling, exocytosis, endoplasmic reticulum, calcium-modulating cyclophilin ligand, cyclophilin B
Introduction
Regulated trafficking of GABAARs in intracellular compartments and at the plasma membrane is critically important for modulation of GABAergic inhibitory transmission and hence for adaptation of neuronal excitability. Moreover, subunit-specific interactions of GABAARs with diverse trafficking factors are thought to contribute to accumulation of these receptors at either synaptic or nonsynaptic membrane sites. Of particular interest are postsynaptic receptor subtypes, which contain the γ2 subunit in diverse combinations with α1-3 and β1-3 subunits and carry GABAergic fast synaptic currents (Fritschy and Brunig, 2003; Luscher and Keller, 2004; Moss and Smart, 2001). The γ2 subunit is essential for trafficking and accumulation of GABAARs in the postsynaptic membrane and for GABAergic synaptic transmission (Essrich et al., 1998; Li et al., 2005; Schweizer et al., 2003). Moreover, modest developmental deficits in γ2 subunit-containing GABAARs are implicated in the etiology of anxiety and depressive disorders (Crestani et al., 1999; Earnheart et al., 2007).
Proteins involved in trafficking of γ2 subunit containing GABAARs to synapses are implicated in functional plasticity of GABAergic synapses. They include the GABAAR-associated protein (GABARAP) (Chen and Olsen, 2007) and the palmitoyltransferase Golgi-specific DHHC zinc finger protein (GODZ) (Fang et al., 2006; Keller et al., 2004), which bind to adjacent domains in the major cytoplasmic loop region between the third (M3) and fourth transmembrane (M4) domains of the γ2 subunit. These proteins were both identified by yeast two-hybrid interaction screening. In a second approach designed to identify GABAAR domains important for trafficking, chimeric subunit constructs containing different portions of the γ2 subunit in an α2 subunit backbone were tested for their ability to cluster GABAAR at GABAergic synapses of γ2 subunit knock-out neurons (Alldred et al., 2005). In particular these experiments revealed that a chimeric subunit construct containing the γ2 subunit cytoplasmic domain in an α2 subunit backbone failed to accumulate at synapses, unless the construct also included the M4 domain from the γ2 subunit. The M4 domain of the γ2 subunit therefore is essential for clustering and function of GABAARs at synapses (Alldred et al., 2005).
We here report the isolation of Calcium-Modulating cyclophilin Ligand (CAML) as a novel GABAAR trafficking factor that interacts with these receptors in a γ2 M4 domain-dependent manner. CAML was originally described as a cyclophilin B-binding protein whose overexpression in Jurkat T cells causes a rise in intracellular calcium, thus activating transcription factors responsible for the early immune response (Bram and Crabtree, 1994; Holloway and Bram, 1996). Subsequent work showed that CAML is involved in intracellular trafficking of diverse receptors and signaling proteins and essential for thymocyte development (Tran et al., 2005; Tran et al., 2003; von Bulow and Bram, 1997). CAML is an endoplasmic reticulum (ER)-resident integral membrane protein that contains a hydrophilic N-terminal cytoplasmic domain, followed by three putative transmembrane domains (Holloway and Bram, 1998). CAML mRNA and protein are highly expressed in many tissues and cell types including astrocytes, microglia and neurons (Lee et al., 2001). Data presented here suggest an important function of CAML in trafficking of postsynaptic GABAARs. CAML contributes to recycling of endocytosed GABAARs to the plasma membrane and is essential for normal accumulation and function of γ2-containing GABAARs at inhibitory synapses.
Results
Identification of CAML as a γ2 subunit-specific GABAAR interacting protein
Transfection of γ2 subunit knockout neurons with chimeric subunit constructs had revealed an important function of the γ2 subunit M4 domain for clustering and function of postsynaptic GABAARs (Alldred et al., 2005). In order to search for proteins involved in trafficking of GABAARs in a γ2 M4 domain-dependent manner we performed yeast two-hybrid screenings of a mouse brain cDNA library with a bait encoding the C-terminal 68-amino-acids of the γ2 subunit (amino acids 361-428 of the mature polypeptide, see Experimental Methods). These experiments yielded 14 independent clones containing partial or full-length cDNA sequences of a 299-amino acid protein previously described as CAML (Bram and Crabtree, 1994). To verify this interaction in mammalian cells, we first tested for colocalization of FLAG-tagged CAML (FL-CAML) and GABAARs in transfected 293T cells. GABAARs (α2β3mycγ2) were faithfully expressed on the cell surface, as shown by immunofluorescent staining with an antiserum directed against the α2 subunit or the myc epitope tag of the mycγ2 subunit (Fig. 1A). On cotransfection with FL-CAML, however, α2β3mycγ2 receptors were trapped in a CAML-positive intracellular compartment, as revealed by colocalization of FL-CAML with the mycγ2 and α2 subunits (Fig. 1B). In contrast, α2β3 receptors that were cotransfected with FL-CAML accumulated at the cell surface, with no discernable colocalization of the α2 or β3 subunit and FL-CAML and similar to cells transfected with α2β3 receptors alone (Fig. 1C-F). The data indicate that CAML interacts with GABAARs in mammalian cells in a γ2 subunit-specific manner.
Figure 1.
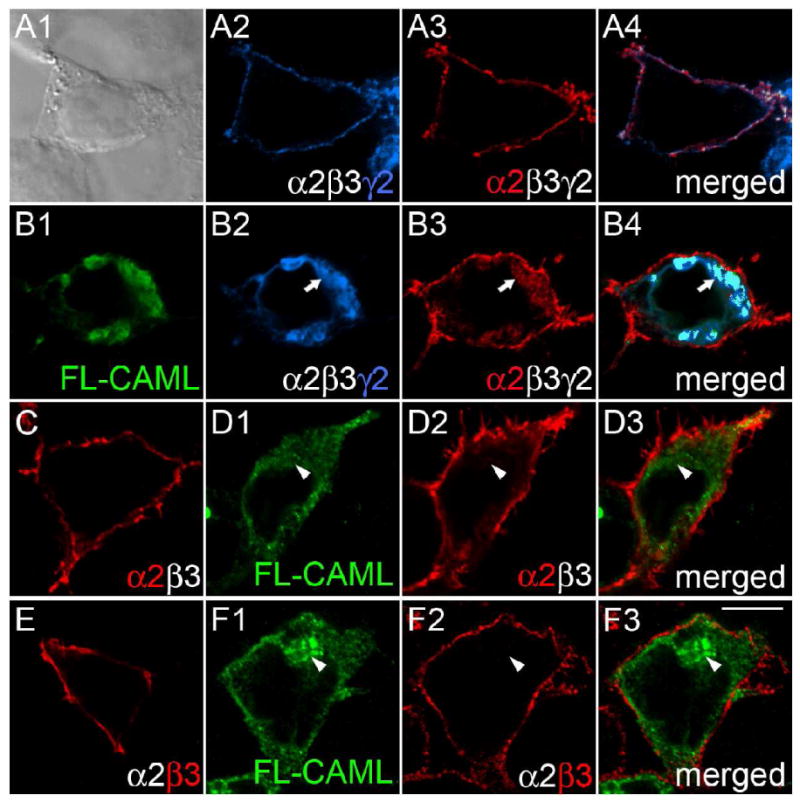
CAML interacts with the γ2 but not α2 and β3 subunits in transfected 293T cells. A-F, Human embryo kidney 293T cells were transfected with α2β3mycγ2 (A, B) or α2β3 receptors (C - F), either alone (A, C, E) or together with FL-CAML (B, D, F) and stained with antibodies specific for the mycγ2 subunit (A2, B2, blue), the α2 subunit (A3, B3, C, D2, red) or β3 subunit (E, F2) and FL-CAML (B1, D1, F1, green). Panel A1 shows a bright field image; the other panels show single optical sections of representative cells imaged by confocal microscopy. Merged images representing two or three separate fluorescent channels of cells in A, B, D, and F are shown in the right column. Note the colocalization of α2 and γ2 subunits at the cell surface of the α2β3mycγ2 receptor-transfected cell in (A) evident in the merged image (A4) that is absent upon cotransfection of CAML with α2β3mycγ2 receptors in the cell shown in (B) (merged image B4). Whereas cells cotransfected with FL-CAML and α2β3mycγ2 show intracellular trapping of the γ2 (B2, B4) and α2 subunits (B3), virtually no intracellular GABAAR subunit staining is seen when α2β3mycγ2 receptors are transfected alone (A2-4), or upon cotransfection of α2β3 receptors with FL-CAML (D2, D3, F2, F3). Arrows point to colocalization of FL-CAML and GABAAR subunits, arrowheads indicate lack of colocalization. Scale bar, 10 μm.
CAML forms stable complexes with γ2 subunit-containing GABAARs
To test whether GABAA receptors (α2β3mycγ2 subunit composition) and CAML can exist as a stable complex in mammalian cells, we first analyzed the biochemical association of these two proteins following cotransfection into 293T cells. Immunoprecipitation of FL-CAML from lysates of 293T cells expressing FL-CAML and α2β3mycγ2 receptors with rabbit anti FLAG antiserum resulted in efficient coimmunoprecipitation of the mycγ2 subunit (Fig. 2A), along with FL-CAML (not shown). In contrast, no coimmunoprecipitation was detected in negative control experiments using rabbit IgG instead of anti-FLAG antibody or in the absence of FL-CAML. Conversely, immunoprecipitation of α2β3mycγ2 receptors with anti-myc mAb resulted in efficient coimmunoprecipitation of cotransfected FL-CAML (Fig. 2B). No coimmunoprecipitation was detected in negative controls using mouse IgG instead of anti-myc mAb or in the absence of the mycγ2 subunit.
Figure 2.
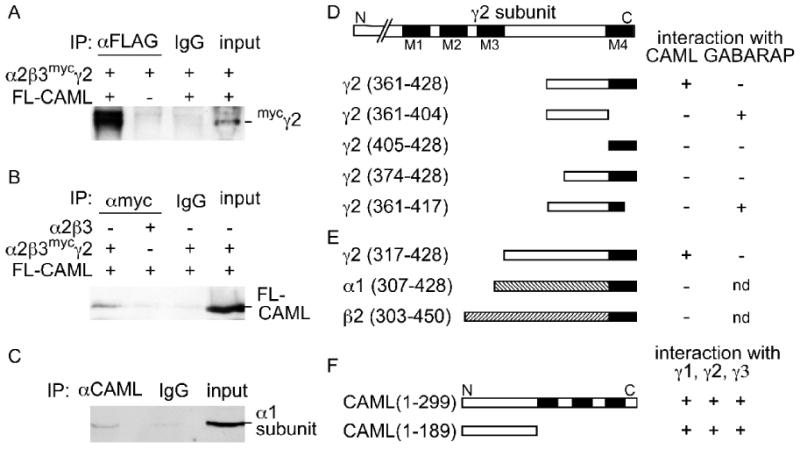
Interaction between the γ2 subunit and CAML requires the cytoplasmic and 4th transmembrane domains of the γ2 subunit and the N-terminal cytoplasmic domain of CAML. A, B, Co-immunoprecipitation of CAML and GABAARs from lysates of transfected 293T cells. HEK 293T cells were cotransfected with FL-CAML and GABAARs (α2β3mycγ2) and cell lysates subjected to immunoprecipitation with anti-FLAG (A) or anti-myc antisera (B) or IgG as a negative control as indicated. Coimmunoprecipitation of GABAARs with anti-FLAG was dependent on cotransfected FL-CAML, as revealed by analysis of immunopurified samples with guinea pig anti γ2 antiserum (A). Conversely, copurification of FL-CAML with anti-myc mAb required cotransfection of GABAARs containing the mycγ2 subunit, as reveled by analysis of immunoblots with rabbit anti-CAML antibody (B). C, Endogenous CAML was immunoprecipitated from DSP-cross-linked mouse brain lysates with mAb anti-CAML and analyzed by western blot for coimmunoprecipitation of GABAARs using a rabbit anti α1 subunit antiserum (Benke et al., 1996). No coimmunoprecipitation was observed in reactions using unspecific IgG instead of anti-FLAG, anti-myc or anti-CAML (A-C). D, E, Interaction of CAML with the γ2 subunit required both cytoplasmic and 4th transmembrane domains of the γ2 subunit and the N-terminal cytoplasmic region of CAML. Various fragments derived from the C-terminal portion of the γ2 subunit (D) or α1 and β2 subunits of GABAARs (E) were tested as bait constructs for interaction with full-length CAML in yeast two-hybrid interaction assays. In parallel the γ2 subunit bait constructs were tested for proper interaction and with GABARAP(36-117) as a positive control. Construct names with numbers in parentheses refer to amino acid numbers of the mature subunit present in the construct. F, Full-length CAML and CAML(1-189) were analogously tested as a prey constructs for interaction with baits containing γ1(398-465), γ2(361-428) and γ3(400-467). Transmembrane regions of the γ2 subunit and CAML are indicated as black boxes. The region between M3 and M4 of the γ2 subunit and CAML(1-189) represent the cytoplasmic regions of the γ2 subunit and CAML, respectively. nd, not determined.
To verify that GABAARs occur in complexes with CAML in vivo, we performed coimmunoprecipitation experiments of CAML and GABAARs from brain lysates. However, detection of complexes in this case is hampered by their predicted transient existence in the secretory pathway and by the low steady state concentration of GABAARs in intracellular compartments. Moreover, complexes between integral membrane proteins such as CAML and the γ2 subunit tend to be sensitive to detergents used to solubilize membrane proteins. To overcome these problems we resorted to crosslinking of crude brain extracts with the reversible crosslinker dithiobis(succinimidyl)propionate (DSP) before detergent extraction. Subsequent immunoprecipitation of CAML from detergent solubilized membranes with mAb anti-CAML but not unspecific IgG resulted in coimmunoprecipitation of GABAARs, as evidenced by detection of the GABAAR α1 subunit in CAML immunoprecipitates by western blot (Fig. 2C). These experiments suggest that CAML forms stable complexes with GABAARs in neurons.
CAML interacts with cytoplasmic loop and M4 domains of the γ2 subunit
Postsynaptic clustering of GABAARs requires both the cytoplasmic loop and M4 domains of the γ2 subunit (Alldred et al., 2005). We therefore examined whether interaction of CAML with GABAARs could explain the role of the M4 domain in postsynaptic accumulation of GABAARs. In yeast two-hybrid assays that employed a series of N-terminally and C-terminally truncated γ2 subunit bait constructs, none showed evidence for interaction with CAML (Fig. 2D). Specifically, truncation of the original γ2 subunit bait fragment by 14-amino-acids from the N-terminus (γ2 374-428) or deletion of the 11 C-terminal amino acids (γ2 361-417) completely abolished interaction with CAML. Similarly, fragments corresponding to the cytoplasmic domain of the original bait [γ2(361-404)] or the M4 domain [γ2(405-428)] of the γ2 subunit also failed to interact. Absence of interaction between CAML and the two M4 domain-lacking γ2 fragments [γ2(361-404) and γ2(361-417)] is significant as both these constructs showed faithful interaction with a bait construct containing amino acids 36-117 of GABARAP, which is known to interact with the C-terminal end of the γ2 subunit cytoplasmic domain (Wang et al., 1999). Collectively, these data indicate that both the C-terminal 44 amino acids of the γ2 subunit major cytoplasmic loop region and the M4 domain are required for interaction with CAML. Consistent with these findings, a longer construct containing the entire γ2 subunit cytoplasmic loop and M4 domain [γ2(317-428)] showed faithful CAML-dependent growth in yeast, similar to the bait construct used for library screening, although the same construct failed to interact with GABARAP for unknown reasons (Fig. 2D, E).
Importantly, α1 and β2 subunit-derived C-terminal fragments that were homologous to this γ2 subunit fragment failed to interact with CAML. These results are consistent with colocalization assays in 293T cells and indicate selective interaction of CAML with the γ2 subunit but not with α and β subunits. A C-terminally truncated CAML construct (CAML1-189) encoding the N-terminal cytoplasmic domain of CAML interacted with the γ2 subunit similarly to full-length CAML (Fig. 2F). Interestingly, yeast two-hybrid interaction tests with bait constructs derived from the γ1 [γ1(398-465)] and γ3 subunits [γ3(400-467)] and homologous to γ2(361-428) interacted with full-length CAML or CAML(1-189) similar to the γ2 construct. Therefore, CAML likely interacts with all γ subunit-containing GABAARs.
CAML colocalizes with GABAARs in an ER-related intracellular compartment
We next analyzed the subcellular distribution of CAML in cultured neurons using immunofluorescent staining. CAML immunoreactivity of cultured cortical neurons showed an intracellular punctate distribution suggesting association with vesicular structures. Moreover CAML was extensively colocalized with the ER marker protein disulfide isomerase (PDI) (Fig. 3A), suggesting distribution to ER and other intracellular membranes. However, CAML was not significantly colocalized with immunoreactivity for the lysosomal-associated membrane protein 1 (LAMP1) (Fig. 3B). Consistent with an exclusively intracellular localization, CAML immunoreactivity showed no overlap with punctate immunoreactivity for the γ2 subunit (Fig. 3C), which, under the culture conditions used, is known to represent postsynaptic GABAAR clusters (Alldred et al., 2005; Essrich et al., 1998; Fang et al., 2006). CAML therefore does not contribute to anchoring of GABAARs to the subsynaptic protein scaffold.
Figure 3.
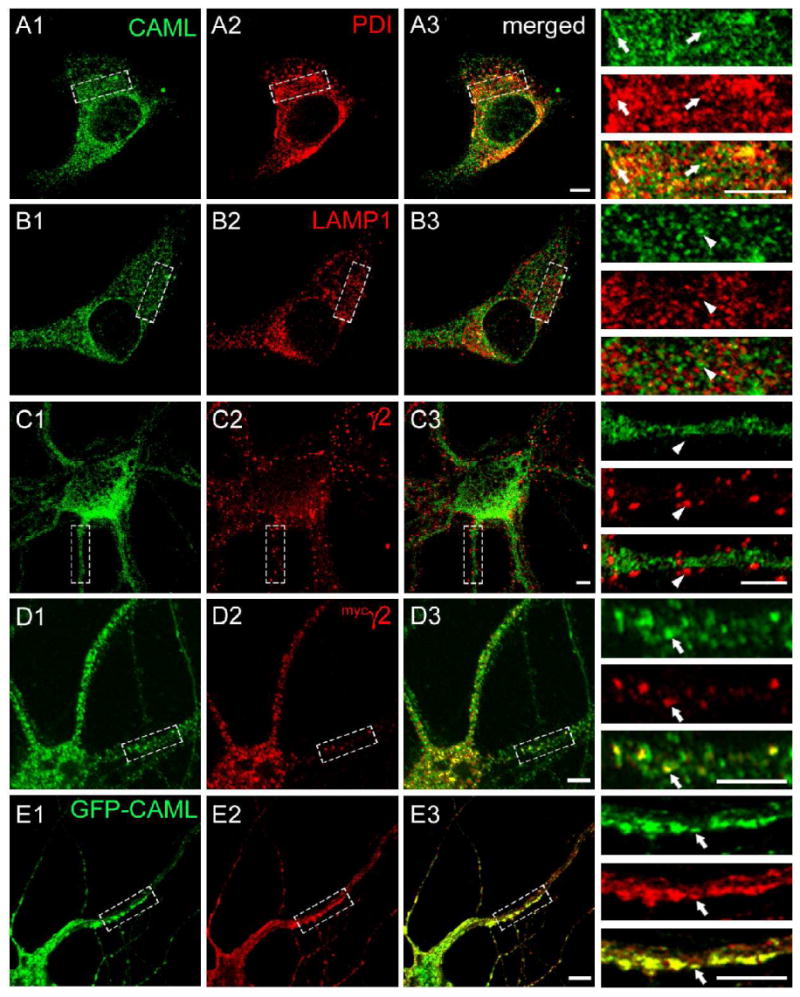
Colocalization of CAML and the γ2 subunit in an intracellular compartment. A-C. Cultured cortical neurons (DIV 18) were fixed, permeabilized and subjected to immunofluorescent staining for CAML (A1, B1, C1, green) together with the ER marker PDI (A2, red), the lysosomal marker LAMP1 (B2, red), or the endogenous γ2 subunit (C2, red) and analyzed by confocal microscopy. Merged images are shown in A3, B3 and C3, respectively, with enlarged bracketed areas of each panel depicted on the right. Note the extensive colocalization of punctate CAML immunoreactivity with staining for the ER marker PDI (A3), whereas there is no discernable colocalization of CAML with lysosomal LAMP1 (B3) or punctate immunoreactivity for the γ2 subunit (C3), representative of postsynaptic GABAARs. D, E. Cortical neurons (DIV16) were transfected with the mycγ2 subunit (D) or GFP-CAML and mycγ2 (E). At DIV18 they were fixed, permeabilized and imaged as above and analyzed for colocalization of immunoreactivity for endogenous CAML (D1, green) or GFP-CAML fluorescence (E1, green) with immunoreactivity for mycγ2 (D2, E2, red). Note the extensive colocalization of punctate intracellular mycγ2 immunoreactivity with vesicular immunoreactivity or GFP fluorescence representing CAML, in both the soma and dendrites of merged images (D3, E3) and detailed in the enlarged dendritic segments. Arrowheads point to lack of colocalization, arrows point to colocalized γ2 subunit and CAML signals. Scale bars, 5 μm.
Given the intracellular localization of CAML and lack of colocalization with GABAARs at synapses, we examined whether CAML would colocalize with GABAARs in intracellular neural compartments. However, currently available antibodies directed against the γ2 subunit are not suitable to specifically visualize intracellular γ2 subunit immunoreactivity. We therefore resorted to transfection of neurons with a myc epitope-tagged γ2 subunit (mycγ2). This construct traffics to postsynaptic sites and restores postsynaptic clustering of GABAARs and gephyrin and inhibitory postsynaptic currents in γ2 subunit knockout neurons, within three days after transfection (Alldred et al., 2005). However, when transfected neurons were analyzed one day after transfection, immunoreactivity for the mycγ2 subunit was largely confined to intracellular compartments and extensively colocalized with a subset of immunoreactive puncta for endogenous CAML in both soma and dendrites (Fig. 3D). The data are consistent with interaction of CAML with γ2 subunit-containing GABAARs in CAML-positive vesicles of the secretory pathway. Moreover, cotransfection of cultured neurons with mycγ2 and GFP-tagged CAML (GFP-CAML, Fig. 3E) resulted in an apparent enlargement of GFP-CAML and mycγ2-containing structures into tubular vesicle-like dendritic aggregates and extensive colocalization of the two proteins. Interestingly, the majority of neurons transfected with GFP-CAML showed overt CAML toxicity and correspondingly low transfection efficiency (not shown), thereby preventing gain of function type analyses of CAML. This toxicity is presumably due to CAML-induced increases in cytoplasmic free calcium (Bram and Crabtree, 1994) and depletion of intracellular Ca2+ stores (Holloway and Bram, 1998). Together with the notion that CAML is an integral membrane protein (Holloway and Bram, 1998), the data suggest that CAML interacts with γ2 subunit-containing GABAARs in an intracellular ER-related vesicular compartment of the neuronal secretory pathway.
CAML is required for normal accumulation of GABAARs at synapses
To address the role of CAML in expression and function of GABAARs, we developed CAML shRNA to knock down expression of CAML in cultured neurons. The efficacy of a CAML cDNA-derived shRNA construct was tested in 293T cells by cotransfection with GFP-CAML (Fang et al., 2006). An unrelated shRNA construct lacking homology to any known mouse mRNAs was used as a negative control. CAML shRNA was found to reduce the average GFP fluorescence of cells cotransfected with GFP-CAML by 98 ± 0.8% compared to control shRNA and GFP-CAML-transfected cells (n = 3 experiments, Supplementary Fig. 1A). Corresponding reductions in GFP-CAML were also seen in lysates of transfected 293T cells analyzed by western blot (Supplementary Fig. 1B).
We first used CAML shRNA to test for possible roles of CAML in expression of GABAARs at synapses. Cultured cortical neurons [days in vitro (DIV) 14] were transfected with CAML shRNA or control shRNA and double stained for the γ2 subunit and the presynaptic GABAergic marker glutamic acid decarboxylase (GAD), using transfection conditions that result in < 5% transfected neurons. Transfected cells were identified by the fluorescence of dsRed encoded by a second transcription unit on the shRNA plasmids. Under these conditions, any effect on GABAergic synapses of shRNA-transfected neurons can be attributed unambiguously to the transfected neuron analyzed and therefore to postsynaptic CAML deficits. Immunofluorescent analyses of CAML shRNA-transfected neurons revealed an overt reduction in punctate staining for the γ2 subunit, indicating reduced expression or maintenance of postsynaptic GABAARs (Fig. 4A). The number of γ2 subunit puncta apposed to punctate GAD staining representing GABAergic terminals was reduced by 34.1 ± 2.2% (n = 110) compared to control shRNA-transfected neurons (n = 47, p < 0.001) (Fig. 4B). In contrast, the size of puncta remained unchanged (Fig. 4C). However, reduced expression of γ2 subunit-containing GABAARs at synapses was associated with a significant reduction in the number of GABAergic synapses, as indicated by a 23.8 ± 1.6% reduction in the number of GAD-positive puncta along dendrites of CAML shRNA-transfected neurons (n = 25) compared to controls (n = 25, p < 0.001)(Fig. 4D). Reduced GABAergic innervation is also seen upon postsynaptic knockdown of the γ2 subunit (Li et al., 2005) or GODZ (Fang et al., 2006), a palmitoyltransferase required for normal accumulation of GABAARs at synapses. Collectively, the data indicate that CAML is required for normal exocytosis and/or membrane stability of γ2 subunit containing GABAARs.
Figure 4.
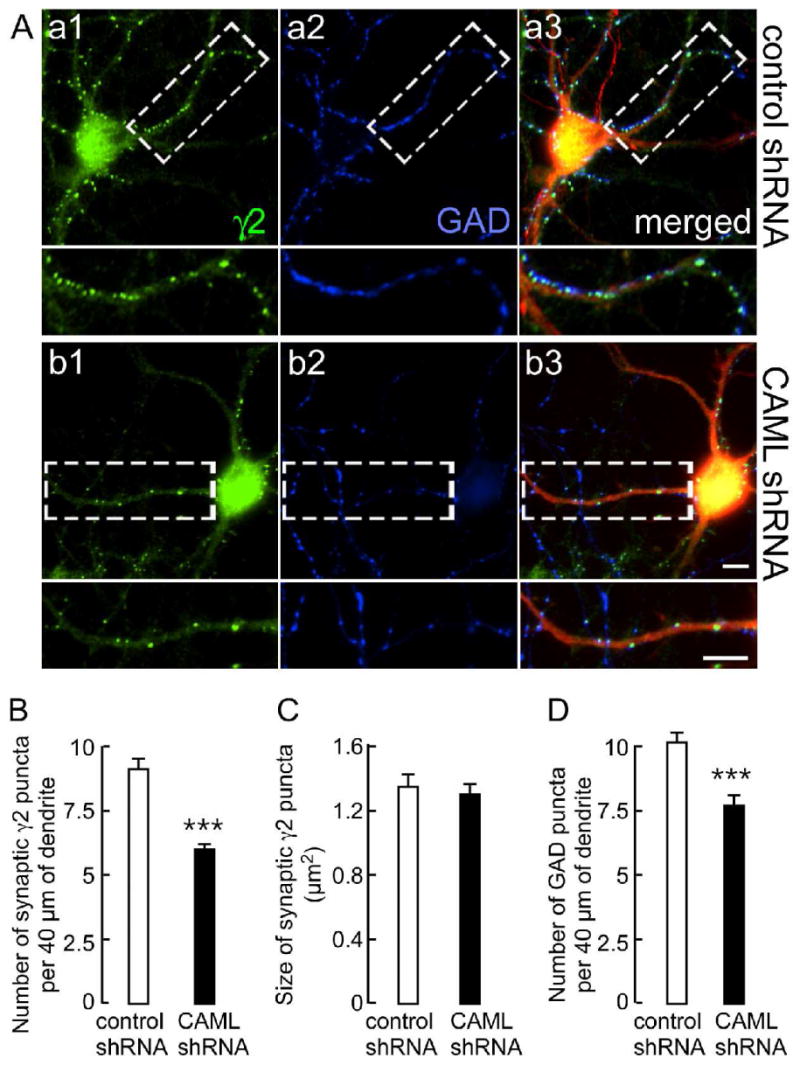
CAML shRNA interferes with expression of postsynaptic γ2 subunit-containing GABAARs and, indirectly, with GABAergic innervation. A. Cortical neurons transfected with control shRNA (Aa1-Aa3) or CAML shRNA (Ab1–Ab3) were stained for the γ2 subunit (green, Aa1, Ab1) and GAD (blue, Aa2, Ab2). Transfected dendrites were identified based on expression of DsRed encoded by the shRNA plasmids, as evident in the merged images (red). Boxed dendritic segments (40 μm) are shown enlarged in the panels below for either γ2, GAD alone, or as merged images. Scale bars, 5 μm. B-D. Semiquantitative analyses of the number (B) and size (C) of immunoreactive puncta for the γ2 subunit apposed to GAD and the number of GABAergic terminals immunopositive for GAD (D) in 40 μm of dendritic segments. Note the prominent reduction in the number of γ2 subunit puncta (CAML shRNA: 6.0 ± 0.2, n = 110; control shRNA: 9.1 ± 0.4, n = 47 cells, p < 0.001) but unaltered size (CAML shRNA: 1.30 ± 0.07 μm2, n = 109; control, 1.35 ± 0.08 μm2, n = 47, p = 0.41). Similarly, the number of GAD puncta was significantly reduced (CAML shRNA, 7.7 ± 0.4; control, 10.1 ± 0.4, n = 25, p < 0.001). Data represent means ± S.E.; ***, p < 0.001, Student t-test.
CAML is essential for normal GABAergic but not glutamatergic transmission
To address possible functions of CAML in synaptic transmission, we first recorded neurotransmitter-evoked whole cell currents of CAML shRNA-transfected cortical neurons, using neurons transfected with an unrelated shRNA as a control. We used a low rate of transfection (< 5% of cells) as above, such that changes in neurotransmission could be unambiguously attributed to CAML deficits in the postsynaptic neurons analyzed. Interestingly, the GABA (20 μM)-evoked current density of CAML shRNA-transfected neurons was greatly reduced to 68.1 ± 10.0% of controls (n = 14 cells per group, p < 0.01, Students t-test) (Fig. 5A). In contrast, the glutamate (1 mM)-evoked current density remained unaffected in CAML shRNA-transfected neurons compared to controls (n = 14, p = 0.37) (Fig. 5B). Moreover, analyses of synaptic currents of CAML shRNA-transfected neurons revealed a dramatic reduction in the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs, 29.5 ± 9.0% of controls, n = 15, p < 0.01), whereas the amplitude of sIPSCs and both the amplitude and frequency of spontaneous excitatory postsynaptic currents (sEPSCs) remained unaffected by CAML shRNA (n = 15, p = 0.42, 0.75 and 0.68 for the three parameters) (Fig. 5C-H). A reduction in the frequency but not the amplitude of sIPSCs is consistent with the reduced number but not size in postsynaptic GABAAR clusters, and the reduced number of GABAergic synapses observed by immunofluorescent staining of CAML shRNA-transfected neurons. These data demonstrate that CAML regulates the trafficking of γ2 subunit-containing GABAARs but not glutamate-gated ion channels.
Figure 5. Functional analyses of CAML shRNA-transfected neurons.
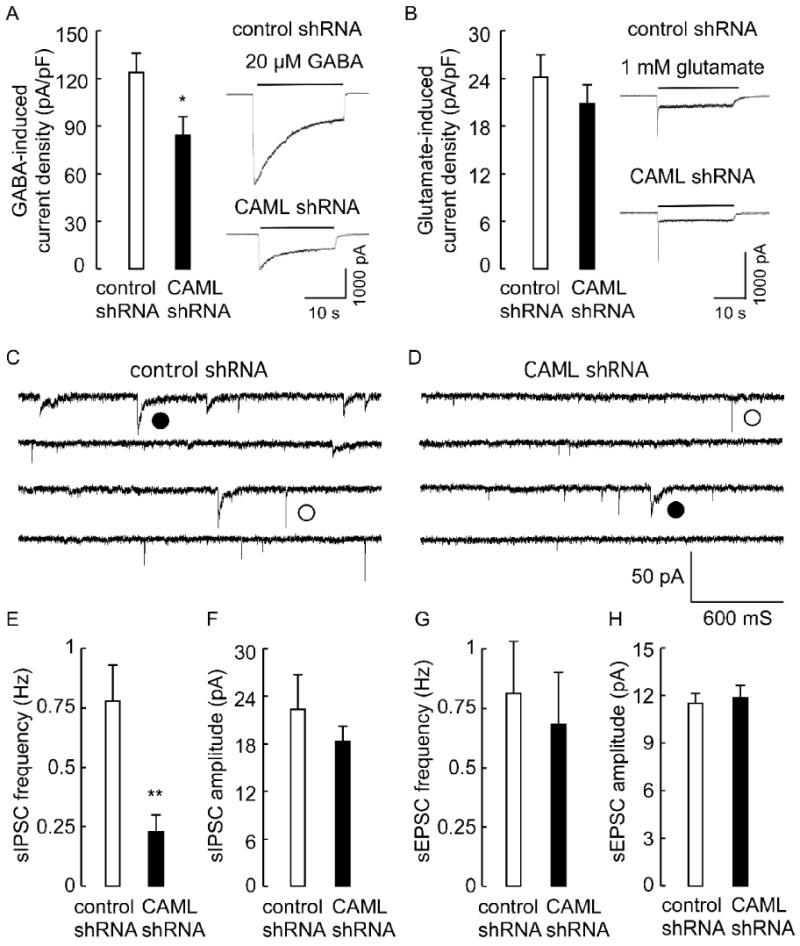
A, B. Analysis of neurotransmitter-evoked whole cell currents revealed a significant reduction in the GABA (20 μM)-induced whole cell current density of CAML shRNA-transfected neurons (84.2 ± 12.3 pA/pF, n = 14 cells) compared to control shRNA-transfected neurons (123.6 ± 11.2 pA/pF, n = 16, p < 0.05) (A). In contrast, the glutamate (1 mM)-induced whole cell current density of CAML shRNA transfected neurons (20.8 ± 2.4 pA/pF, n = 14) was indistinguishable from controls (24.2 ± 2.8 pA/pF, n = 14, p = 0.37)(B). C, D, Current traces recorded from control (C) and CAML shRNA-transfected neurons (D), with representative sIPSCs and sEPSCs denoted with filled and open circles, respectively. E, F, The frequency of sIPSCs recorded from CAML shRNA-transfected neurons (0.23 ± 0.07 Hz) was significantly reduced compared to controls (0.78 ± 0.15 Hz, n = 15, p < 0.01)(E), whereas the amplitude of sIPSCs of CAML shRNA-transfected neurons was unaltered (CAML shRNA, 18.3 ± 1.9 pA, control 22.3 ± 4.4 pA, n = 15, p = 0.42)(D). G, H, The frequency and amplitude of sEPSCs recorded from CAML shRNA-transfected neurons (0.68 ± 0.22 Hz, 11.9 ± 0.78 pA, n = 15) was not different from controls (0.81 ± 0.22 Hz, p = 0.68, 11.5 ± 0.61 pA, n = 15, p = 0.75). Data represent means ± S.E; * p < 0.05, ** p < 0.01, Student's t-test.
CAML is required for normal endocytic recycling of GABAARs
CAML is prominently expressed in the ER and ER-related vesicular compartments in the soma and dendrites of neurons. Moreover, previous analyses of CAML in non-neural cells revealed a critically important function of CAML in endocytic recycling of epidermal growth factor (EGF) receptor (Tran et al., 2003). We therefore addressed whether functional deficits in GABAergic transmission observed in CAML-deficient neurons might reflect deficits in membrane stability or endocytic trafficking of GABAARs. Knock-out mice with a global deletion of CAML exhibit early embryonic lethality (Tran et al., 2003) that precludes preparation and analyses of neurons cultured from corresponding embryos. Therefore, to further investigate the function of CAML biochemically, we took advantage of mice with a CAML locus that is flanked (floxed) by loxP sites (Tran et al., 2005). CAML-mutant neurons were prepared using 4-hydroxytamoxifen-induced conditional deletion of the CAML gene from differentiated neuron cultures derived from CAGGCre-ER™ × CAMLf/f or CAGGCre-ER™ × CAMLf/- embryos (for mating scheme to generate these embryos, see Experimental Methods). Neurons cultured from such embryos express a cytoplasmic and therefore functionally silent version of Cre recombinase (Cre-ER™) that is fused to the hormone-binding domain of the estrogen receptor (Hayashi and McMahon, 2002). Treatment of cultures with 4-hydroxytamoxifen disrupts the interaction of Cre-ER™ with endogenous heat shock protein 90 (Mattioni et al., 1994), thereby allowing it to enter the nucleus and to induce recombination and functional inactivation of the loxP site-containing (floxed) CAML locus.
To determine the deletion efficiency of Cre-ER™-mediated recombination, cortical neuron cultures (DIV 7) from CAGGCre-ER™ × CAMLf/- and CAGGCre-ER™ × CAMLf/f embryos were subjected to chronic treatment with 4-hydroxytamoxifen for 5 days (CAML-mutant cultures) and compared to pseudo WT CAMLf/f or CAMLf/+ or CAMLf/- control cultures that were treated identically in parallel. Quantitative analyses of CAML protein in whole cell lysates of CAML-mutant cultures by western blotting revealed highly effective depletion of CAML to 10 ± 0.27% of CAML protein levels in controls (n = 8, p < 0.001) (Fig. 6A). Moreover, consistent with results from shRNA-transfected neurons, genetic inactivation of the CAML gene in differentiated neurons resulted in reduced punctate immunofluorescent staining of the γ2 subunit (Supplementary Fig. 2A). Whereas the number of puncta was more modestly reduced than in shRNA-treated cultures, loss of CAML in mutant cultures also modestly reduced the size of GABAAR clusters, which was unchanged in CAML shRNA transfected neurons (Supplementary Fig. 2B). A more modest synaptic deficit in CAML mutant than shRNA-transfected neurons is likely due to lack of competition for innervation of neurons in CAML mutant cultures (see Discussion).
Figure 6. Deficits in endocytic recycling of GABAARs in CAML mutant neurons.
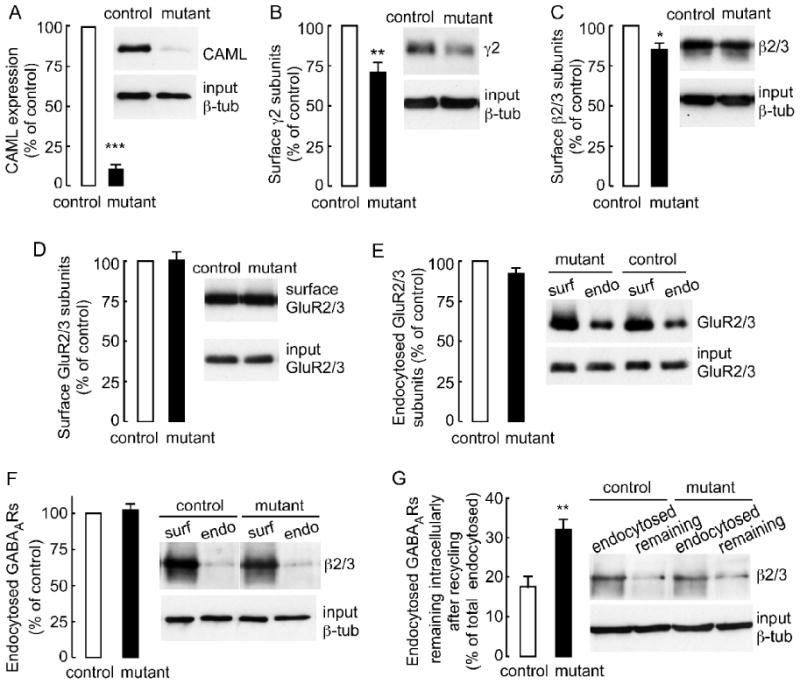
A, The expression of CAML in 4-hydroxytamoxifen-treated CAML mutant (CAGGCre-ER™ × CAMLf/- or CAGGCre-ER™ × CAMLf/f) normalized and compared to pseudo-WT (CAMLf/f) control neuron cultures (DIV 12) was reduced to 10.4 ± 0.3% of controls (= 100%) (n = 8 experiments, p < 0.001). B, The cell surface expression of γ2 subunit-containing GABAARs purified using cell surface biotinylation of CAML mutant cultures was reduced to 72.6 ± 4.7% of CAMLf/- and CAMLf/f controls (n = 5, p < 0.01). C, The cell surface levels of GABAAR β2/3 subunits in CAML mutant cultures were similarly reduced to 84.8 ± 6.1% of controls (n = 5, p < 0.05). D, E, The cell surface expression (D) and rate of endocytosis (E) of GluR2 and/or GluR3-containing AMPA receptors were unaltered in mutants compared to controls (GluR2/3 surface expression in mutants: 100.5 ± 5.1% of controls, n = 5; GluR2/3 endocytosed in mutants during 30 min: 92.0 ± 3.9% of controls, n = 3, p > 0.05). F, The rate of endocytosis of GABAARs in CAML mutant neuron cultures was not different from controls (CAML mutant, 102.6 ± 4.5% of controls, n = 5, p > 0.05). G, The portion of endocytosed GABAARs that remained intracellular after recycling for 40 min was greatly increased in CAML mutant (31.4 ± 2.8% of endocytosed receptors) compared to control cultures (17.1 ± 2.1%, n = 5, p < 0.01). Data represent means ± S.E; * p < 0.05, ** p < 0.01, *** p < 0.001, Student's t-test.
We next used cell surface biotinylation of neurons (DIV12) with sulfo-NHS-SS-biotin to assess possible changes in the surface expression, rates of endocytosis and recycling to the plasma membrane of endocytosed GABAARs in CAML-mutant neurons (Experimental Methods). The mean value for surface biotinylated GABAARs quantified by western blotting with an antiserum specific for the γ2 subunit was lower in CAML mutant (72.6 ± 4.7% of controls, n = 5, p < 0.01) compared to CAMLf/- and CAMLf/f control cultures (Fig. 6B), consistent with the reduction of postsynaptic GABAARs determined by immunofluorescent staining (Fig. 4A, B, Supplementary Fig. 2A, B). Similarly, the mean value for surface biotinylated β2/3 subunit-containing GABAARs quantified with β2/3 subunit specific mAb bd17 was lower in CAML mutant neurons (84.8 ± 6.1% of controls, n = 5 experiments, p < 0.05) (Fig. 6C). In contrast the surface expression of AMPA receptors assessed by quantification of surface biotinylated GluR2 and GluR3 subunit was unaltered in CAML mutant neurons (100.5 ± 5.1% of controls, n = 5 experiments) (Fig 6D). Collectively the data indicate a significant reduction in surface expression of β2/3 and γ2 subunit-containing GABAARs in CAML mutant compared to control cultures. In contrast and consistent with unaltered glutamate-mediated whole cell and synaptic currents (Fig. 5), the expression of AMPA receptors remained unaffected, thereby confirming that the functional impairment of CAML-deficient neurons is selective for inhibitory synapses.
To further address whether reduced surface expression of GABAARs was due to alterations in endocytosis or recycling of receptors we first measured the fraction of surface biotinylated receptors that endocytosed over the duration of 30 min. The fractions of GluR2/3 subunit-containing AMPA receptors and β2/3-containing GABAARs that endocytosed from the cell surface of CAML mutant neurons were indistinguishable from control cultures [endocytosed GluR2/3 subunits (mutant) = 43.6 ± 6.8% of surface GluR2/3 subunits, (control) = 47.6 ± 8.1%, n = 3, p > 0.05; endocytosed β2/3 subunits (mutant) = 10.5 ± 2.6% of surface β2/3, (control) =10.3 ± 2.3%, n = 5, p > 0.05) (Fig. 6E, F). Therefore, reduced accumulation of GABAARs at the cell surface of CAML-deficient neurons is not due to increases endocytosis of cell surface receptors, consistent with absence of CAML immunoreactivity at the plasma membrane of neurons (Fig. 3, and data not shown). In contrast, the portion of endocytosed β2/3-containing GABAARs that remained intracellularly after recycling of endocytosed receptors for 40 min was greatly increased in CAML mutant (31.4 ± 2.8%) compared to control cultures (17.1 ± 2.1%, n = 5 experiments, p < 0.01) (Fig. 6G). The data suggest that reduced accumulation and function of GABAARs at synapses of CAML-deficient neurons is at least in part due to deficits in recycling of internalized GABAARs to the neural cell surface, perhaps with additional deficits caused by reduced exocytosis and impaired function of de novo synthesized GABAARs.
Discussion
Increasing evidence suggests that postsynaptic GABAARs are segregated from non-synaptic receptor subtypes by γ2 subunit-specific protein interactions that regulate the vesicular sorting of these receptors in the secretory pathway. The γ2 subunit interacting trafficking factor GABARAP and its paralogs are subject to C-terminal lipidation, which regulates their vesicular membrane association (Kabeya et al., 2004), a mechanism that is implicated in transport of GABAARs to the plasma membrane (Chen et al., 2007). Furthermore, γ2 subunit-containing GABAARs are subject to palmitoylation by GODZ, a Golgi-restricted membrane protein required for normal accumulation of GABAARs at synapses (Fang et al., 2006; Keller et al., 2004; Rathenberg et al., 2004). Here we have identified CAML as an integral membrane protein that regulates surface expression of GABAARs through interaction with the C-terminal region of the γ2 subunit. Importantly, the M4 domain at the C-terminus of this region is known to be required for accumulation of GABAARs at synapses (Alldred et al., 2005). Our experiments therefore suggest that γ2 M4 domain-dependent trafficking and accumulation of GABAARs at synapses reflects a functional interaction between GABAARs and CAML in the secretory pathway. Consistent with this interpretation, electrophysiological recordings of CAML shRNA - transfected neurons revealed that CAML is required in postsynaptic neurons for normal GABAergic transmission.
In addition to the γ2 M4 domain, interaction of CAML with the γ2 subunit requires the C-terminal end of the γ2 subunit cytoplasmic domain, which includes the γ2 subunit palmitoylation sites (Rathenberg et al., 2004) as well as the interaction sites for GODZ (Fang et al., 2006; Keller et al., 2004) and GABARAP (Chen and Olsen, 2007). Whereas all these proteins are implicated in regulated exocytosis of GABAARs, further investigation is needed to determine whether they act synergistically or antagonistically, whether they interact with each other, and whether CAML regulates GODZ-mediated palmitoylation of the γ2 subunit, or vice versa. Moreover, yeast two-hybrid assays indicate that γ1 and γ3 subunit containing GABAARs interact with CAML in a manner similar to γ2 containing GABAARs. The γ3 subunit can substitute for the γ2 subunit in targeting of GABAARs to postsynaptic sites (Baer et al., 1999). CAML therefore is likely to be relevant for trafficking of all postsynaptic GABAAR subtypes.
CAML immunoreactivity was significantly colocalized with a subset of PDI positive ER membranes. However, CAML was also found in PDI negative vesicular structures, suggesting that its function is not limited to ER membranes. Localization of CAML to ER-related membranes is consistent with earlier studies showing copurification of CAML with membrane fractions containing the sarcoplasmic/endoplasmic reticulum calcium/ATPase-2 (SERCA) and the ER-resident chaperone calreticulin, as well as evidence that overexpression of CAML results in release of Ca2+ from intracellular stores (Holloway and Bram, 1998). Interaction between GABAARs and CAML therefore points to a novel type of interaction of GABAARs with intracellular calcium signaling pathways. Indeed, a GABAAR activation-mediated but membrane depolarization-independent mechanism that leads to elevation of intracellular calcium has been described recently (Chavas et al., 2004). In addition, depolarization- and NMDA receptor-dependent calcium entry is also implicated in regulated trafficking and function of γ2 subunit-containing GABAARs (Marsden et al., 2007; Wang et al., 2003).
CAML deficits induced in cultured neurons by CAML shRNA or by conditional inactivation of the CAML gene demonstrate a role of CAML in normal surface expression of γ2 subunit-containing GABAARs. Furthermore, plasma membrane trafficking assays indicate that deficits in expression and function of GABAARs are at least in part due to reduced endocytic recycling of GABAARs. Interestingly, CAML has previously been suggested to contribute to endocytic recycling of the epidermal growth factor (EGF) receptor (Tran et al., 2003) similar to the function of CAML in the context of GABAARs. The function of CAML in endocytic recycling therefore is not limited to GABAARs or neurons but is likely to be relevant for diverse membrane proteins and cell types. Nevertheless, glutamatergic transmission of CAML shRNA transfected neurons was unaltered, indicating that CAML exerts considerable specificity, in particular for postsynaptic subtypes of GABAARs. Double staining of cultured cortical neurons for CAML and GAD or the pan-neural marker NeuN indicated that CAML is expressed in most or all neurons (data not shown). Therefore, CAML is expected to contribute to trafficking of γ2 subunit-containing GABAARs in most or all neurons.
ShRNA-mediated knockdown of CAML in postsynaptic neurons was associated with loss of GABAergic innervation, in parallel with loss of postsynaptic GABAAR clusters. Such presynaptic deficits have recently been demonstrated upon shRNA-mediated knockdown of the γ2 subunit (Li et al., 2005), or postsynaptic depletion of the γ2 subunit by knock down of GODZ (Fang et al., 2006). Interestingly, significant deficits in GABAergic innervation are also seen in γ2+/- knockout neurons, provided they are cocultured with an excess of wildtype neurons (R. Lal and B.L., unpublished results). In contrast, GABAergic innervation is unaffected in response to reduction of γ2 subunit expression in pure γ2-/- or γ2+/- cultures and in brain of conditional γ2 subunit knockout mice (Essrich et al., 1998; Schweizer et al., 2003). Therefore, presynaptic deficits revealed by recordings from postsynaptic CAML shRNA-transfected neurons are not due to shRNA off target effects but reflect a failure of postsynaptic CAML- and GABAAR-deficient neurons to compete with non-transfected neurons for innervation by GABAergic axons. In contrast to shRNA-transfected cultures, CAML knock-out cultures exhibit CAML and GABAAR deficits in all neurons, thereby avoiding this competitive situation. Further experimentation will be needed to address whether CAML contributes to presynaptic function, in addition to the postsynaptic mechanism analyzed here.
The exact mechanism by which CAML contributes to exocytosis and recycling of GABAARs and accumulation at synapses remains unknown. However, the function of CAML in endocytic trafficking of GABAARs is reminiscent of Huntingtin-Associated Protein 1 (HAP1), a GABAAR β subunit-interacting factor recently shown to promote the recycling of endocytosed GABAARs by inhibiting their degradation (Kittler et al., 2004). In contrast to HAP1, our analyses of GABAAR membrane trafficking in CAML mutant neurons were conducted in the presence of leupeptin, which blocks GABAAR degradation (Kittler et al., 2004). Therefore, CAML appears to differ from HAP1 in that it promotes plasma membrane insertion of GABAARs independent of lysosomal degradative pathways, an interpretation that is consistent with lack of significant colocalization of CAML with the lysosomal marker, LAMP1 (Fig. 3). We speculate that CAML promotes the distribution of GABAARs to ER-derived vesicles of the secretory pathway, thereby facilitating its transport to the cell surface. Once inserted into the plasma membrane, CAML-independent mechanisms such as interaction of GABAARs with subsynaptic gephyrin (Tretter et al., 2001) or synaptic adhesion proteins are likely to ensure maintenance of GABAARs in the postsynaptic membrane. Consistent with this idea, GABAAR exocytosis to and endocytosis from the plasma membrane has recently been demonstrated to occur exclusively at non-synaptic sites (Bogdanov et al., 2006).
In summary, we have identified a novel and selective interaction of CAML with γ2 subunit-containing GABAARs that is required for normal accumulation and function of these receptors at inhibitory synapses. In contrast, CAML is dispensable for normal expression and function of AMPA receptors. CAML may have profound effects on neural excitability via its ability to regulate the endocytic recycling and surface expression of γ2 subunit-containing GABAARs.
Experimental Methods
Isolation of CAML and generation of plasmid constructs
The Matchmaker™ Gal4 Two-Hybrid System 3 was used to screen a mouse brain cDNA library for γ2 subunit-interacting proteins (all reagents from Clontech, Paolo Alto, CA, unless indicated otherwise). The bait vector consisted of the 3′ half of the major cytoplasmic loop and M4 regions of the mouse γ2S subunit (amino acids 361-428 of mature polypeptide) in pGBKT7. Fourteen independent clones were identified and confirmed by sequencing and/or restriction digest to contain part or the entire open reading frame of the CAML cDNA (NM_007596) (Kim et al., 1995). The N-terminal fragment CAML (1-189) was isolated by digestion of the original cDNA with Ssp I and Nco I and inserted into the Sma I and Nco I digested pACT2. An N-terminally truncated fragment of mouse GABARAP (amino acids 36 – 117) was PCR amplified from IMAGE clone AA637305 (MRC Human Genome Mapping Project Resource Centre, Hinxton, UK) and inserted between Nco I and Xho I sites of pACT2. PCR-generated fragments of the γ2 subunit tested as bait construct were inserted between EcoRI and Sal I polylinker sites of either pAS2-1 [γ2 (405-428) and 317-428)] or pGBKT7 [γ2 (361-404), (361-417) and 374-428)]. The C-terminal fragments of the mouse α1 (307-428), mouse β2 subunit (303-450), rat γ1 (aa398-465) and rat γ3 (aa400-467) were analogously inserted into the poly-linker of pAS2-1 or pGBKT7.
Mammalian expression vectors for CAML and GFP-CAML contained the mouse CAML cDNA in pCMV-Tag2C and pEGFP-C3 (Clontech), respectively. Cytomegalovirus based expression vectors for the α2, β3 and mycγ2 subunits have been previously described (Alldred et al., 2005; Benson et al., 1998; Malherbe et al., 1990). The CAML shRNA plasmid was designed based on the mouse CAML cDNA sequence (NM_007596) using the online RNAi design service provided by Integrated DNA Technologies (www.idtDNA.com). It contained the sequence 5′-GATCC GCACT TTGTA TTTCT GTCAT TCCTT TTCAA GAGAA AGGAA TGACA GAAAT ACAAA GTGCT TTTTT G-3′ (underlined nucleotides refer to mouse CAML cDNA sequence) in the Bam HI site of pSIREN-DNR-DsRed-Express (Clontech). This sequence shows 72% identity with the human CAML cDNA. The control shRNA construct was described previously (Fang et al., 2006) and contained an unrelated sequence that lacks homology to transcripts present in the public mouse EST database.
Mouse strains and genotyping
Knockout mice with a deletion of the CAML gene in the germ line (CAML+/- or CAML-/-, Tran et al., 2003) and the mouse line containing a floxed CAML allele (CAMLf/f, Tran et al., 2005) have been previously described. CAGGCre-ER™ transgenic mice (Hayashi and McMahon, 2002) were obtained from JAX mice (Bar Harbor, ME). For 4-hydroxytamoxifen-inducible conditional deletion of CAML in cultured neurons, CAML+/-, with CAMLf/f and CAGGCre-ER™ transgenic mice were interbred to generate CAGGCre-ER™ × CAMLf/- mice and further mated with CAMLf/f mice to produce CAGGCre-ER™ × CAMLf/- and CAGGCre-ER™ × CAMLf/f (inducible mutants) as well as CAMLf/+, CAMLf/-, and CAMLf/f control littermates.
Tissue culture and transfections
Human embryonic kidney 293T (293T) cells were maintained in Dulbecco's Modified Eagle Medium (DMEM), 4 mM Glutamax I and 100 u/ml penicillin/streptomycin supplemented with 10% fetal bovine serum (FBS, all reagents from Invitrogen, Carlsbad, CA, unless indicated otherwise) and transfected using a standard CaPO4 transfection protocol. Cultured cortical neurons were prepared from embryonic day 14/15 mouse embryos essentially as described (Alldred et al., 2005). Upon harvesting, the embryo heads were stored in Hibernate E (Brain Bits, Springfield, IL) supplemented with 2% B27 for maximally 16 h to allow time for genotyping. Cortices of embryos selected for proper genotypes were dissected and treated with 0.5 mg/ml papain (Sigma, Saint Louis, MO) and 10 μg/ml DNase I in PBS containing 10 mM glucose and 0.1% BSA at room temperature for 15 min. The tissue was triturated with a fire polished Pasteur pipette and the cells seeded in Minimal Essential Medium (MEM) supplemented with 2 mM Glutamax I, 2% FBS, 1 mM pyruvic acid, 5.2 mg/ml glucose and 100 u/ml penicillin/streptomycin at 3.7 × 104 cells/cm2 onto poly-L-lysine-coated coverslips (for immunofluorescence analyses) or at 6 × 104 cells/cm2 directly into poly-L-lysine-coated Petri dishes (for biochemical analyses) in incubators set at 37°C and 10% CO2. One h after seeding, the medium was replaced by fresh medium containing 10% FBS for overnight incubation, and starting from the second day, the cells were maintained in Neurobasal-A (NBA) medium containing 2% B27, 2 mM Glutamax I, 100 u/ml penicillin/streptomycin at 37°C and 10% CO2. Cultures on coverslips were maintained upside down on top of a confluent layer of rat glial cells prepared as described (Alldred et al., 2005). Neurons were transfected on DIV14 or 16 using the Calphos Kit (Clontech) as described (Alldred et al., 2005) and processed for immunofluorescence analyses 24-48 h after transfection.
Immunoprecipitations
Lysates of transfected 293T cells in 150 mM NaCl, 2 mM EDTA, 50 mM Tris-HCl pH 7.6, 1% IGEPAL CA630, 100 μM sodium orthovanadate, and protease inhibitors] were cleared by centrifugation and the supernatant diluted with PBS to 0.4% IGEPAL CA630. Protein aliquots were incubated (4°C for 2 h) with IgG, rabbit anti-Flag antiserum or anti-myc mAb that had been conjugated to protein A or G agarose beads as described (Harlow and Lane, 1988). The beads were washed in the above binding buffer (4 × 20 min at 4°C), eluted with SDS-containing sample buffer and subjected to SDS-PAGE/western blot probed with rabbit anti-CAML (1:500) (Tran et al., 2003) or guinea pig anti-γ2 antiserum (Benke et al., 1996) (1:500, generously provided by D. Benke, University of Zurich). For immunoprecipitation of complexes from brain, one mouse brain was homogenized in 3 ml of ice-cold sodium borate buffer (100 mM Na-borate, 1 mM phenylmethylsulfonyl fluoride (PMSF), pH 8.0) and centrifuged at 700 × g for 10 min at 4°C. The supernatant was treated with 0.3 mM DSP at 4°C for 20 min and the reaction stopped by addition of 1/10 volume of 1 M Tris-HCl, pH 7.4. The extract was diluted to 25 ml with buffer A (5 mM Tris-HCl pH 7.4, 5 mM EDTA and protease inhibitors) and the membrane fraction collected by centrifugation at 12,000 × g for 20 min. The pellet was extracted on ice for 20 min in 1 ml 1% Triton X-100, 20 mM HEPES pH 7.4, 5 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 100 μM sodium orthovanadate, protease inhibitors as above) and cleared by centrifugation at 100,000 × g for 30 min. Aliquots of the supernatant were subjected to immunoprecipitation in extraction buffer using an anti-CAML mAb directed against GST-CAML1-189, preconjugated to protein G as above. Immunoprecipitates were analyzed by immunoblot using a rabbit anti-α1 subunit antiserum generously provided by D. Benke, Univ. of Zurich (Benke et al., 1996).
Immunofluorescence analyses of cultured cells
Transfected 293T cells and cortical neurons were processed for immunofluorescence analyses under permeabilized conditions as described (Alldred et al., 2005). The following primary antibodies were used: guinea pig anti-α2 (1:700) and guinea pig anti-γ2 (1:1500) and mAb bd17 specific for β2 and β3 subunits of GABAARs (all provided by J.-M. Fritschy, University of Zurich, Switzerland), mouse anti-GAD (GAD-6, 1:75, Developmental Studies Hybridoma Bank, University of Iowa), mAb anti-myc (1:500, MBL, Woburn, MA), rabbit anti-FLAG (1:1000, Sigma), rabbit anti-CAML (Tran et al., 2003), mAb ER marker PDI and mAb lysosomal marker LAMP1 (1:100, Stressgen Bioreagents, Victoria, Canada). For detection of primary antibodies, Alexa Fluor 488-conjugated goat anti guinea pig, Alexa Fluor 647-conjugated goat anti mouse (Molecular Probes, Eugene, OR), Alexa Fluor 488-conjugated goat anti rabbit, and Cy3 donkey anti-mouse or Cy3 donkey anti-guinea pig antibodies (Jackson ImmunoResearch, West Grove, PA) were used as appropriate. Fluorescent images were captured from a Zeiss Axiophot 2 microscope equipped with a 40 × 1.3 numerical aperture objective and an ORCA-100 video camera linked to an OpenLab imaging system (Improvision, Lexington, MA), or with a confocal laser-scanning microscope (Olympus FlowView FV300). The immunofluorescent staining of images was quantitated as described (Alldred et al., 2005; Fang et al., 2006). Representative images were adjusted for contrast using OpenLab or FlowView software, respectively, and assembled into figure palettes using Adobe Photoshop.
Electrophysiology
For electrophysiological recordings, dissociated neurons were seeded directly onto glial feeders and media changed on the second day to MEM containing 100 mg NaHCO3, 20 mM D-glucose, 5% FBS, 25 u/ml penicillin/streptomycin, 0.5 mM L-glutamine, 4 μM Ara-C, and 2% B27. Neurons maintained at 37°C and 5% CO2 were transfected with shRNA constructs (1 μg) and pEGFP-C1 (0.5 μg, Clontech) on DIV 8 or 10 using the Calphos Kit (Clontech) as described (Jiang and Chen, 2006). A total volume of 40-50 μl of DNA-containing transfection mixture was added to neurons in 500 μl of transfection medium (MEM containing 30 mM D-glucose) for 30-45 min, followed by washing with warm medium preequilibrated at 10% CO2 and culture at 5% CO2 for 2 more days. Conventional whole-cell patch clamp recordings were performed as described (Yao et al., 2006). Signals were amplified by Multiclamp 700A patch clamp amplifier (Axon Instruments). Patch pipettes were pulled from borosilicate glass and fire-polished (4-6 MΩ). The recording chamber was continuously perfused with a bath solution containing 128 mM NaCl, 30 mM glucose, 25 mM HEPES, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, pH 7.3 adjusted with NaOH. The pipette was filled with 147 mM KCl, 5 mM Tris-phosphocreatine, 2 mM EGTA, 10 mM HEPES, 4 mM MgATP, 0.5 mM Na2GTP, pH 7.3 adjusted with KOH. The series resistance was typically 10-20 MΩ and compensated by 15-40%. The membrane potential was held at -70 mV. Data were acquired using pClamp 9 software, sampled at 5 kHz and filtered at 1 kHz. Off-line data analysis was done with Clampfit 9 software (Axon Instrument) and MiniAnalysis software (Synaptosoft). All data were expressed as means ± SE and Student's t test was used for statistical analysis. Spontaneous postsynaptic currents were recorded and categorized into excitatory or inhibitory events based on their kinetic characteristics. Spontaneous EPSCs were defined as synaptic events with a decay time constant of 3-6 ms that were blocked by 10 μM 6-cyano-7-nitoquinoxaline-2,3-dione (CNQX). Spontaneous IPSCs were defined as synaptic events with a decay time constant of 10-20 ms that could be blocked by 20 μM bicuculline. Recordings of GABA- and glutamate-induced current densities were performed as described (Fang et al., 2006; Qi et al., 2006).
Analysis of endocytic recycling by surface biotinylation of CAML-deficient neurons
Cortical neurons derived from CAGGCre-ER™ × CAMLf/- or CAGGCre-ER™ × CAMLf/f embryos and CAMLf/+, CAMLf/- or CAMLf/f control embryos were plated into poly-L-lysine-coated Petri dishes (6 cm, 6 × 104 cells/cm2), differentiated to DIV7 and treated with 4-hydroxytamoxifen as described above for 3-6 days. Endocytic recycling assays were performed essentially as described (Ehlers, 2000; Kittler et al., 2004). For measurements of total surface expression, cortical neurons (DIV 10-13, ≥ 3 replicate 6-cm plates/genotype) were incubated for 30 min with 100 μg/ml leupeptin in media, washed with PBS (+ CaCl2/MgCl2), followed by incubation with 1 mg/ml sulfo-NHS-SS-biotin (Pierce, Rockford, IL) in PBS for 20 min on ice. Unreacted biotin was quenched with 50 mM glycine in PBS for 5 min on ice and the cells lysed in a buffer containing 1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1 mM PMSF, 10 μg/ml each of leupeptin, pepstatin, and aprotinin. The biotinylated proteins were purified over UltraLink NeutrAvidin (Pierce) and quantified by immunoblot using mAb bd17 specific for β2/β3 subunits or antisera specific for the γ2 subunit (Sigma, Saint Louis, MO). The band intensities of western blots were quantified using Image J software and normalized against the total amount of protein in each sample assessed by western blot of unpurified lysates (1/30) on the same gel using antibodies against β-tubulin (SIGMA). For quantification of GluR2/3 subunits the same blots were stripped and reprobed with affinity-purified rabbit anti GluR2/3 subunit antiserum (1:500, Chemicon, Temecula, CA) and the surface protein bands were normalized against the GluR2/3 amounts in aliquots of unpurified lysates analyzed on the same membrane. Surface GABAAR and AMPA receptor subunit amounts in mutant neurons were then normalized to values of control cultures analyzed in parallel on the same membrane. For measurements of endocytosed receptors, the surface expression of β2/3 and GluR2/3 subunits was analyzed as above. In addition, the biotinylated proteins of a second plate of cells processed in parallel were allowed to endocytose in modified Ringer solution (119 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 0.001 mM glycine, 30 mM glucose, 25 m HEPES pH7.4) at 37°C for 30 min. Surface biotin was cleaved with glutathione (75 mM glutathione, 75 mM NaCl, 10 mM EDTA, 1% BSA pH 8.0, 2 × 15 min on ice), followed by purification of endocytosed biotinylated proteins and quantitation of β2/β3 or GluR2 subunits as above.
The amounts of endocytosed GABAA and AMPA receptors were normalized to the amounts of total surface receptors in each sample and expressed as % of controls. A third plate used for measurement of endocytosed minus recycled GABAAR pools was labeled, subjected to endocytosis and glutathione-treated identically but then returned to modified Ringer solution (37°C, 40 min) containing 75 mM glutathione and 1% BSA. Under these conditions, the biotin moiety of endocytosed proteins that recycle back to the cell surface are cleaved off, such that the pool of biotinylated proteins remaining and quantified by western blot corresponds to the fraction of intracellular endocytosed GABAARs that failed to recycle back to the cell surface. The fraction of endocytosed GABAAR subunits in mutant neurons remaining after recycling was expressed as % of total endocytosed GABAARs determined simultaneously. Experiments were repeated five times for statistical analyses.
Acknowledgments
We are grateful to Drs. D. Benke and J.-M. Fritschy for gifts of GABAAR antisera and to Yao Guo, Lauren Kerr, Sue Lingenfelter, Michelle Martin, and Junlei Sun for technical assistance. This work was supported by grants from the NIMH (MH62391, MH60989) to BL and a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alldred MJ, Mulder-Rosi J, Lingenfelter SE, Chen G, Luscher B. Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAA receptors and gephyrin. J Neurosci. 2005;25:594–603. doi: 10.1523/JNEUROSCI.4011-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy JM, Luscher B. Postsynaptic clustering of GABAA receptors by the γ3 subunit in vivo. Proc Natl Acad Sci USA. 1999;96:12860–12865. doi: 10.1073/pnas.96.22.12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benke D, Honer M, Michel C, Mohler H. GABAA receptor subtypes differentiated by their gamma-subunit variants: prevalence, pharmacology and subunit architecture. Neuropharmacology. 1996;35:1413–1423. doi: 10.1016/s0028-3908(96)00068-8. [DOI] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant γ-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABA(A) receptors are directly recruited from their extrasynaptic counterparts. Embo J. 2006;25:4381–4389. doi: 10.1038/sj.emboj.7601309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram RJ, Crabtree GR. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature. 1994;371:355–358. doi: 10.1038/371355a0. [DOI] [PubMed] [Google Scholar]
- Chavas J, Forero ME, Collin T, Llano I, Marty A. Osmotic tension as a possible link between GABA(A) receptor activation and intracellular calcium elevation. Neuron. 2004;44:701–713. doi: 10.1016/j.neuron.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Chang CS, Leil TA, Olsen RW. C-terminal modification is required for GABARAP-mediated GABA(A) receptor trafficking. J Neurosci. 2007;27:6655–6663. doi: 10.1523/JNEUROSCI.0919-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZW, Olsen RW. GABA Receptor Associated Proteins: a key factor regulating GABA receptor function. J Neurochem. 2007;100:279–294. doi: 10.1111/j.1471-4159.2006.04206.x. [DOI] [PubMed] [Google Scholar]
- Crestani F, Lorez M, Baer K, Essrich C, Benke D, Laurent JP, Belzung C, Fritschy JM, Luscher B, Mohler H. Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat Neurosci. 1999;2:833–839. doi: 10.1038/12207. [DOI] [PubMed] [Google Scholar]
- Earnheart JC, Schweizer C, Crestani F, Iwasato T, Itohara S, Mohler H, Luscher B. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity- dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Essrich C, Lorez M, Benson J, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci. 2006;26:12758–12768. doi: 10.1523/JNEUROSCI.4214-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Brunig I. Formation and plasticity of GABAergic synapses: physiological mechanisms and pathophysiological implications. Pharmacol Ther. 2003;98:299–323. doi: 10.1016/s0163-7258(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A laboratory Manual. Cold Spring Harbor Laboratory; Cold Spring Harbor: 1988. [Google Scholar]
- Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244:305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- Holloway MP, Bram RJ. A hydrophobic domain of Ca2+-modulating cyclophilin ligand modulates calcium influx signaling in T lymphocytes. J Biol Chem. 1996;271:8549–8552. doi: 10.1074/jbc.271.15.8549. [DOI] [PubMed] [Google Scholar]
- Holloway MP, Bram RJ. Co-localization of calcium-modulating cyclophilin ligand with intracellular calcium pools. J Biol Chem. 1998;273:16346–16350. doi: 10.1074/jbc.273.26.16346. [DOI] [PubMed] [Google Scholar]
- Jiang M, Chen G. High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc. 2006;1:695–700. doi: 10.1038/nprot.2006.86. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred MJ, Sassoè-Pognetto M, Luscher B. The γ2 subunit of GABAA receptors is a substrate for palmitoylation by GODZ. J Neurosci. 2004;24:5881–5891. doi: 10.1523/JNEUROSCI.1037-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Morales VM, Dass C, Encinas J, Teitell M, Blumberg RS. Cloning of the gene encoding the mouse homologue of the human calcium signal-modulating ligand. Gene. 1995;163:323–324. doi: 10.1016/0378-1119(95)00393-k. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci U S A. 2004;101:12736–12741. doi: 10.1073/pnas.0401860101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Drabik K, Benveniste EN. Cloning of rat calcium-modulating cyclophilin ligand. DNA Seq. 2001;12:209–213. doi: 10.3109/10425170109080777. [DOI] [PubMed] [Google Scholar]
- Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J Neurochem. 2005;95:756–770. doi: 10.1111/j.1471-4159.2005.03426.x. [DOI] [PubMed] [Google Scholar]
- Luscher B, Keller CA. Regulation of GABAA receptor trafficking and channel activity in functional plasticity of inhibitory synapses. Pharmacol Ther. 2004;102:195–221. doi: 10.1016/j.pharmthera.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Malherbe P, Draguhn A, Multhaup G, Beyreuther K, Mohler H. GABAA-receptor expressed from rat brain alpha- and beta-subunit cDNAs displays potentiation by benzodiazepine receptor ligands. Mol Brain Res. 1990;8:199–208. doi: 10.1016/0169-328x(90)90017-8. [DOI] [PubMed] [Google Scholar]
- Marsden KC, Beattie JB, Friedenthal J, Carroll RC. NMDA receptor activation potentiates inhibitory transmission through GABA receptor-associated protein-dependent exocytosis of GABA(A) receptors. J Neurosci. 2007;27:14326–14337. doi: 10.1523/JNEUROSCI.4433-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioni T, Louvion JF, Picard D. Regulation of protein activities by fusion to steroid binding domains. Meth Cell Biol. 1994;43(Pt A):335–352. doi: 10.1016/s0091-679x(08)60611-1. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Qi J, Yao J, Fang C, Luscher B, Chen G. Downregulation of tonic GABA currents following epileptogenic stimulation of rat hippocampal cultures. J Physiol. 2006;577:579–590. doi: 10.1113/jphysiol.2006.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neurosci. 2004;26:251–257. doi: 10.1016/j.mcn.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Schweizer C, Balsiger S, Bluethmann H, Mansuy M, Fritschy JM, Mohler H, Luscher B. The γ2 subunit of GABAA receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- Tran DD, Edgar CE, Heckman KL, Sutor SL, Huntoon CJ, van Deursen J, McKean DL, Bram RJ. CAML is a p56Lck-interacting protein that is required for thymocyte development. Immunity. 2005;23:139–152. doi: 10.1016/j.immuni.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Tran DD, Russell HR, Sutor SL, van Deursen J, Bram RJ. CAML is required for efficient EGF receptor recycling. Dev Cell. 2003;5:245–256. doi: 10.1016/s1534-5807(03)00207-7. [DOI] [PubMed] [Google Scholar]
- Tretter V, Hauer B, Nusser Z, Mihalek RM, Hoger H, Homanics GE, Somogyi P, Sieghart W. Targeted disruption of the GABAA receptor delta subunit gene leads to an upregulation of gamma2 subunit-containing receptors in cerebellar granule cells. J Biol Chem. 2001;276:10532–10538. doi: 10.1074/jbc.M011054200. [DOI] [PubMed] [Google Scholar]
- von Bulow GU, Bram RJ. NF-AT activation induced by a CAML-interacting member of the tumor necrosis factor receptor superfamily. Science. 1997;278:138–141. doi: 10.1126/science.278.5335.138. [DOI] [PubMed] [Google Scholar]
- Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW. GABAA-receptor-associated protein links GABAA receptors and the cytoskeleton. Nature. 1999;397:69–72. doi: 10.1038/16264. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, Paw J, Wang Y, Mansuy I, Salter MM, Lu YM. Interaction of calcineurin and type-A GABA receptor gamma2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003;23:826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Qi J, Chen G. Actin-dependent activation of presynaptic silent synapses contributes to long-term synaptic plasticity in developing hippocampal neurons. J Neurosci. 2006;26:8137–8147. doi: 10.1523/JNEUROSCI.1183-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


