Abstract
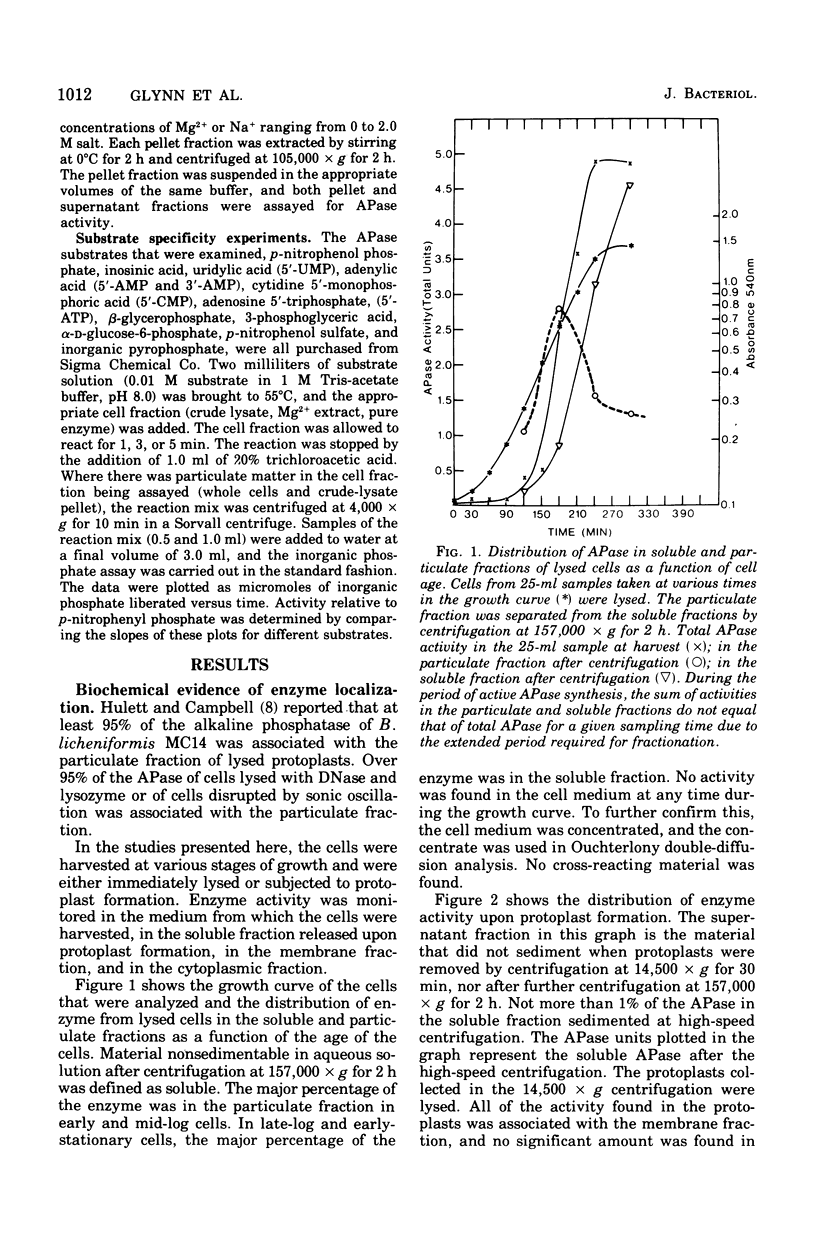
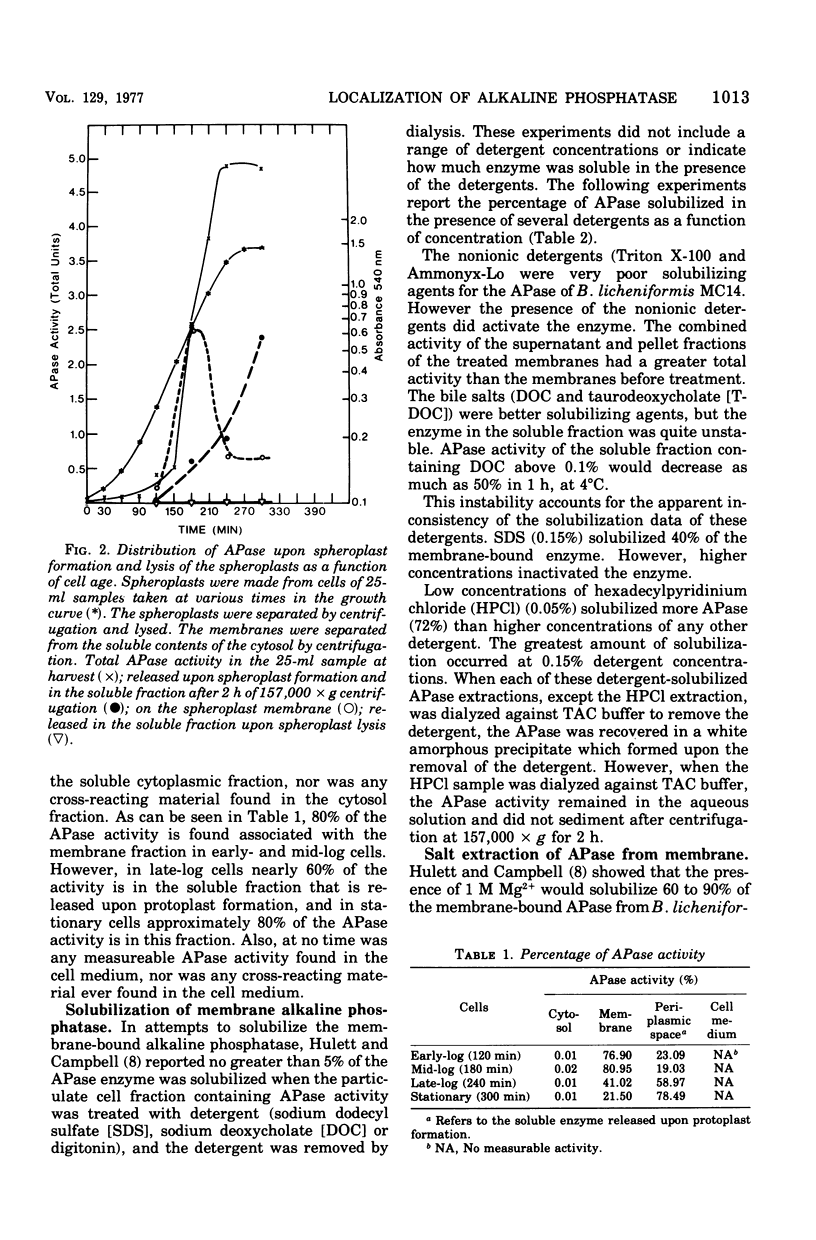
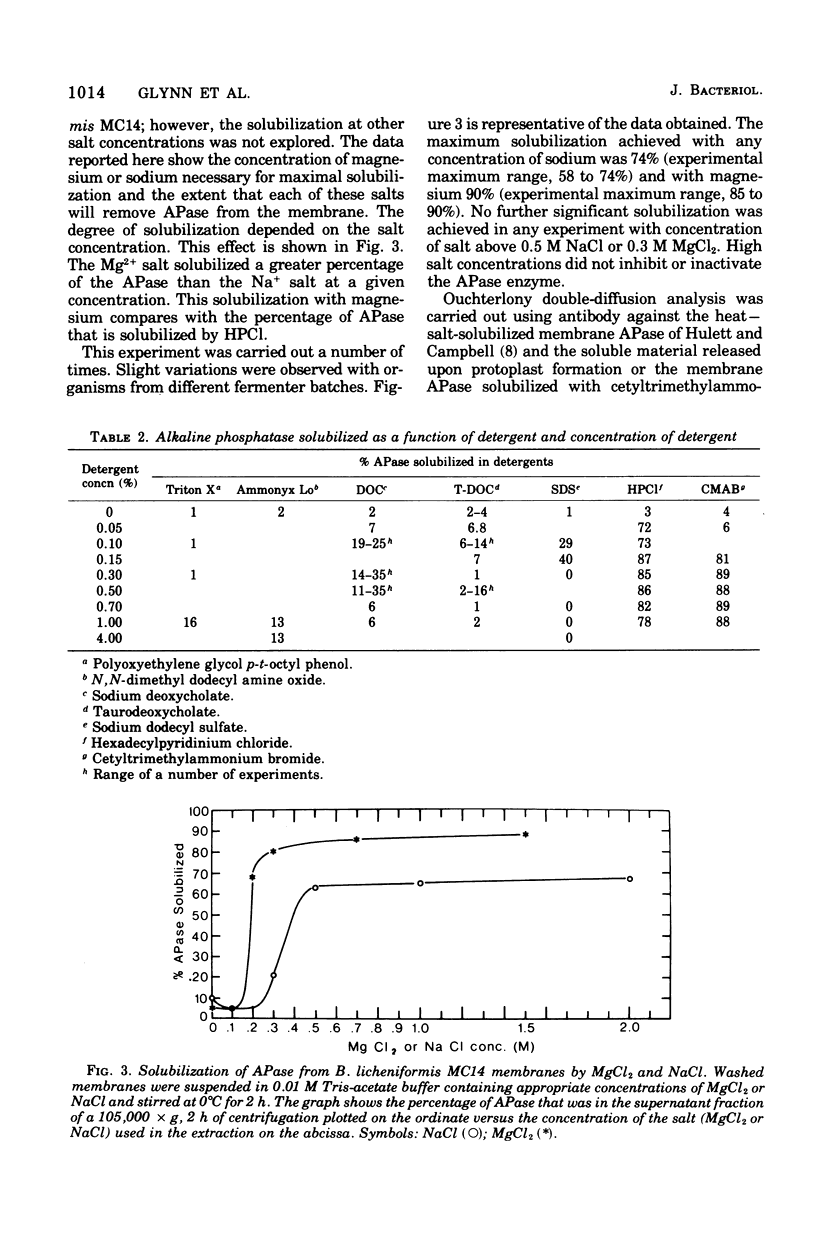
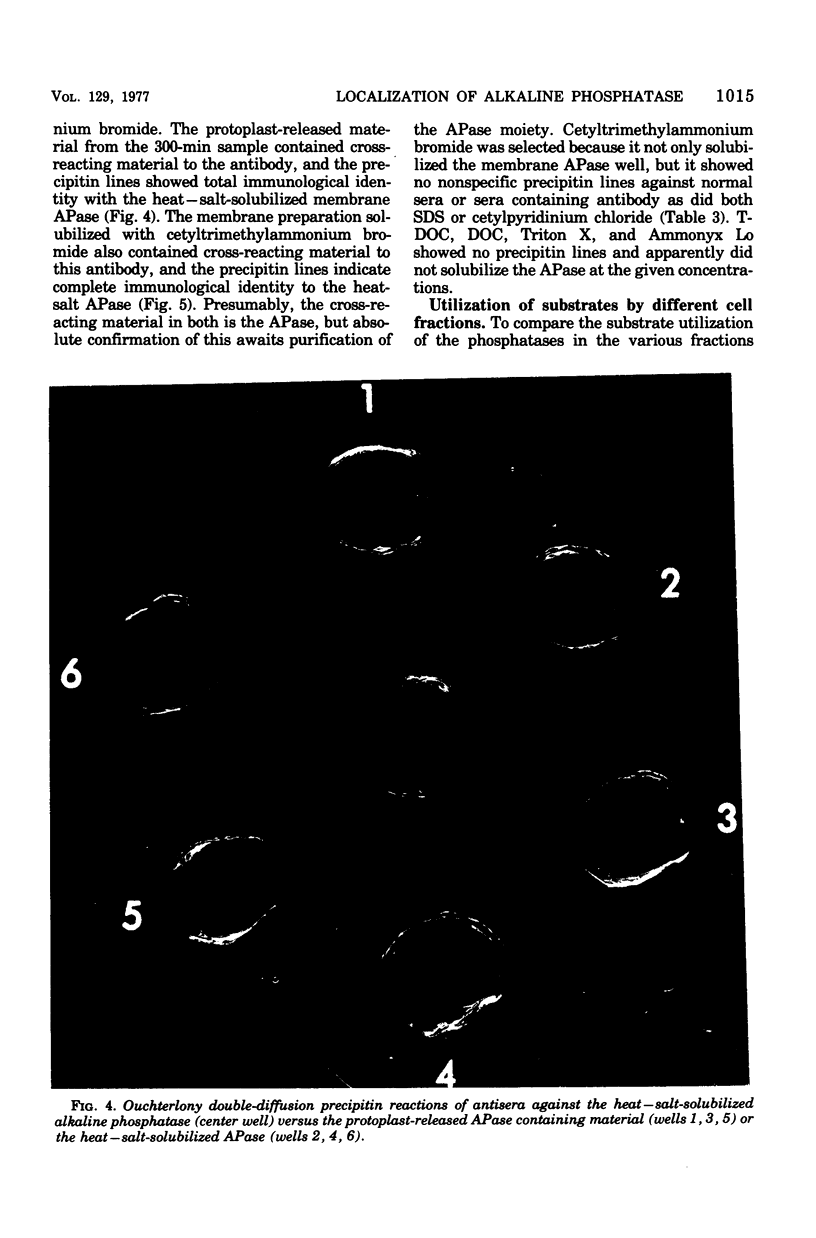
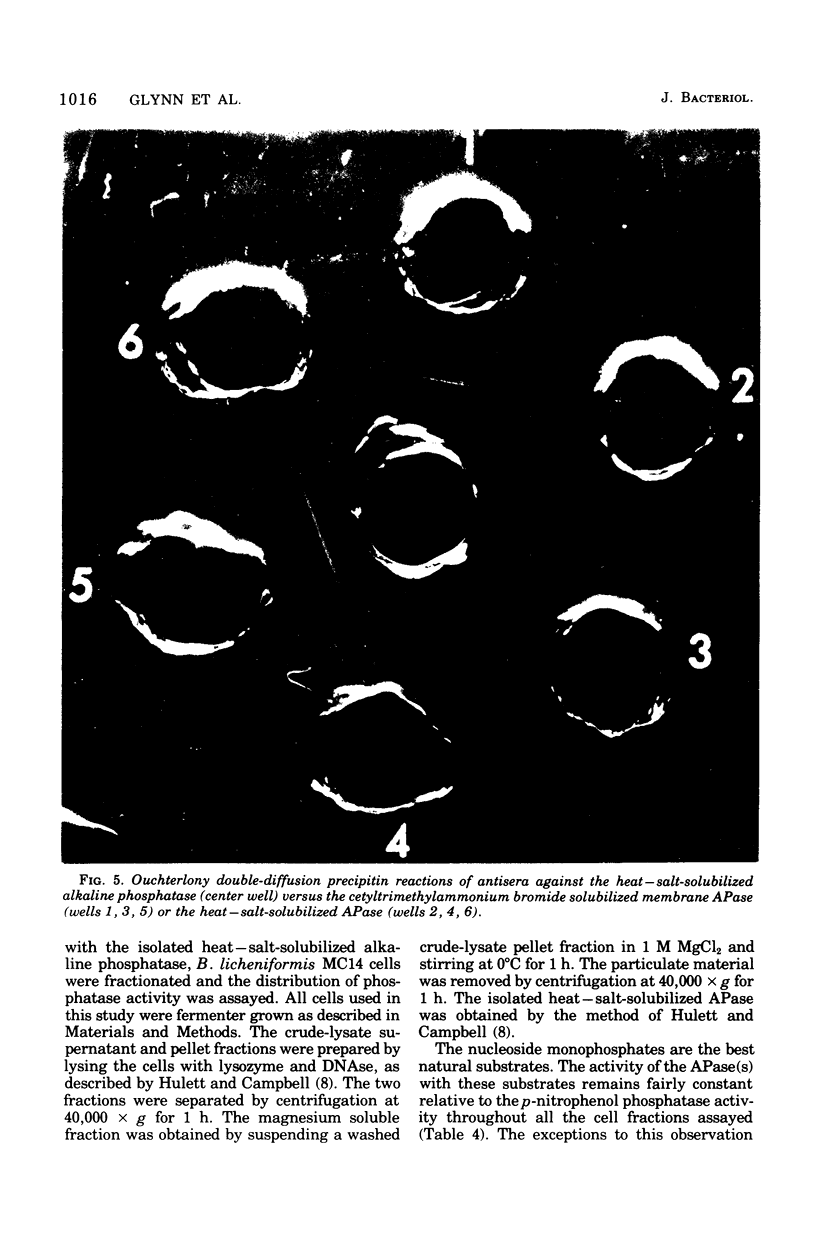
Biochemical localization of the enzyme as a function of age of cell culture showed the alkaline phosphatase (orthophosphoric monoester phosphohydrolase, EC 3.1.3.1) activity of Bacillus licheniformis MC14 predominantly in the particulate cell fraction in early- and mid-log cells. However, in late-log and stationary cells, increasing amounts of activity were found in the soluble fraction of lysed cells. Upon protoplast formation of these cells, the activity was released into the soluble fraction. No alkaline phosphatase activity was found in either the cytoplasmic fraction or in the cell medium during any phase of cell growth. The soluble fraction released on protoplast formation that contained alkaline phosphatase activity showed immunological cross-reactivity with antibody to the purified heat--salt-solubilized membrane alkaline phosphatase (F. M. Hulett-Cowling and L. L. Campbell, 1971). Theparticulate membrane fraction containing a firmly associated alkaline phosphatase also showed similar cross-reactivity. Further, the effectiveness of nonionic detergents, ionic detergents, bile salts, and various concentrations of magnesium and sodium as solubilizing agents for this membrane-bound alkaline phosphatase was investigated. Hexadecyl pyridinium chloride (0.03 M) and magnesium and sodium salts (above 0.2 M) were effective solubilizing agents. The substrate specificities of the various fractions were determined and compared to the substrate specificities of the purified membrane alkaline phosphatase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capaldi R. A., Green D. E. Membrane proteins and membrane structure. FEBS Lett. 1972 Sep 15;25(2):205–209. doi: 10.1016/0014-5793(72)80486-1. [DOI] [PubMed] [Google Scholar]
- Cashel M., Freese E. Excretion of alkaline phosphatase of Bacillus subtilis. Biochem Biophys Res Commun. 1964 Aug 11;16(6):541–544. doi: 10.1016/0006-291x(64)90189-5. [DOI] [PubMed] [Google Scholar]
- Chesbro W. R., Lampen J. O. Characteristics of secretion of penicillinase, alkaline phosphatase, and nuclease by Bacillus species. J Bacteriol. 1968 Aug;96(2):428–437. doi: 10.1128/jb.96.2.428-437.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. I. Isolation and characterization. J Biol Chem. 1971 Mar 25;246(6):1556–1565. [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Hulett-Cowling F. M., Campbell L. L. Molecular weight and subunits of the alkaline phosphatase of Bacillus licheniformis. Biochemistry. 1971 Apr 13;10(8):1371–1376. doi: 10.1021/bi00784a015. [DOI] [PubMed] [Google Scholar]
- Hulett-Cowling F. M., Campbell L. L. Purification and properties of an alkaline phosphatase of Bacillus licheniformis. Biochemistry. 1971 Apr 13;10(8):1364–1371. doi: 10.1021/bi00784a014. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- Schlesinger M. J. Secretion of alkaline phosphatase subunits by spheroplasts of Escherichia coli. J Bacteriol. 1968 Sep;96(3):727–733. doi: 10.1128/jb.96.3.727-733.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Singer S. J. The molecular organization of membranes. Annu Rev Biochem. 1974;43(0):805–833. doi: 10.1146/annurev.bi.43.070174.004105. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tsugita A. Phosphoesterases of Bacillus subtilis. II. Crystallization and properties of alkaline phosphatase. J Biochem. 1967 Feb;61(2):231–241. doi: 10.1093/oxfordjournals.jbchem.a128535. [DOI] [PubMed] [Google Scholar]
- Wood D. A., Tristram H. Localization in the Cell and Extraction of Alkaline Phosphatase from Bacillus subtilis. J Bacteriol. 1970 Dec;104(3):1045–1051. doi: 10.1128/jb.104.3.1045-1051.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]