Abstract
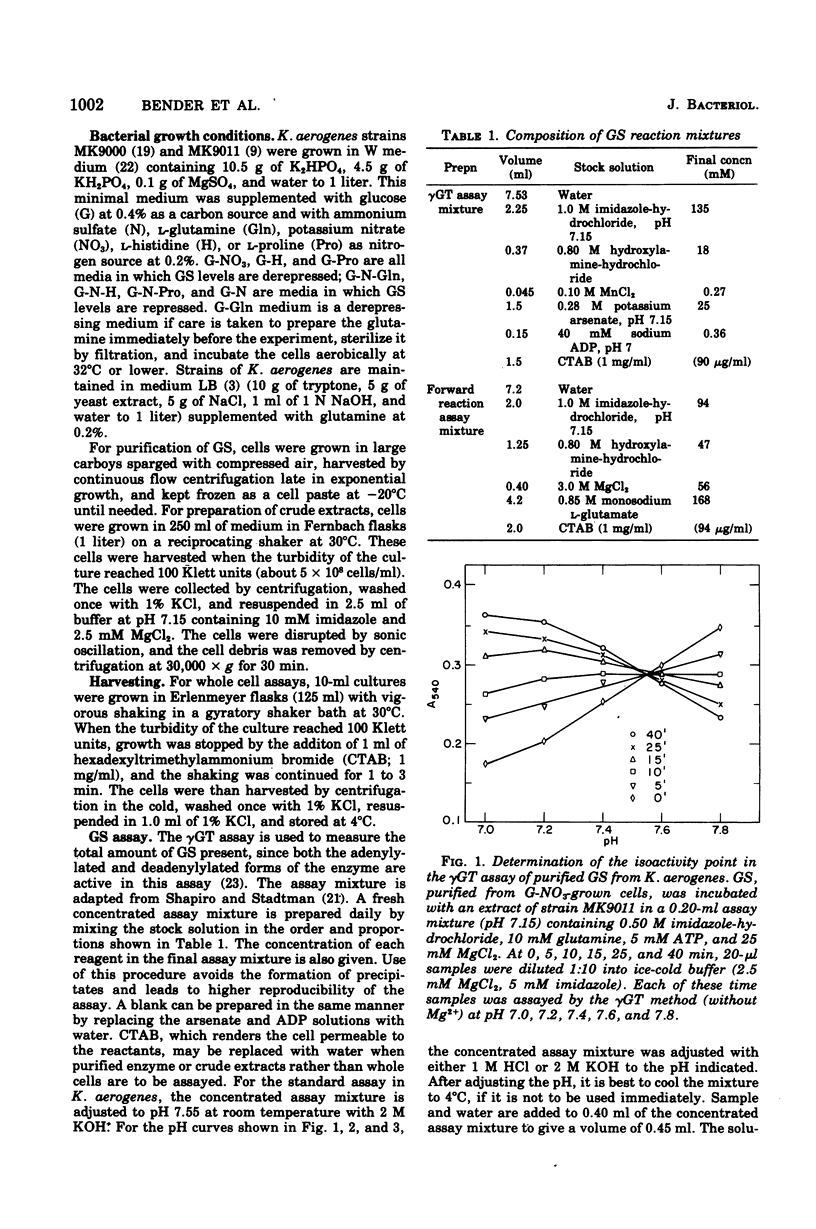
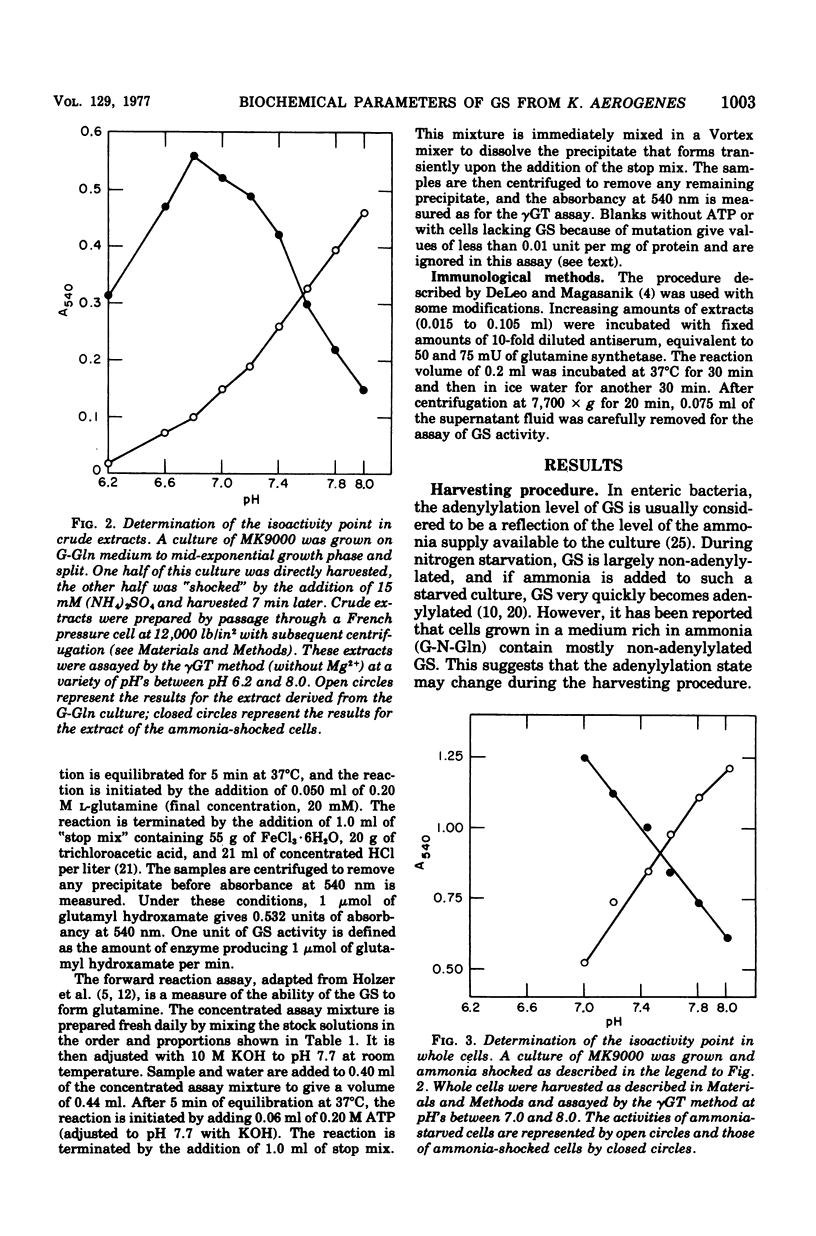
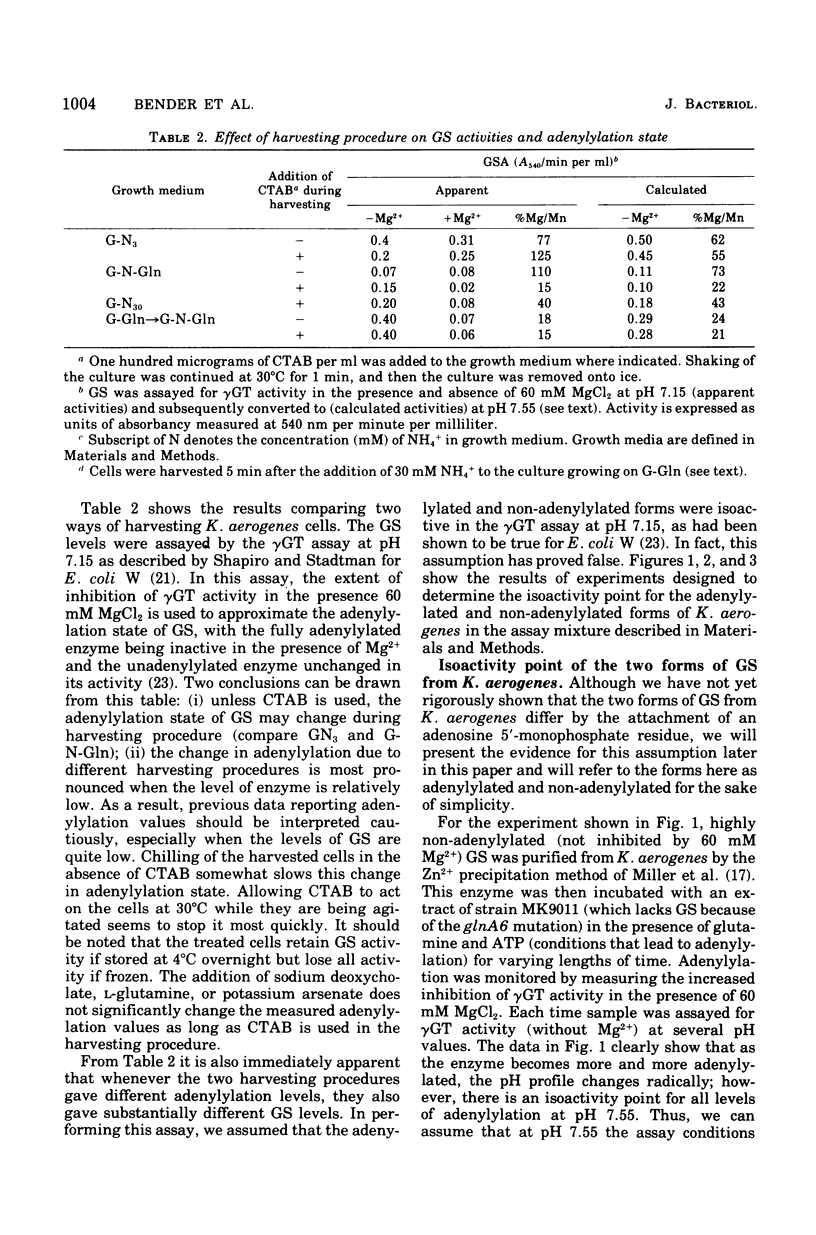
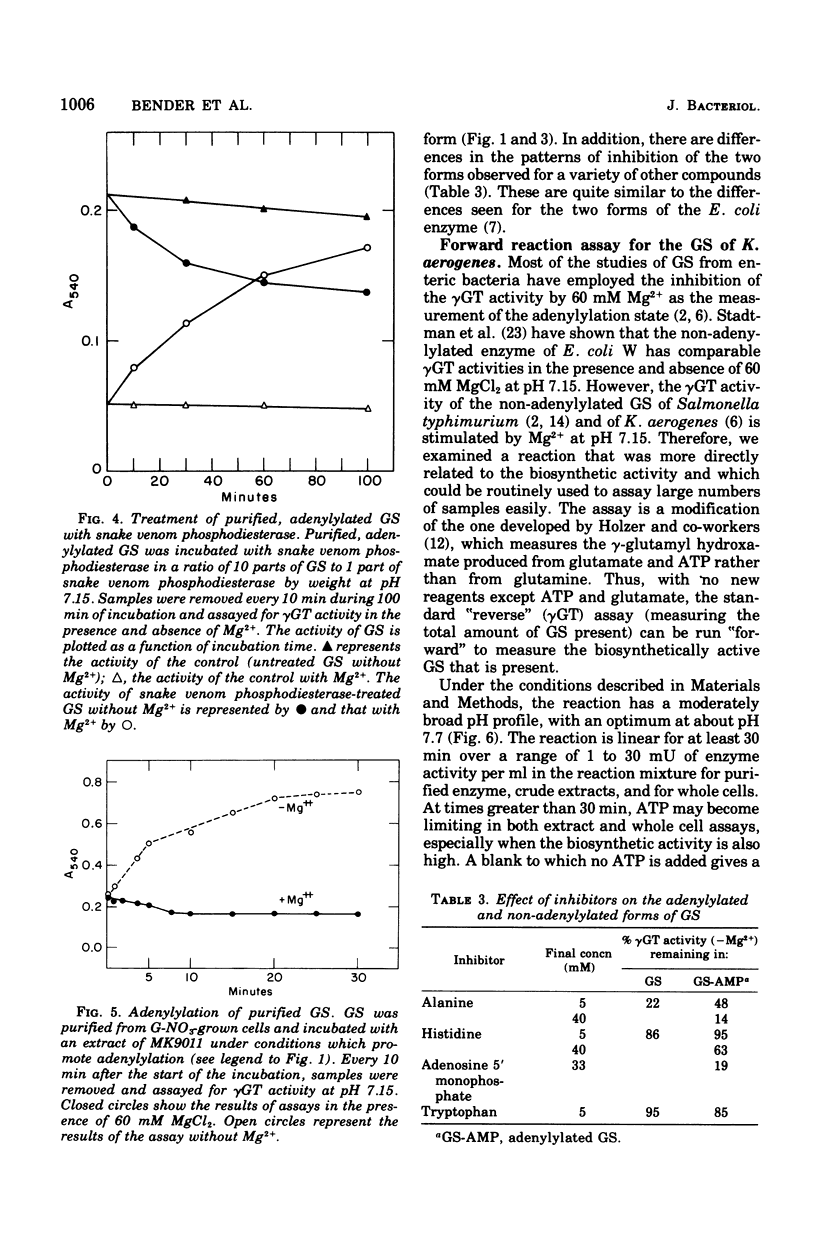
The glutamine synthetase (GS) from Klebsiella aerogenes is similar to that from Escherichia coli in several respects: (i) it is repressed by high levels of ammonia in the growth medium; (ii) its biosynthetic activity is greatly reduced by adenylylation; and (iii) adenylylation lowers the pH optimum and alters the response of the enzymes to various inhibitors in the gamma-glutamyl transferase (gammaGT) assay. There are, however, several important differences: (i) the isoactivity point for the adenylylated and non-adenylylated forms in the gammaGT assay occurs at pH 7.55 in K. aerogenes and at pH 7.15 in E. coli; (ii) the non-adenylylated form of the GS from K. aerogenes is stimulated by 60 mM MgCl2 in the gammaGT assay at pH 7.15. A biosynthetic reaction assay that correlates well with number of non-adenylylated enzyme subunits, as determined by the method of Mg2+ inhibition of the gammaGT assay, is described. Finally, we have found that it is necessary to use special methods to harvest growing cells to prevent changes in the adenylylation state of GS from occurring during harvesting.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Purich D., Stadtman E. R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975 Aug 25;250(16):6264–6272. [PubMed] [Google Scholar]
- Brenchley J. E., Baker C. A., Patil L. G. Regulation of the ammonia assimilatory enzymes in Salmonella typhimurium. J Bacteriol. 1975 Oct;124(1):182–189. doi: 10.1128/jb.124.1.182-189.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley J. E., Prival M. J., Magasanik B. Regulation of the synthesis of enzymes responsible for glutamate formation in Klebsiella aerogenes. J Biol Chem. 1973 Sep 10;248(17):6122–6128. [PubMed] [Google Scholar]
- Deleo A. B., Magasanik B. Identification of the structural gene for glutamine synthetase in Klebsiella aerogenes. J Bacteriol. 1975 Jan;121(1):313–319. doi: 10.1128/jb.121.1.313-319.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foor F., Janssen K. A., Magasanik B. Regulation of synthesis of glutamine synthetase by adenylylated glutamine synthetase. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4844–4848. doi: 10.1073/pnas.72.12.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg A. Conformational changes in glutamine synthetase from Escherichia coli. II. Some characteristics of the equilibrium binding of feedback inhibitors to the enzyme. Biochemistry. 1969 Apr;8(4):1726–1740. doi: 10.1021/bi00832a056. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Bender R. A., Streicher S. L. Direct selection for P1-sensitive mutants of enteric bacteria. J Bacteriol. 1974 Jun;118(3):810–814. doi: 10.1128/jb.118.3.810-814.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen K. A., Magasanik B. Glutamine synthetase of Klebsiella aerogenes: genetic and physiological properties of mutants in the adenylylation system. J Bacteriol. 1977 Feb;129(2):993–1000. doi: 10.1128/jb.129.2.993-1000.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLHAW G., DRAEGERT W., HOLZER H. PARALLEL-REPRESSION DER SYNTHESE VON GLUTAMIN-SYNTHETASE UND DPN-ABHAENGIGER GLUTAMAT-DEHYDROGENASE IN HEFE. Biochem Z. 1965 Feb 8;341:224–238. [PubMed] [Google Scholar]
- Kingdon H. S., Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. 8. ATP: glutamine synthetase adenylyltransferase, an enzyme that catalyzes alterations in the regulatory properties of glutamine synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1703–1710. doi: 10.1073/pnas.58.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach J. S., Berberich M. A., Venetianer P., Goldberger R. F. Repression of the histidine operon: effect of the first enzyme on the kinetics of repression. J Bacteriol. 1969 Mar;97(3):1283–1290. doi: 10.1128/jb.97.3.1283-1290.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustu S. G., McKereghan K. Mutations affecting glutamine synthetase activity in Salmonella typhimurium. J Bacteriol. 1975 Jun;122(3):1006–1016. doi: 10.1128/jb.122.3.1006-1016.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magasanik B., Prival M. J., Brenchley J. E., Tyler B. M., DeLeo A. B., Streicher S. L., Bender R. A., Paris C. G. Glutamine synthetase as a regulator of enzyme synthesis. Curr Top Cell Regul. 1974;8(0):119–138. doi: 10.1016/b978-0-12-152808-9.50010-9. [DOI] [PubMed] [Google Scholar]
- Mangum J. H., Magni G., Stadtman E. R. Regulation of glutamine synthetase adenylylation and deadenylylation by the enzymatic uridylylation and deuridylylation of the PII regulatory protein. Arch Biochem Biophys. 1973 Oct;158(2):514–525. doi: 10.1016/0003-9861(73)90543-2. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Umbarger H. E. Isoleucine and valine metabolism in Escherichia coli. XXII. A pleiotropic mutation affecting induction of isomeroreductase activity. J Bacteriol. 1973 Apr;114(1):195–207. doi: 10.1128/jb.114.1.195-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prival M. J., Magasanik B. Resistance to catabolite repression of histidase and proline oxidase during nitrogen-limited growth of Klebsiella aerogenes. J Biol Chem. 1971 Oct 25;246(20):6288–6296. [PubMed] [Google Scholar]
- Schutt H., Holzer H. Biological function of the ammonia-induced inactivation of glutamine synthetase in Escherichia coli. Eur J Biochem. 1972 Mar 15;26(1):68–72. doi: 10.1111/j.1432-1033.1972.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Smith G. R., Halpern Y. S., Magasanik B. Genetic and metabolic control of enzymes responsible for histidine degradation in Salmonella typhimurium. 4-imidazolone-5-propionate amidohydrolase and N-formimino-L-glutamate formiminohydrolase. J Biol Chem. 1971 May 25;246(10):3320–3329. [PubMed] [Google Scholar]
- Stadtman E. R., Ginsburg A., Ciardi J. E., Yeh J., Hennig S. B., Shapiro B. M. Multiple molecular forms of glutamine synthetase produced by enzyme catalyzed adenylation and deadenylylation reactions. Adv Enzyme Regul. 1970;8:99–118. doi: 10.1016/0065-2571(70)90011-7. [DOI] [PubMed] [Google Scholar]
- Tyler B., Deleo A. B., Magasanik B. Activation of transcription of hut DNA by glutamine synthetase. Proc Natl Acad Sci U S A. 1974 Jan;71(1):225–229. doi: 10.1073/pnas.71.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]



