Abstract
Heterotrimeric G proteins are comprised of a guanine nucleotide binding Gα subunit and the Gβγ dimers that link G protein-coupled receptors (GPCRs) to effectors. This study focuses on the expression and localization patterns for certain Gβ and Gγ subunits in neonatal and adult cardiomyocytes. We identify developmental downregulation of Gβ1, Gβ2 and Gγ2, and a switch in the molecular form of Gγ3, in cardiomyocytes. Gβ1 is highly localized to caveolae membranes, whereas Gβ2 is identified in caveolae and other membrane fractions. Gβ3 is not detected in neonatal cardiomyocytes, but rather Gβ3 is upregulated in adult cardiomyocytes and detected in the caveolae and soluble fractions. The observation that cardiomyocytes co-express multiple Gβ and Gγ subunits in a developmentally regulated manner, and that these Gβ and Gγ subunits assume distinct subcellular localization patterns, provides for a high level of signaling specificity in the heart.
Keywords: G proteins, cardiomyocytes, development, caveolae
INTRODUCTION
Heterotrimeric G proteins are a family of signaling proteins that link G protein-coupled receptors (GPCRs) to the activation of effectors [1]. G proteins are composed of a guanine nucleotide binding Gα subunit that associates with the dimeric Gβγ complex; GPCR activation catalyzes GDP exchange for GTP on the Gα subunit, resulting in the dissociation of GTP-liganded Gα from Gβγ. Freed Gα subunits and Gβγ dimers both regulate effector responses; Gβγ dimers also play a role in targeting Gα subunits to membrane and in controlling G protein interactions with GPCRs and effectors.
At present, 5 Gβ subunits and more than 12 Gγ subunit genes have been identified in the human genome. Gβ1–4 share over 80% identity with one another in their primary amino acid sequence, whereas Gβ5 is an outlier by structural and functional criteria. Early studies relied largely on in vitro reconstitution assays and characterized β1–β4 subunits as interchangeable components of the βγ dimer. βγ dimer signaling specificity was attributed largely to differences in the functional properties of the structurally divergent Gγ subunits. However, recent studies identify effects of Gβ subunits to direct heterotrimeric G protein interactions with GPCRs and effectors [2]. The large number of Gβ and Gγ subunits expressed by most tissues provides, at least in theory, the potential to assemble a dauntingly large number of structurally unique βγ dimers. However, the number of structurally distinct βγ dimers in vivo is limited by [1] tissue-restricted expression of certain Gβ and Gγ subunits and [2] selectivity in Gβ-Gγ interactions, such that only certain βγ combinations form functional dimers. For example, using a yeast two-hybrid system, Yan et al. published that most Gγ subunits interact well with Gβ1 and Gβ2 subunits, but poorly with Gβ3 and Gβ4 subunits [3]. This is consistent with studies in transfected cells or in vitro translation systems, where all Gγ subunits interact with Gβ1, most Gγ subunits (with the notable exception of Gγ1, and the related Gγ11) interact with Gβ2, but no Gγ subunit binds to Gβ3 [4–6]. However, these βγ dimer pairs forming during reconstitution experiments in cell-free systems (or following heterologous overexpression in undifferentiated cell lines) may not necessarily faithfully mimic βγ dimer formation in native tissues (at endogenous levels of protein expression and with potential differences in the subcellular localization of individual Gβ and Gγ subunits). Of note, there is still only limited information on Gβγ dimer expression in heart and β3γ interactions in native tissues have not been examined. The absence of information on Gβ3γ dimer expression is frustrating in view of evidence that a polymorphism in the Gβ3 subunit gene (the GNB3 825T allele) has been associated with several metabolic and cardiovascular disorders [7].
Gβ and Gγ subunit expression in ventricular myocardium (or isolated cardiomyocytes preparations) is reported to change during development. Hansen et al. identified Gβ1, Gβ2, Gγ3, Gγ5, and Gγ7 expression in cholate extracts of membranes prepared from neonatal ventricles (and neonatal rat cardiomyocyte cultures), with lower levels of Gβ2, Gγ5, and Gγ7 (and no Gβ1 or Gγ3) in adult ventricular preparations; Gβ3 and Gγ2 were not detected in the ventricle at either age [8]. Since recent studies identified caveolae (or lipid rafts) as membrane subdomains that spatially organize GPCR-activated signaling pathway, and G protein βγ dimers expressed at low levels (or compartmentalized to lipid rafts) might have evaded detection in previous studies, we examined G protein β and γ subunit expression in ventricular cardiomyocytes. This study identifies a striking developmental switch in Gβ and Gγ subunit expression in the ventricle that (in theory) provides a novel mechanism to specify GPCR signaling pathways in the heart.
METHODS
Cardiomyocytes were isolated from the hearts of 2-day-old Wistar rats by a trypsin dispersion procedure according to a protocol that incorporates a differential attachment procedure to enrich for cardiomyocytes followed by irradiation [9]. Cells were plated at a density of 0.5 × 106 cells/ml (high-density, to generate a confluent monolayer) or 0.25 × 106 cells/ml (low-density, conditions associated with markedly reduced cell-cell contacts) on protamine sulfate-coated culture dishes and cultured in MEM (Gibco BRL) with 10% fetal calf serum, 5 × 10−6 M hypoxanthine, and 12 mM NaHCO3. Adult rat ventricular myocytes were disaggregated according to methods described previously and used within 1–6 hr of isolation [10].
Caveolin-rich membranes were prepared according to a detergent-free purification scheme described previously [9]. Briefly, cells from five 100-mm diameter dishes were scraped into 0.5 M sodium carbonate, pH 11.0 (0.5 ml per dish) and combined for each preparation. The extract was sequentially disrupted by homogenization with a Dounce homogenizer, a Polytron tissue grinder, and a tip sonicator. The homogenate was adjusted to 40% sucrose by adding an equal volume of 80% sucrose prepared in Mes-buffered saline (MBS; 25 mM Mes, pH 6.5, 0.15 M NaCl), placed on the bottom of an ultracentrifuge tube, overlaid with a 5–35% continuous sucrose gradient, and centrifuged at 38,000 rpm for 16–18 hrs in a SW40 rotor (Beckman). After centrifugation, aliquots of fractions were dissolved in sample buffer containing SDS and 2-mercaptoethanol and heated prior to electrophoresis in SDS-PAGE gels. Samples were then transferred to nitrocellulose and immunoblotted with anti-caveolin-3 (mAb 26; BD Transduction Laboratories). The polyclonal anti-Gβ1, -Gβ2, -Gβ3, -Gγ2 and -Gγ3 were from Santa Cruz. Immunoblot analysis was limited to these proteins as preliminary studies indicated that the commercially available reagents for other Gβ or Gγ subunits do not detect endogenous levels of Gβ and Gγ subunits in heart. Immunodetection was with chemiluminescence.
Results
Developmental changes in Gβ and Gγ subunit expression in ventricular myocardium
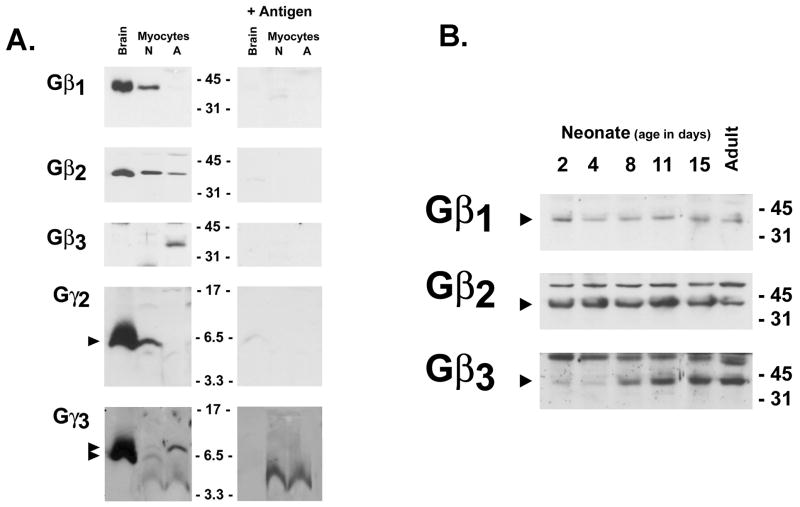
Gβ and Gγ subunit expression generally has been examined at the mRNA level, since antibodies that are sufficiently sensitive/specific to detect the endogenous proteins at physiologically relevant levels of expression are relatively recent. Figure 1A shows that Gβ1, Gβ2, and Gβ3 proteins are detected in a developmentally regulated manner in extracts from post-natal day 2 and adult cardiomyocytes; high levels of Gβ1 and Gβ2 (but not Gβ3) also are detected by immunoblot analysis in extracts from rat brain (included as a control preparation in the experiments). In each case immunoreactivity is specific; bands are not detected when immunoblot analysis is performed with antibodies preadsorbed with competing peptide antigen. Gβ1 and Gβ2 are readily detected in neonatal cardiomyocytes. Gβ2 expression also is detected in adult cardiomyocytes, although at considerably lower levels; Gβ1 expression is at the limits of detection in extracts from isolated adult cardiomyocytes. While immunoblotting on extracts from intact ventricular tissue also shows a developmental decline in Gβ1 and Gβ2 protein expression, these differences are rather modest when compared to the more striking developmental decreases in Gβ1 and Gβ2 protein expression in purified cardiomyocyte preparations (Figure 1B). These results suggest that the significant amounts of Gβ subunits detected in the adult ventricle derive from contaminating cell types (such as fibroblasts). In striking contrast, Gβ3 expression is confined to adult cardiomyocytes; Gβ3 is not detected in neonatal cardiomyocytes. Gβ3 is detected only at trace levels in post-natal day 2 ventricles; Gβ3 expression increases by post-natal days 8–11, reaching adult levels by 2 weeks of age.
Figure 1. Developmental changes in Gβ and Gγ expression in the ventricle.
100 μg of total cell protein extracted from day 5 neonatal cardiomyocyte cultures, or freshly isolated adult rat cardiomyocytes (Panel A) or from ventricles from rats at the indicated ages (Panel B) or were subjected to SDS-PAGE and immunoblot analysis with the indicated antibodies. An extract from brain (100 μg) was included as a positive control in Panel A. Epitope specific immunoreactivity was established by immunoblotting with antibody complexed with competing antigen peptide; note smaller proteins detected by the Gγ3 antibody are non-specific. Arrow denote epitope specific bands, with positions of the molecular weight standards (in kDa) indicated.
Gβ subunit expression also was tracked in neonatal cardiomyocytes maintained in culture for 10–14 days, to determine whether Gβ subunit expression is regulated in vitro. The observation that Gβ1 and Gβ2 expression is maintained at high levels and Gβ3 expression is not induced when cardiomyocytes are aged in vitro (for an interval that would be associated with downregulation of Gβ1/Gβ2 and the induction of Gβ3 in vivo in the intact ventricle) indicates that the developmental programs that regulate Gβ expression in vivo are not triggered when cardiomyocytes are aged in vitro (and that neonatal cardiomyocyte cultures are a valid surrogate model for studies of Gβ subunit function in the neonatal ventricle).
Certain developmental changes in gene expression have been attributed to the postnatal surge in thyroid hormone secretion that regulates the expression of thyroid hormone-responsive gene products. Given the marked effects of thyroid hormone on autonomic (and GPCR) responsiveness, we tracked Gβ expression in neonatal cardiomyocytes cultured for 5 days in serum free medium containing a range of thyroid hormone concentrations (10−12 to 10−8 M tri-iodothyronine). The observation that stepwise increments in thyroid hormone concentrations lead to the predictable increase in β1-adrenergic receptor expression, without a fall in Gβ1/Gβ2 expression or the induction of Gβ3 effectively excludes a role for thyroid hormone as a major mediator of developmental changes in cardiomyocyte Gβ subunit expression (data not shown).
Gγ subunits that introduce an additional level of complexity to signaling pathways also were examined. Figure 1A shows that Gγ2 and Gγ3 are detected at high levels in brain. Gγ2 is detected in neonatal cardiomyocytes, and at much lower levels in the adult cardiomyocytes. Gγ3 is resolved as an epitope specific doublet in brain and neonatal cardiomyocytes; similar molecular heterogeneity for Gγ3 (and other Gγ subunits) has been identified in studies on endogenous proteins in tissues, but its significance remains uncertain. Both forms of Gγ3 are detected in equal amounts in neonatal cardiomyocytes; the slower migrating form of Gγ3 predominates in adult cardiomyocytes.
β and γ subunit targeting to lipid raft/caveolae
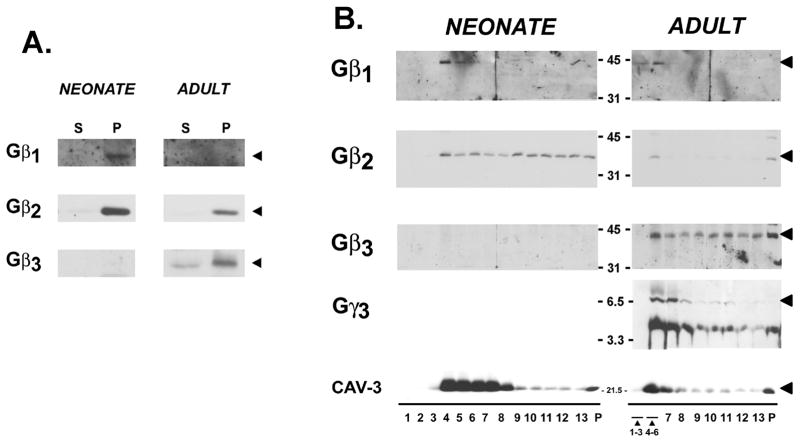
Gβ subunit partitioning between soluble and particulate fractions was examined as an initial strategy to identify differences in Gβγ dimer localization in cardiomyocytes. Figure 2 shows that Gβ1 and Gβ2 are recovered exclusively in the particulate fraction of neonatal and adult cardiomyocytes; no Gβ1 or Gβ2 immunoreactivity is detected in the soluble fractions (even with increased protein loading or long exposures of the gel). In contrast, Gβ3 (which is confined to adult cardiomyocytes) partitions to both particulate and soluble fractions.
Figure 2. Gβ and Gγ partitioning to membranes and low-density caveolae in cardiomyocytes.
Panel A: Neonatal cardiomyocyte cultures and acutely isolated adult cardiomyocytes were partitioned into soluble and particulate fractions and then subjected to immunoblot analysis for Gβ subunits as indicated. Panel B: Neonatal cardiomyocyte cultures and isolated adult cardiomyocytes were homogenized in sodium carbonate buffer and subjected to sucrose gradient centrifugation as described in methods. Fractions were collected from the top of the gradient and analyzed by SDS-PAGE and immunoblot analysis with the indicated antibodies. Fractions 1–3 and 4–6 in profiles from adult cardiomyocytes were pooled, due to the limiting amounts of protein recovered in these fractions. Immunoblot analysis was on 35 μg of protein from each fraction. Results are representative of data from three separate experiments.
Recent studies identify caveolae (or lipid rafts) as structures that spatially organize certain components of GPCR-activated signaling pathways [11–13]. Caveolae are 50–100 nm flask-shaped invaginations of the plasma membrane that are particularly abundant in terminally differentiated cells (including cardiomyocytes) and provide a mechanism to dynamically regulate specialized functions at the cell surface. We previously demonstrated that β2-adrenergic receptors and pertussis toxin-sensitive Gα subunits are confined to the caveolae fraction of quiescent neonatal and adult cardiomyocytes [9;14]. In contrast, Gαs and Gβ (identified with a antibody that does not discriminate between individual Gβ isoforms) partition between caveolae and other cell fractions [14]. We examined the subcellular distribution of individual Gβ subunits using a similar biochemical fractionation scheme that involves equilibrium centrifugation through a discontinuous sucrose gradient to separate buoyant lipid vesicles (F4-7 of the gradient, that represents ~0.5% of total cell protein and is enriched in the muscle-specific caveolae marker protein caveolin-3) from other cell membranes and cytosolic proteins (that are recovered in the heavy sucrose gradient fractions and pellet [P]).
Figure 2 shows that Gβ1 is recovered exclusively in the low-density buoyant vesicle fraction isolated from neonatal and adult cardiomyocytes. Gγ3 also is recovered mostly in the caveolin-3-enriched F4-7 fraction. In contrast, Gβ2 (in neonatal and adult cardiomyocytes) and Gβ3 (in adult cardiomyocytes) distribute to both low-density caveolin-3-enriched membranes and heavy sucrose gradient fractions (which contain the bulk of the cellular protein).
Discussion
This study demonstrates that cardiomyocytes co-express multiple Gβ and Gγ subunits in a developmentally regulated manner. We identify a developmental downregulation of Gβ1, Gβ2 and Gγ2, a switch in the molecular form of Gγ3, and a developmental induction of Gβ3. There is little-to-no information on developmental controls of Gβ and Gγ subunit expression (and the available information has focused mainly on Gγ2, which increases during the late embryonic period in the brain and is induced in human leukemia HL-60 cells during differentiation into granulocyte [15]). The molecular mechanism underlying developmental changes in Gβγ dimer expression remain uncertain; while certain age-dependent changes in gene expression can be attributed to a perinatal surge in thyroid hormone, the Gβγ subunits examined in this study do not appear to be thyroid hormone-responsive gene products. However, developmental differences in Gβ and Gγ expression are predicted to lead to the formation of βγ dimer pairs of varying composition in distinct subcellular compartments (caveolae vs. non-caveolae membranes) in neonatal and adult cardiomyocytes. Current concepts regarding the role of Gβγ dimers in the regulation of GPCR signaling pathways is largely based upon studies that pair many Gγ subunits with a single Gβ subunit (or a single Gγ with structurally/functionally diverse Gβ’s such as Gβ1 and Gβ5). Reconstitution experiments that examine the signaling repertoire of a particular cell type (i.e., that address physiologically relevant questions related to Gβγ dimer assembly and function in a particular tissue), based upon the Gβ and Gγ subunits that are expressed and co-localize in any particular cell type have not been attempted. Studies reported herein, which identify Gβ and Gγ subunit expression and localization in neonatal and adult cardiomyocytes, provide the basis for such an analysis in future studies.
This study identified distinct expression patterns for the structurally related Gβ1, Gβ2, and Gβ3 proteins; technical constraints (the lack of a sufficiently sensitive/specific antibody) precluded studies of Gβ4; Gβ5 is an outlier by structural and functional criteria and was not considered. While Gβ1 and Gβ2 share considerable structural homology, and these Gβ subunits function interchangeably in the regulation of a range of effectors (including G protein-activated inwardly rectifying potassium channels and high-voltage-activated calcium channels), Gβ1 and Gβ2 display distinct expression and localization patterns in cardiomyocytes. Gβ1 is highly localized to caveolae and undergoes a pronounced developmental down-regulation. Of note, our previous studies showed that β2-ARs also localize to caveolae membranes. The co-localization of β2-ARs and Gβ1 subunits to caveolae may be significant, given recent evidence that β2-adrenergic receptor activation leads to the internalization of Gαs-Gβ1-Gγ7 complexes from the plasma membrane to the cytoplasm [16] and that Gβ1-containing dimers are more potent than Gβ2-containing dimers at activating the Gαs-adenylyl cyclase pathway [2]. These results suggest that caveolae may play a particularly important role as nucleation centers for a β2AR-Gβ1γ-AC pathway. Studies from the Robishaw laboratory suggest that Gγ7 is Gβ1’s preferred dimerization partner in the β2-AR signaling pathway [17;18]. On the basis of these studies, we would predict that Gγ7 also localizes (with β2-ARs and Gβ1 subunits) to caveolae membranes, but available anti-Gγ7 antibodies were not sufficiently sensitive/specific to recognize native Gγ7 proteins at physiologic levels of expression to test this hypothesis (data not shown).
Gβ2 shows a considerably more modest developmental downregulation and a different localization pattern; Gβ2 partitions to both to caveolae and non-caveolae membranes. The distinct subcellular localization patterns for Gβ2 and Gβ1 may be significant, given recent evidence that even the highly structurally homologous Gβ1 and Gβ2 subunits are not necessarily functionally redundant. For example, while Gβ1 and Gβ2 act interchangeably to regulate many effectors, only Gβ2 regulates low-voltage-activated Ca2+ currents carried by α1H Ca2+ channels (due to a cluster of surface-exposed residues that are unique to Gβ2 and form a surface contact point for α1H channels [19]).
Finally, we show that Gβ3 is detected only in the adult heart. The developmental upregulation of Gβ3 is unusual; most developmentally regulated signaling proteins (such as Gβ1 and Gβ2) are abundant in the neonatal (less differentiated) heart and undergo a developmental down-regulation. While Gβ3 expression has been detected in retinal cone cells, brain, heart, skeletal muscle, platelets, liver and lung, developmental regulation of Gβ3 expression has not previously been reported. Gβ3 also displays a unique subcellular distribution, relative to other Gβ subunits; Gβ3 is not exclusively a membrane protein, but is recovered in substantial amounts in the cytosolic fraction. A similar cytosolic localization pattern for Gβ3 has been reported previously, although a unique functional role for cytosolic Gβ3 (distinct from other Gβ subunits, not necessarily requiring dimerization with Gγ subunits) has not been considered. Rather, attempts to delineate Gβ3 function have used reconstitution approaches focusing on traditional GPCR signaling mechanisms. Most attempts to identify Gβ3 dimerization partners have been thwarted by the generally weak interaction between Gβ3 and most Gγ subunits [3–6]; Gβ3 does not mimic the actions of Gβ1 and Gβ2 to interact with Gγ2 or Gγ3 or to support signaling by Gαs [20]. However, effects of Gβ3 to reconstitute some traditional GPCR signaling functions have been identified. For example, Gβ3γ4 dimers mediate mAChR-dependent calcium channel inhibition, Gβ3γ5 dimers (but not Gβ1γ5 dimers) support α2A-AR-coupling to Gαi, and Gβ3 translocates from the cytosol to the membrane fraction in response to β AR activation in rat heart [21;22]. These results are most consistent with the notion that Gβ3 plays a unique role (distinct from Gβ1 or Gβ2) and that Gβ3 may preferentially participate in signaling pathway mediated by PTX-sensitive G proteins.
Recent studies identify a polymorphism in the human Gβ3 gene (the GNB3 825T allele) associated with a complex phenotype consisting of essential hypertension, obesity, diabetes and other metabolic disorders, and altered drug responses [7;23]. Gβ subunits are comprised of seven WD repeating motif proteins that define a seven blade propeller-like structure. The GNB3 825T allele encodes an alternatively spliced (41 amino acid shorter) variant of the β3 subunit; this in-frame deletion results in the expression of a protein that lacks the equivalent of one entire WD repeat domain (i.e., a Gβ3s subunit with only 6 propeller blades). The Siffert laboratory has published evidence that this truncated Gβ3 protein is functional, with evidence that Gβ3s dimerizes with certain Gγ subunits (Gγ5 and the related Gγ12, as well as Gγ8 in retinal cone cells) and it enhances signaling by PTX-sensitive heterotrimeric G proteins [24;25]. Based upon these findings, the enhanced chemotaxis and cardiac potassium channel regulation that has been identified in individuals carrying the 825-T allele has been attributed to dimers containing truncated Gβ3s subunits (that are believed to interact with enhanced efficacy with effectors, compared to dimers containing full-length Gβ3). However, other investigators have failed to identify Gβ3s dimerization with Gγ subunits (or Gβ3s modulation of channel function), and have argued that the C825T-allele produces a ‘functional Gβ3 knockout’ by expressing a ‘junk Gβ3s mRNA’ that is not translated into protein. While our studies do not address this controversy, the observation that Gβ3 is developmentally-induced in the ventricle (and therefore may subserve a unique function in the mature differentiated heart) provides an additional rationale to resolve the functional role of this somewhat eccentric signaling protein.
Acknowledgments
This work was supported by U.S.P.H.S.-N.H.L.B.I. grants HL-28958 and HL-74161.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Farfel Z, Bourne HR, Iiri T. The expanding spectrum of G protein diseases. N Engl J Med. 1999;340:1012–1020. doi: 10.1056/NEJM199904013401306. [DOI] [PubMed] [Google Scholar]
- 2.McIntire WE, MacCleery G, Garrison JC. The G protein beta subunit is a determinant in the coupling of Gs to the β1-adrenergic and A2a adenosine receptors. Journal of Biological Chemistry. 2001;276:15801–15809. doi: 10.1074/jbc.M011233200. [DOI] [PubMed] [Google Scholar]
- 3.Yan K, Kalyanaraman V, Gautam N. Differential ability to form the G protein βγ complex among members of the β and γ subunit families. Journal of Biological Chemistry. 1996;271:7141–7146. doi: 10.1074/jbc.271.12.7141. [DOI] [PubMed] [Google Scholar]
- 4.Pronin AN, Gautam N. Interaction between G-protein β and γ subunit types is selective. Proc Natl Acad Sci USA. 1992;89:6220–6224. doi: 10.1073/pnas.89.13.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray K, Kunsch C, Bonner LM, Robishaw JD. Isolation of cDNA clones encoding eight different human G protein γ subunits, including three novel forms designated the γ4, γ10, and γ11 subunits. Journal of Biological Chemistry. 1995;270:21765–21771. doi: 10.1074/jbc.270.37.21765. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt CJ, Thomas TC, Levine MA, Neer EJ. Specificity of G protein β and γ subunit interactions. Journal of Biological Chemistry. 1992;267:13807–13810. [PubMed] [Google Scholar]
- 7.Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, Erbel R, Sharma AM, Ritz E, Wichmann HE, Jakobs KH, Horsthemke B. Association of a human G-protein β3 subunit variant with hypertension. Nat Genet. 1998;18:45–48. doi: 10.1038/ng0198-45. [DOI] [PubMed] [Google Scholar]
- 8.Hansen CA, Schroering AG, Robishaw JD. Subunit expression of signal transducing G proteins in cardiac tissue: Implications for phospholipase C-β regulation. J Mol Cell Cardiol. 1995;27:471–484. doi: 10.1016/s0022-2828(08)80043-0. [DOI] [PubMed] [Google Scholar]
- 9.Rybin VO, Xu X, Lisanti MP, Steinberg SF. Differential targeting of β-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae: A mechanism to functionally regulate the cAMP signaling pathway. Journal of Biological Chemistry. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- 10.Kuznetsov V, Pak E, Robinson RB, Steinberg SF. β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circulation Research. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- 11.Zhao YY, Feron O, Dessy C, Han X, Marchionni MA, Kelly RA. Neuregulin signaling in the heart. Dynamic targeting of erbB4 to caveolar microdomains in cardiac myocytes. Circulation Research. 1999;84:1380–1387. doi: 10.1161/01.res.84.12.1380. [DOI] [PubMed] [Google Scholar]
- 12.Rybin VO, Xu X, Steinberg SF. Activated protein kinase C isoforms target to cardiomyocyte caveolae. Circulation Research. 1999;84:980–988. doi: 10.1161/01.res.84.9.980. [DOI] [PubMed] [Google Scholar]
- 13.Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. Journal of Biological Chemistry. 2001;276:42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- 14.Rybin VO, Pak E, Alcott S, Steinberg SF. Developmental changes in β2-adrenergic receptor signaling in ventricular myocytes: the role of Gi proteins and caveolae microdomains. Mol Pharmacol. 2003;63:1338–1348. doi: 10.1124/mol.63.6.1338. [DOI] [PubMed] [Google Scholar]
- 15.Iiri T, Homma Y, Ohoka Y, Robishaw JD, Katada T, Bourne HR. Potentiation of Gi-mediated phospholipase C activation by retinoic acid in HL-60 cells. Possible role of G gamma 2. Journal of Biological Chemistry. 1995;270:5901–5908. doi: 10.1074/jbc.270.11.5901. [DOI] [PubMed] [Google Scholar]
- 16.Hynes TR, Mervine SM, Yost EA, Sabo JL, Berlot CH. Live cell imaging of Gs and the beta2-adrenergic receptor demonstrates that both alphas and beta1gamma7 internalize upon stimulation and exhibit similar trafficking patterns that differ from that of the beta2-adrenergic receptor. Journal of Biological Chemistry. 2004;279:44101–44112. doi: 10.1074/jbc.M405151200. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Mullah B, Hanson C, Asundi J, Robishaw JD. Ribozyme-mediated suppression of the G protein γ7 subunit suggests a role in hormone regulation of adenylylcyclase activity. Journal of Biological Chemistry. 1997;272:26040–26048. doi: 10.1074/jbc.272.41.26040. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Mullah BK, Robishaw JD. Ribozyme approach identifies a functional association between the G protein β1γ7 subunits in the β-adrenergic receptor signaling pathway. Journal of Biological Chemistry. 1999;274:17365–17371. doi: 10.1074/jbc.274.24.17365. [DOI] [PubMed] [Google Scholar]
- 19.DePuy SD, Yao J, Hu C, McIntire W, Bidaud I, Lory P, Rastinejad F, Gonzalez C, Garrison JC, Barrett PQ. The molecular basis for T-type Ca2+ channel inhibition by G protein beta2gamma2 subunits. Proc Natl Acad Sci USA. 2006;103:14590–14595. doi: 10.1073/pnas.0603945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evanko DS, Thiyagarajan MM, Siderovski DP, Wedegaertner PB. Gβ γ Isoforms Selectively Rescue Plasma Membrane Localization and Palmitoylation of Mutant Gαs and Gαq. Journal of Biological Chemistry. 2001;276:23945–23953. doi: 10.1074/jbc.M101154200. [DOI] [PubMed] [Google Scholar]
- 21.Richardson M, Robishaw JD. The α2A-adrenergic receptor discriminates between Gi heterotrimers of different βγ subunit composition in Sf9 insect cell membranes. Journal of Biological Chemistry. 1999;274:13525–13533. doi: 10.1074/jbc.274.19.13525. [DOI] [PubMed] [Google Scholar]
- 22.Kageyama K, Murakami T, Iizuka K, Sato K, Ichihara K, Tokumitsu Y, Kitabatake A, Kawaguchi H. Translocation of G-protein beta3 subunit from the cytosol pool to the membrane pool by beta1-adrenergic receptor stimulation in perfused rat hearts. Biochemical Pharmacology. 1999;58:1497–1500. doi: 10.1016/s0006-2952(99)00230-0. [DOI] [PubMed] [Google Scholar]
- 23.Baumgart D, Naber C, Haude M, Oldenburg O, Erbel R, Heusch G, Siffert W. G protein β3 subunit 825T allele and enhanced coronary vasoconstriction on alpha(2)-adrenoceptor activation. Circulation Research. 1999;85:965–969. doi: 10.1161/01.res.85.10.965. [DOI] [PubMed] [Google Scholar]
- 24.Rosskopf D, Manthey I, Habich C, Kielbik M, Eisenhardt A, Nikula C, Urban M, Kohnen S, Graf E, Ravens U, Siffert W. Identification and characterization of Gβ3s2, a novel splice variant of the G-protein β3 subunit. Biochem J. 2003;371:223–232. doi: 10.1042/BJ20021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Virchow S, Ansorge N, Rosskopf D, Rubben H, Siffert W. The G protein beta3 subunit splice variant Gβ3-s causes enhanced chemotaxis of human neutrophils in response to interleukin-8. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:27–32. doi: 10.1007/s002109900040. [DOI] [PubMed] [Google Scholar]