Abstract
Women have greater temporal summation of experimental pain stimuli and also have a higher propensity for developing chronic visceral pain conditions. Sex hormone-mediated regulation of NMDA receptors (NMDARs) in nociceptive pathways is a plausible mechanism that may underlie these phenomena. The aim of this study was to compare the effect of 17-ß-estradiol (E2) in modulation of NMDAR activity in adult male and female rat dorsal root ganglia (DRG) neurons. DRG neurons were collected from adult male or female rats and grown in short-term culture in steroid-free media. NMDAR currents were recorded on small to medium size neurons by whole cell patch clamp using rapid perfusion with saturating concentrations of NMDA and glycine in the absence of extracellular Mg2+. We found that the average density of NMDAR currents was 2.8-fold larger in DRG neurons from female rats compared to male rats (p<0.0001). Addition of 100nM E2 increased NMDAR currents 55 ± 15% in female neurons, but only 19 ± 7% in male neurons. Potentiation was maximal after 20-40 mins and dose dependent with an apparent EC50 of 17-23nM. This effect was mimicked by E2 conjugated to BSA and attenuated by pretreatment with the protein tyrosine kinase inhibitor lavendustin A (1μM) or the estrogen receptor (ER) antagonist, ICI 182,780 (1μM), strongly suggesting activation of a cell surface ER acting through a non-genomic mechanism involving protein tyrosine kinases to increase NMDAR currents. These results identify sex-based differences in both the basal expression and the regulation of the NMDARs in DRG neurons.
Keywords: sex differences, estrogen, pain
Introduction
Women are more likely than men to develop certain chronic visceral and somatic pain syndromes, including irritable bowel syndrome (IBS), chronic pelvic pain and fibromyalgia (Chang and Heitkemper, 2002; Heitkemper et al., 2003; Aloisi, 2003). Women also show greater temporal summation of repeated somatic stimuli, suggesting sex based differences in spinal procesing of acute pain stimuli. Even though many factors are likely to contribute to the observed differences, sex hormones have been suggested as an important mediator of biologically based differences (Sarlani et al., 2004; Robinson et al., 2004). Sex-based differences in pain perception may result from genetic, sex chromosome-determined traits, from permanent, “organizing” effects of early gonadal secretions, or from transient, “activational” modulation of receptor function in the adult organism (Becker et al., 2005). Depending on the time course of the effect, the activational effects may result from genomic and non-genomic effects of Estrogen (E). For example, E (in particular the potent estrogen 17-ß-estradiol, E2) is a circulating steroid hormone that has marked biological effect on many cells and tissues, including neurons (Woolley, 1998; McEwen, 2002). Considerable evidence suggests that female sex hormones influence somatic and visceral sensory processing and the perception of pain, although with considerable variability depending on the species, tissue, and the type of test employed (Nemmani et al., 2004; LaCroix-Fralish et al., 2005; Ji et al., 2005). There are 2 types of estrogen receptor (ER), ERα and ERβ, and both types of ERs are distributed in regions of the central and peripheral nervous system which are involved in pain perception, including spinal dorsal horn neurons and DRG neurons (Woolley, 1998; Cui and Goldstein, 2000; McEwen, 2002; Papka and Storey-Workley, 2002; Bennett et al., 2003).
In the central nervous system, E has been shown to mediate changes in N-methyl-D-glutamate receptor (NMDAR) expression and activity (Woolley, 1998; Cyr et al., 2001; McEwen, 2002). NMDARs are glutamate and glycine-gated cation channels that play an essential role in neuroplasticity. NMDARs are expressed by nearly all dorsal root ganglia (DRG) neurons (Marvizon et al., 2002) and direct stimulation of peripheral afferent nerve terminal fields with NMDAR agonists causes pain responses in both rodents and humans (Zhou et al., 1996; Du et al., 2003; Cairns et al., 2003a; Cairns et al., 2006). Visceral nociceptive responses have greater sensitivity to peripherally administered NMDAR antagonists than those arising from somatic tissues implying a differentially greater role of these NMDARs in visceral pain transmission (Olivar and Laird, 1999; McRoberts et al., 2001). In the periphery, stimulation of NMDARs causes the release of the neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP) from capsaicin-sensitive peripheral nerve terminals (McRoberts et al., 2001). These neuropeptides contribute to neurogenic inflammation by causing edema and hyperemia. Stimulation of NMDARs on the central projections of these neurons in the spinal cord also mediates neuropeptide release (Liu et al., 1997; Marvizon et al., 1997; Malcangio et al., 1998). SP in particular is known to regulate spinal neuron excitability (Herrero et al., 2000), although recent work has indicated that CGRP (Mogil et al., 2005) and another protein released from peptidinergic neurons, brain-derived nerve growth factor (BDNF) (Zhao et al., 2006) also have important pro-nociceptive roles as well. Thus changes in the NMDAR expression in DRG neurons could have dramatic impact on pain transmission and inflammatory responses. In this study we compared the activity of NMDARs expressed on male and female DRG neurons in short term culture and examined the effect of acute estrogen receptor stimulation.
Materials and Methods
Isolation and primary culture of DRG neurons
All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Chancellor’s Animal Research Committee at UCLA. DRG neurons from adult male or female Sprague-Dawley rats were isolated as previously described (Li et al., 2004). Neurons were plated on poly-L-lysine coated 35 mm culture dishes and cultured at 37°C in 5% CO2 incubator in Dulbecco’s Modified Eagle’s Medium (DMEM cat # D1152; Sigma Chemical Co., St. Louis, MO, USA) supplemented with 10% fetal bovine serum, 2 mM glutamine-penicillin-streptomycin mixture, 1 μg/ml DNAase, 200μM ketamine (all from Sigma), and 5 ng/ml NGF (Invitrogen). The next day, media was replaced with that containing 10% steroid-free (charcoal-filtered) fetal bovine serum. This was done to minimize the effect of endogenous estrogens (and other sex hormones) in the serum. The growth media contained 0.0159 g/L phenol red, which has been reported to have estrogenic effects on cultured cells (Berthois et al., 1986), although this has been refuted (Moreno-Cuevas and Sirbasku, 2000). Neurons were studied 24-48 hours after the media change.
Electrophysiological recordings
Whole cell patch clamp recordings were performed on visualized small to medium sized DRG neurons (< 40 μm in diameter) exactly as described previously (Li et al., 2004) using nominally Mg2+-free extracellular buffer and a holding potential of -60 mV. Series resistance was monitored throughout the experiment. A neuron was considered to have functional NMDARs if the inward current evoked by 250 μM NMDA and 10 μM glycine was greater than 10 pA. Neurons were stimulated for 5 sec at 5 min intervals in order to generate time course experiments. Under these conditions there is very little if any rundown of channel activity (Li et al., 2004).
Drug delivery
NMDA and glycine were prepared as 100 mM stock solutions. Test solutions were prepared daily by diluting stock solutions to desired concentrations in extracellular solution. ICI 182,780, lavendustin, E2 were prepared as 1000-fold stock solutions in dimethyl sulfoxide (DMSO). DMSO was added such that all solutions contained equivalent amounts of vehicle. Test compounds were rapidly perfused onto the patched cells using a gravity-fed multibarrel perfusion system (SF-77B Perfusion Fast-Step, Warner Instrument Corp. Hamden, CT) controlled by a Labmaster board using the pClamp 8.0 software (Axon Instruments, Foster City, CA). The tip of multibarrel was positioned 200-300 μm from the neuron under study. When a test solution was not being applied, the neuron was continuously perfused with extracellular solution.
Materials
ICI 182,780 was purchased from Tocris (Ellisville, MO), NMDA and lavendustin from Calbiochem (La Jolla, CA), and regular and charcoal-filtered fetal bovine serum from Irvine Scientific (Irvine, CA). All other reagents including E2 (17β-Estradiol, cat E2758) and E2 conjugated to BSA (β-Estradiol 6-(O-carboxy-methyl)oxime: BSA, cat E5630) were purchased from Sigma (St. Louis, MO).
Statistical Analysis
Data were analyzed by one way analysis of variance or as unpaired t-tests or nonparametric statistical tests as appropriate. The kinetic and dose-response data were analyzed by nonlinear regression using GraphPad Prism software (version 4, GraphPad Software, Inc. San Diego, CA). The kinetic data were fit to an exponential association/activation equation: I=Ib + (Imax- Ib)(1-e -k*t), where t is time, k is the first order rate constant, Ib is the basal current and Imax is the maximal current. In some cases data were also fit to a mixed association-dissociation equation: I= Ib + (Imax- Ib)(e -ke*t/(ke/ka) - e -ke*t/(ke/ka-1)), where ka and ke are the first order rate constants of association/activation and dissociation/decay, respectively. F-test comparison between different fits were used to test which fit was best, and between two different sets of data to determine whether the curves were significantly different from each other. A P value of ≤ 0.05 was taken as statistically significant. All data are given as the mean ± SEM.
Results
Fast perfusion with 250 μM NMDA combined with 10 μM glycine induced inward currents in the majority of DRG neurons from both male and female rats (62 and 65%, respectively). As shown in the representative traces in Fig. 1A, peak and steady state current amplitudes were generally larger in neurons derived from female rats than those from male rats. Fig. 1B is scatter plot of the current density in 123 individual neurons calculated from the peak current divided by the whole cell capacitance. The average NMDAR current density in female derived neurons was 2.8-fold larger than those from male rats (-13.95 ± 1.36 pA/pS, n=49 vs. -4.93 ± 0.58 pA/pS, n=74, female vs. male respectively). Although there was a great deal of variation within each population of neurons, this difference was highly significant (P<0.0001 using either the unpaired t-test with Welch’s correction for unequal variances, or by the non-parametric Mann Whitney test). This was not due to a difference in the cell size since whole cell capacitance was not significantly different between the female and male neurons studied (46.4 ± 1.4, n=49 vs. 48.8 ± 1.7, n=74, respectively; P∼0.33). In addition, acute stimulation of the neurons with 100 nM E2 rapidly increased NMDAR currents. Maximal potentiation was greater in female neurons than that observed in male neurons with a 67 ± 21% increase at 55 min in female neurons compared to a 19 ± 5% at 20 min in male neurons (fig. 1C).
Figure 1.
Sex dependent differences in NMDAR currents in DRG neurons and effect of 17ß-estridiol (E2). Panel A shows representative tracings of NMDAR currents in male and female neurons. The solid bar indicates the time of rapid perfusion with 250 μM NMDA and 10 μM glycine. Panel B shows a scatter-gram of the data of the NMDAR current density data which was derived from the peak NMDA current in each cell divided by the whole cell capacitance. The heavy solid bar is the mean current density. Panel C shows a time course of NMDAR currents taken every 5 min before and after addition of 100 nM E2. Values were normalized to the basal current at t= 0 min. Mean currents at t=0 were -550 ± 155 pA (n=5) and -284 ± 73 pA (n=7) for female and male neurons, respectively. Nonlinear regression of the data to an exponential association equation (Methods) gave Imax values of 163 ± 10% and 119 ± 5% with first order rate constants of 0.062 min-1 and 0.131 min-1 for female and male neurons, respectively. F-test comparison indicated that the curves were significantly different from each other [P = 0.0007; F=7.773 (2,106)]. The response of male neurons fit significantly better [P<0.0001; F=38.84 (1,72)] to a mixed association-disassociation equation with an Imax value of 129 ± 31% and ka = 0.143 min-1 and ke = 0.062 min-1 indicating that inactivation of E2-enhancement of NMDAR currents was significant in male neurons.
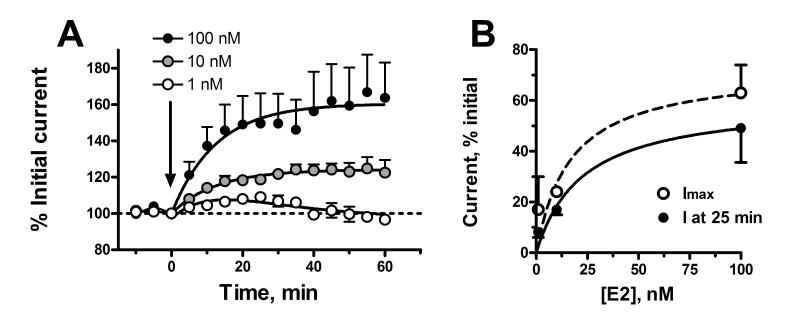
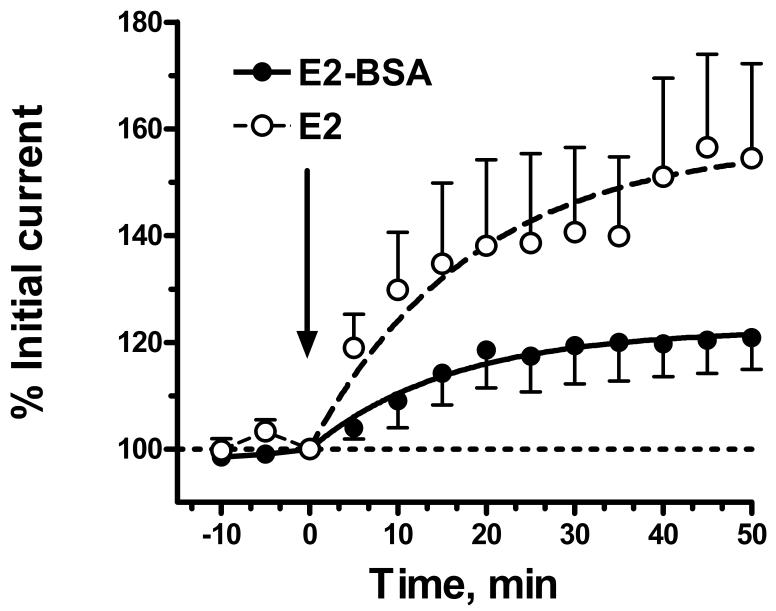
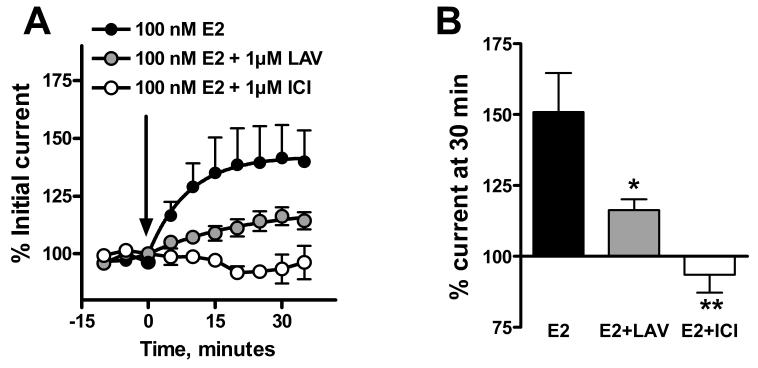
The rapid effect of E2 on NMDAR responses in female neurons was dose dependent with an EC50 of 17-23 nM (fig 2). Addition of E2 conjugated to BSA also increased NMDAR currents, although the effect was significantly smaller in magnitude than that produced by unconjugated E2 (fig. 3). Nonlinear regression analysis of the kinetic data gave an estimated maximal increase in current that was only 39% that of unconjugated E2, however the rate constants were similar (0.054 vs. 0.062 min-1 for E2-BSA compared to E2, respectively). The acute effect of E2 was inhibited by prior addition of 1 μM ICI 182,780, a highly selective estrogen receptor antagonist, and markedly attenuated by 1 μM lavendustin, a selective protein tyrosine kinase inhibitor (fig 4).
Figure 2.
Dose dependent effect of E2 on NMDAR currents in female DRG neurons. Panel A is a time course of the effect of 100, 10 and 1 nM E2 plotted against the normalized current at time 0. Mean currents at t=0 were -550 ± 155 pA, n=5; -512 ± 170 pA, n=5; and -540 ± 209 pA, n=6, respectively. Nonlinear regression of the data to an exponential association equation gave Imax values of 163 ± 10%, 124.1 ± 2% and 117 ± 13% with first order rate constants of 0.062 min-1, 0.080 min-1, and 0.032 min-1 for E2 additions of 100 nM, 10 nM and 1 nM, respectively. The response of neurons to 1 nM E2 fit significantly better [P<0.0001; F=41.3 (1,66)] to a mixed association-disassociation equation with an Imax value of 120 ± 4% and ka = 0.072 min-1 and ke = 0.052 min-1. Panel B shows two nonlinear regression analyses of the dose response data. Using the normalized current 25 min after addition of E2 (solid circles) gave an EC50 of 23 ± 18 nM and a maximal degree of current enhancement of 60 ± 14%. Using the Imax values derived from kinetic curve fitting of the data (open circles) gave an EC50 value of 17 ± 18 nM with a maximal degree of current enhancement of 73 ± 23%.
Figure 3.
Acute effect of BSA-conjugated E2 on NMDAR currents in female DRG neurons. The figure shows a time course of the effect of 100 nM E2 conjugated to BSA compared to that of 100nM E2 on NMDAR currents evoked at 5 min intervals. The mean current at t=0 for the neurons treated with 100 nM E2 conjugated to BSA (E2-BSA) was -473 ± 86 pA (n=6) which was not significantly different from neurons treated with E2 (-550 ± 155 pA; P∼0.7 by unpaired t-test). Nonlinear regression of the E2-BSA data to gave an Imax value of 122 ± 4% (compared 163 ± 10% for E2) with a first order rate constant of 0.054 min-1 (compared to 0.062 min-1 for neurons treated with and E2). F-test comparison indicated that the curves were significantly different [P <0.001; F= 16.24 (2,140)].
Figure 4.
The effect of E2 is estrogen receptor specific and dependent on protein tyrosine phosphorylation. Prior addition of 1μM ICI 182,780 (ICI), a highly specific estrogen receptor antagonist, prevented the effect of E2. Prior treatment with 1μM lavendustin (LAV), a general tyrosine kinase inhibitor, markedly attenuated the response. Right panel shows the time course. Left panel shows the effect at 30 min post E2 addition. Mean currents at t=0 were -452 ± 141 (n=5) and -405 ± 110 (n=5) for the neurons treated ICI 182,780 and lavendustin, respectively. Significantly different from E2-treated neurons: *, P<0.05; **, P<0.01.
Discussion
In this study, we describe two important findings pertaining to sex-based differences in NMDAR activity. The first finding is that small to medium size cultured DRG neurons from female rats have significantly larger NMDAR currents than those observed in neurons from male rats. The second finding is that addition of E2 caused a rapid increase in NMDAR currents that was more pronounced in female compared to male derived neurons.
While there was a great deal of variation within each population of neurons, the mean NMDAR current density in female DRG neurons was 2.8-fold larger that of male neurons. This result cannot be explained by a change in the affinity of the NMDAR for agonists since the concentration of glycine and NMDA used to induce currents would result in better than 85% saturation of the receptor (96% and 85%, respectively)(Li et al., 2004)). Instead, the result could be due to increased expression of NMDARs with a consequent increase in the degree of cell surface expression, or it could be due to greater activity of the individual NMDAR channels in female neurons. The current results do not distinguish between these latter two possibilities. This effect is most likely sex hormone dependent since other studies have shown that E2 supplementation of ovarectomized female rats increased expression of NMDARs in the hippocampus (Gazzaley et al., 1996; Woolley et al., 1997). In the current study both female and male neurons were cultured in steroid hormone-free media ruling out a short-term effect of sex hormones in mediating this effect. Since E2 and other sex hormones act transcriptionally and post-transcriptionally to regulate the expression of a number of genes, the persistence in short term culture of sex hormone mediated long-term changes in expression and activity is not surprising. However, we cannot at this time rule out a direct sex chromosome related effect, unrelated to E2, in mediating the higher level of NMDAR activity in female DRG neurons.
The rapid effect of E2 was dose dependent with an EC50 of 17-23 nM in female neurons. This concentration of E2 is higher than that found in plasma, however the effective concentration of hormone near target cells is not known and could far exceed plasma levels (Schleicher et al., 1998). Furthermore, this dose is in the concentration range generally found to be effective in vitro (Kurata et al., 2001) and is similar to the dose of E2 found to rapidly inhibit L-type calcium channels in DRG neurons (IC50 =27 nM) (Chaban and Micevych, 2005). In both the present study and the latter example, the rapid effect of E2 was mimicked by E2 conjugated to BSA and inhibited by the ER antagonist ICI 182,780, strongly suggesting functional expression of cell surface ER in DRG neurons. That the effect of E2 conjugated to BSA on NMDAR currents was significantly smaller than that of unconjugated E2 suggests that both cell surface and intracellular ER can mediate the rapid effect of E2 and that there may be a limited number of cell surface ER on DRG neurons. Alternatively, conjugated E2 may have a lower affinity for the cell surface ER receptor than E2 and thereby produce only partial stimulation at a concentration of 100 nM. Future studies will be needed to address this issue.
In this study we found that lavendustin could markedly attenuate the effect of E2 on NMDAR activity, strongly suggesting that one or more tyrosine kinases are necessary for ER-mediated increases in NMDAR channel activity. In our previous studies using male rats, we found that addition of sodium orthovanadate, a general tyrosine phosphatase inhibitor, caused a rapid increase in NMDAR activity in DRG neurons which involved a Src-like tyrosine kinase (Li et al., 2006). Given the similarity in the onset and magnitude of the effect of E2 and vanadate in increasing NMDAR activity, it seems likely that the same mechanisms underlie both processes. ER dependent activation of NMDARs in the CNS appears to involve a Src-like kinase and increased tyrosine phosphorylation of the NR2B subunit (Bi et al., 2000). We have shown that DRG neurons express the NR2B and NR2D subunits of the NMDAR along with the obligatory NR1 subunit (Marvizon et al., 2002), however, NMDARs expressed on the cell surface of cultured DRG neurons have functional properties that identify them as containing NR2B subunits (Li et al., 2004). Thus, direct Src kinase mediated phosphorylation of NR2B may be involved in ER-mediated increase in NMDAR activity. Additional experiments to examine this possibility are planned.
The current study used DRG neurons in short-term primary culture in order to measure NMDAR currents using whole cell patch clamping technique. Although there are clearly limitations to extrapolation of these results to the intact tissue, there has been repeated demonstration that regulation of ion channels in the peripheral and central terminals of these neurons are reflected in the cell body (Vyklicky and Knotkova-Urbancova, 1996). The current results demonstrating increased expression of NMDAR activity in female neurons may help to explain sex-based differences in glutamate-mediated nociceptive responses in humans. Both intramuscular and subcutaneous injection of glutamate produce acute and secondary pain responses in human volunteers and this effect is greater in women than men (Svensson et al., 2003; Cairns et al., 2003b). A previous study in men had shown that ketamine, an NMDAR antagonist, inhibited the nociceptive effect of injected glutamate (Cairns et al., 2003a). Since the peripheral terminals of primary afferent neurons express NMDARs (Carlton et al., 1995), the nociceptive effect of glutamate is most likely mediated by direct stimulation of NMDARs on these terminals.
A sex-dependent increase in NMDAR activity on the central terminals of primary afferent neurons in the spinal cord could have important effects in the development and maintenance of chronic pain by enhancing Ca2+ dependent release of neuropeptides such as SP, CGRP and BDNF from nociceptive terminals. These neuropeptides act on dorsal horn neurons to promote long-term central sensitization (Herrero et al., 2000). Thus, sex dependent elevation of NMDARs on the central terminals of sensory neurons could promote long-term sensitization which is believed to underlie the development and maintenance of chronic pain states which are more predominant in women than men. Clinically this may be reflected by greater degree of temporal summation (or “wind-up”) to repeated noxious somatic and visceral pain in women compared to men (Sarlani et al., 2004; Robinson et al., 2004). Although temporal summation as measured clinically is short lived, it is increased in female patients with chronic pain disorders such as irritable bowel syndrome (Berman et al., 2000) and fibromylgia (Staud et al., 2003). Thus, the mechanisms underlying temporal summation are likely to be involved in longer lasting forms of pain. The current results suggest that sex-based modulation of NMDAR activity on spinal afferent nerves may contribute to sex-based differences in peripheral and spinal nociceptive transmission.
Acknowledgements
We thank Dr. Paul Micevych for his helpful comments, Drs. Bruce Naliboff and Roger Bolus for help with the statistical analysis and Ms. Cathy Lui for help with illustrations.
Grant Support: NIH grants DK58173 and 1 P50 DK64539
ABBREVIATIONS
- E
estrogen
- E2
17-ß-estradiol
- ER
estrogen receptor
- NMDA
N-methyl-D-aspartic acid
- NMDAR
NMDA receptor
- DRG
dorsal root ganglia
- EC50
50% excitatory concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section Editor: Dr. Linda S. Sorkin
References
- Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 2003;19:168–174. doi: 10.1097/00002508-200305000-00004. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bennett HL, Gustafsson JA, Keast JR. Estrogen receptor expression in lumbosacral dorsal root ganglion cells innervating the female rat urinary bladder. Auton Neurosci. 2003;105:90–100. doi: 10.1016/S1566-0702(03)00044-4. [DOI] [PubMed] [Google Scholar]
- Berman S, Munakata J, Naliboff BD, Chang L, Mandelkern M, Silverman D, Kovalik E, Mayer EA. Gender differences in regional brain response to visceral pressure in IBS patients. Eur J Pain. 2000;4:157–172. doi: 10.1053/eujp.2000.0167. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol Red in Tissue Culture Media is a Weak Estrogen: Implications concerning the Study of Estrogen-Responsive Cells in Culture. PNAS. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi R, Broutman G, Foy MR, Thompson RF, Baudry M. The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus. Proc Natl Acad Sci U S A. 2000;97:3602–3607. doi: 10.1073/pnas.060034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006;169:467–472. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003a;90:2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Wang K, Hu JW, Sessle BJ, Arendt-Nielsen L, Svensson P. The effect of glutamate-evoked masseter muscle pain on the human jaw-stretch reflex differs in men and women. J Orofac Pain. 2003b;17:317–325. [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- Cui S, Goldstein RS. Expression of estrogen receptors in the dorsal root ganglia of the chick embryo. Brain Res. 2000;882:236–240. doi: 10.1016/s0006-8993(00)02848-1. [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T. Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Rev. 2001;37:153–161. doi: 10.1016/s0165-0173(01)00115-1. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitkemper M, Jarrett M, Bond EF, Chang L. Impact of sex and gender on irritable bowel syndrome. Biol Res Nurs. 2003;5:56–65. doi: 10.1177/1099800403005001006. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Ji Y, Tang B, Traub RJ. Modulatory effects of estrogen and progesterone on colorectal hyperalgesia in the rat. Pain. 2005;117:433–442. doi: 10.1016/j.pain.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Kurata K, Takebayashi M, Kagaya A, Morinobu S, Yamawaki S. Effect of beta-estradiol on voltage-gated Ca(2+) channels in rat hippocampal neurons: a comparison with dehydroepiandrosterone. Eur J Pharmacol. 2001;416:203–212. doi: 10.1016/s0014-2999(01)00880-9. [DOI] [PubMed] [Google Scholar]
- LaCroix-Fralish ML, Rutkowski MD, Weinstein JN, Mogil JS, Deleo JA. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine. 2005;30:1821–1827. doi: 10.1097/01.brs.0000174122.63291.38. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N-methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2006;291:G219–G228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. British Journal of Pharmacology. 1998;125:1625–1626. doi: 10.1038/sj.bjp.0702260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, Hordo C, Messlinger K, Nemmani KV, Rankin AL, Ritchie J, Siegling A, Smith SB, Sotocinal S, Vater A, Lehto SG, Klussmann S, Quirion R, Michaelis M, Devor M, Reeh PW. Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene. Proc Natl Acad Sci U S A. 2005;102:12938–12943. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Cuevas JE, Sirbasku DA. Estrogen mitogenic action. III. is phenol red a “red herring”? In Vitro Cell Dev Biol Anim. 2000;36:447–464. doi: 10.1290/1071-2690(2000)036<0447:EMAIIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific N-methyl-D-aspartate receptor antagonists: dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Olivar T, Laird JM. Differential effects of N-methyl-D-aspartate receptor blockade on nociceptive somatic and visceral reflexes. Pain. 1999;79:67–73. doi: 10.1016/S0304-3959(98)00152-3. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319:71–74. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain. 2004;5:77–82. doi: 10.1016/j.jpain.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Sarlani E, Grace EG, Reynolds MA, Greenspan JD. Sex differences in temporal summation of pain and aftersensations following repetitive noxious mechanical stimulation. Pain. 2004;109:115–123. doi: 10.1016/j.pain.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Schleicher F, Tauber U, Louton T, Schunack W. Tissue distribution of sex steroids: concentration of 17beta-oestradiol and cyproterone acetate in selected organs of female Wistar rats. Pharmacol Toxicol. 1998;82:34–39. doi: 10.1111/j.1600-0773.1998.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Staud R, Cannon RC, Mauderli AP, Robinson ME, Price DD, Vierck CJ., Jr. Temporal summation of pain from mechanical stimulation of muscle tissue in normal controls and subjects with fibromyalgia syndrome. Pain. 2003 Mar;102(12):87–95. doi: 10.1016/s0304-3959(02)00344-5. 102:87-95.2003. [DOI] [PubMed] [Google Scholar]
- Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ. Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain. 2003;101:221–227. doi: 10.1016/S0304-3959(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Vyklicky L, Knotkova-Urbancova H. Can sensory neurones in culture serve as a model of nociception? Physiol Res. 1996;45:1–9. [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006;31:539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7:895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]