Abstract
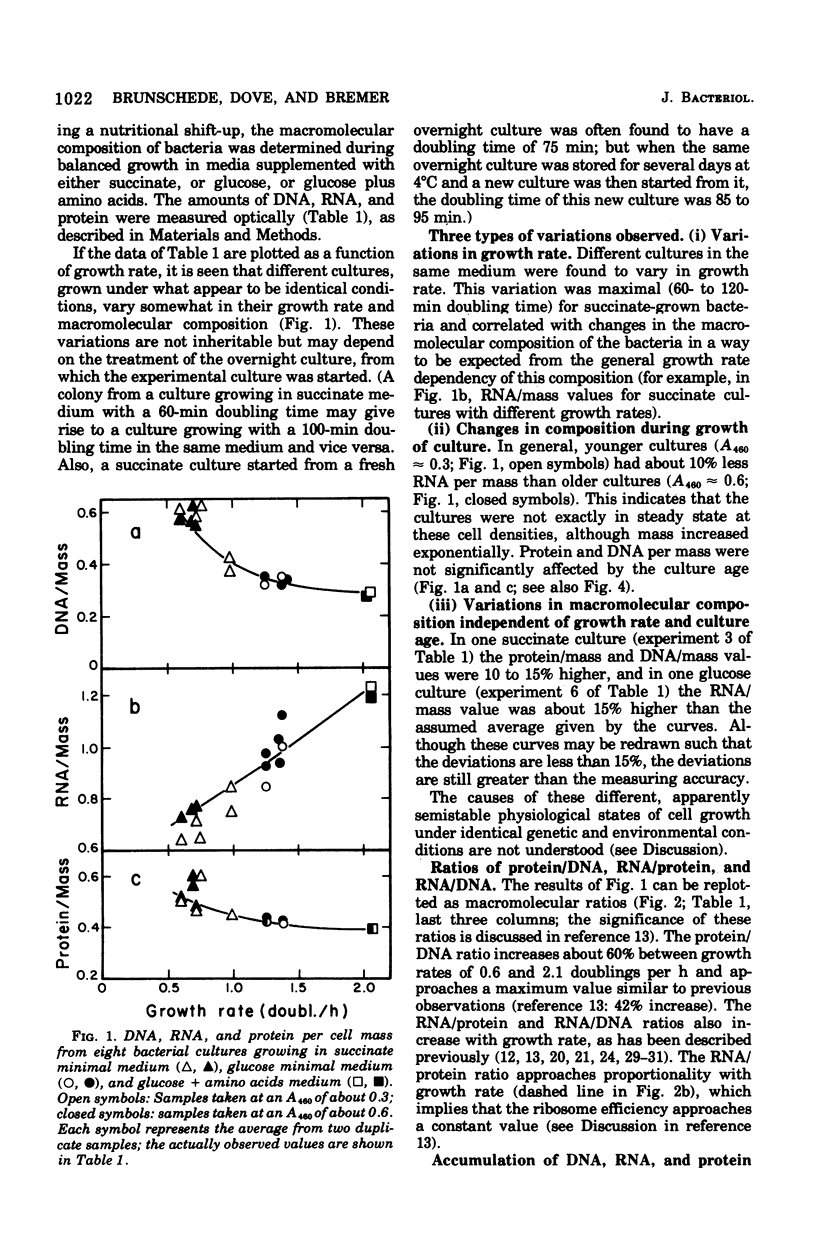
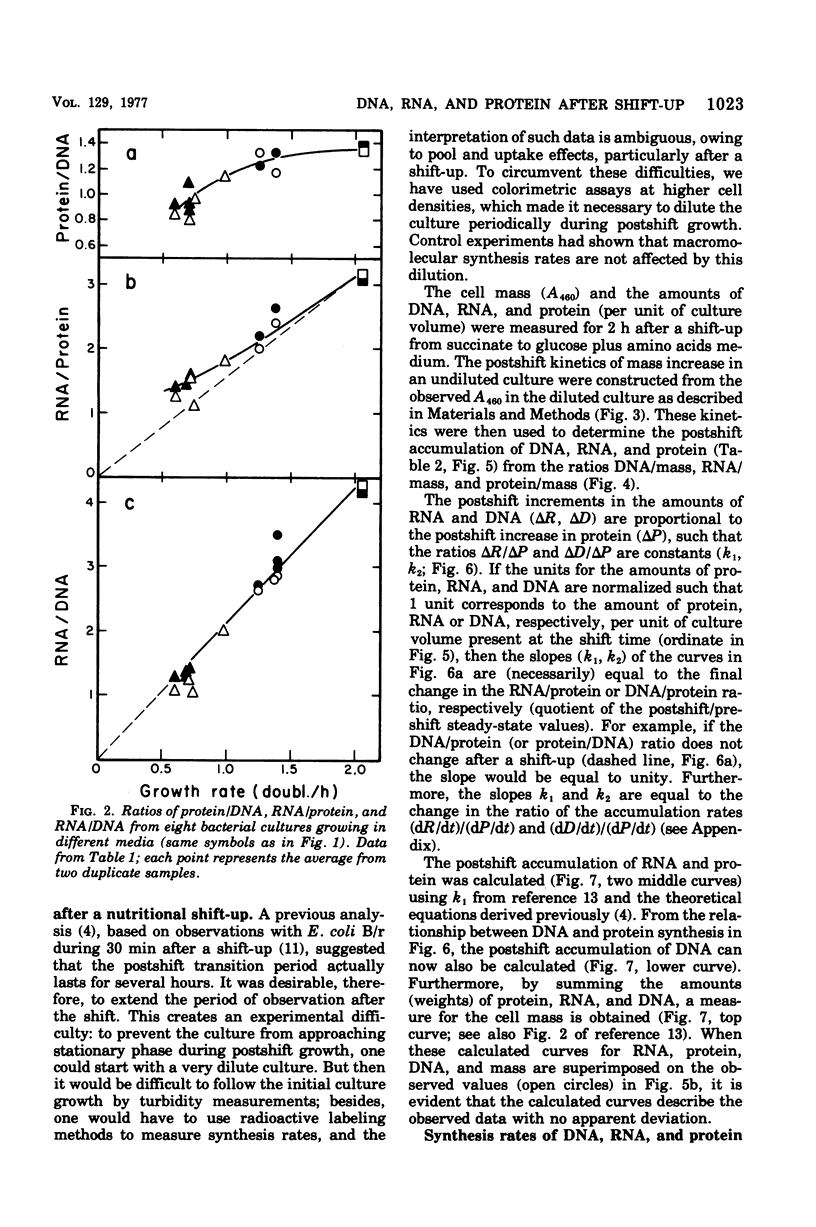
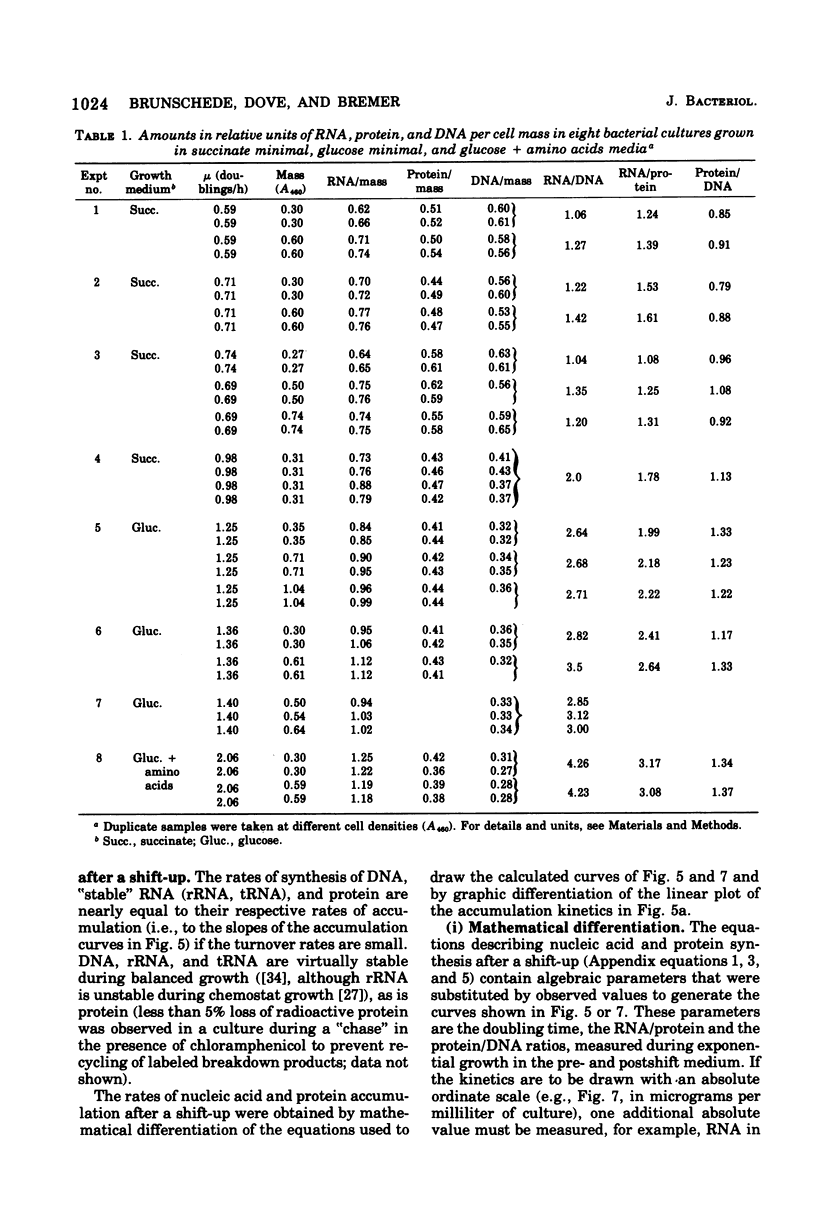
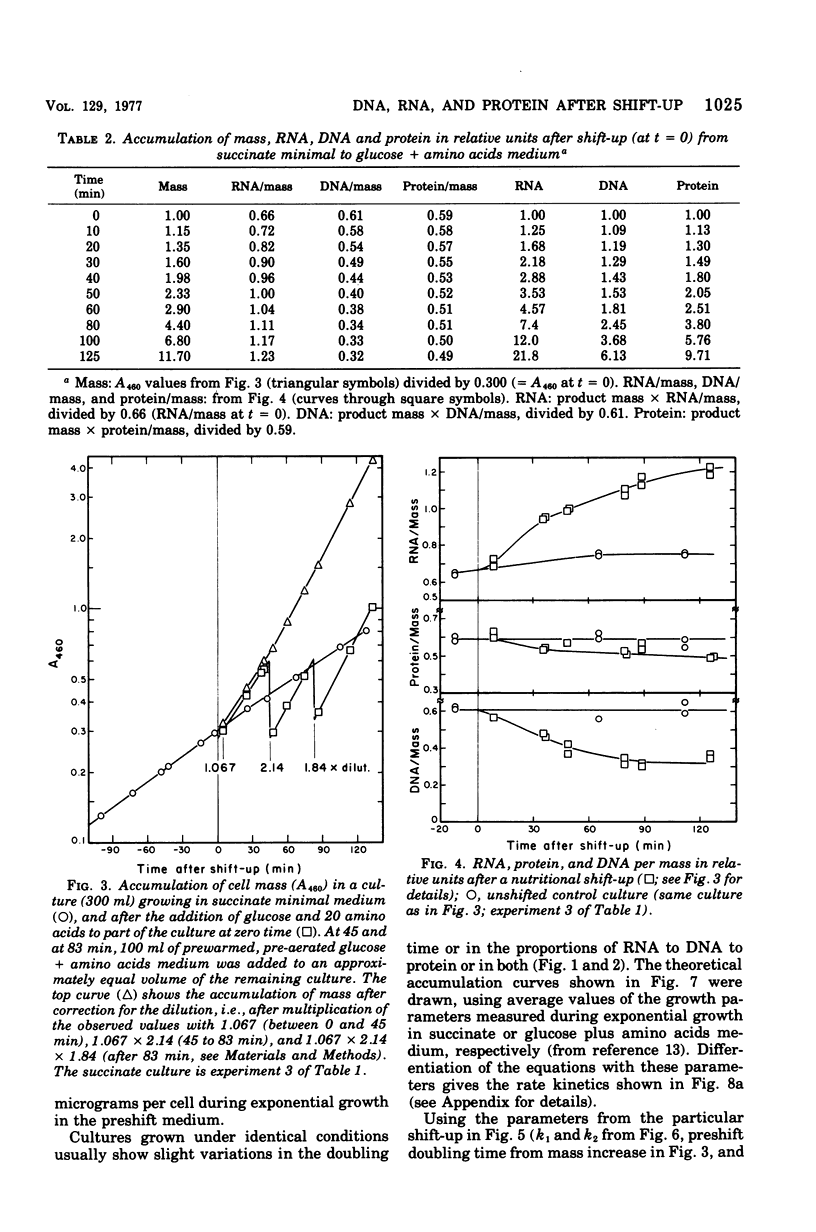
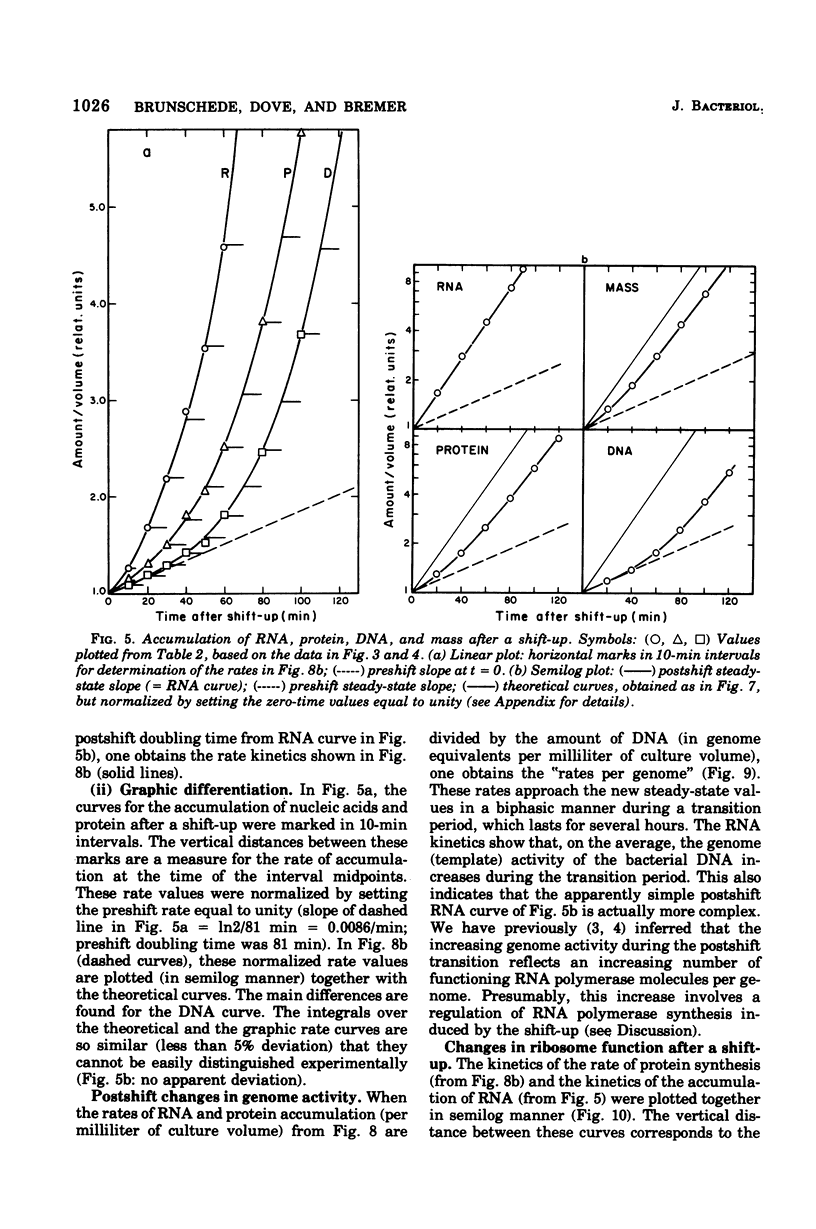
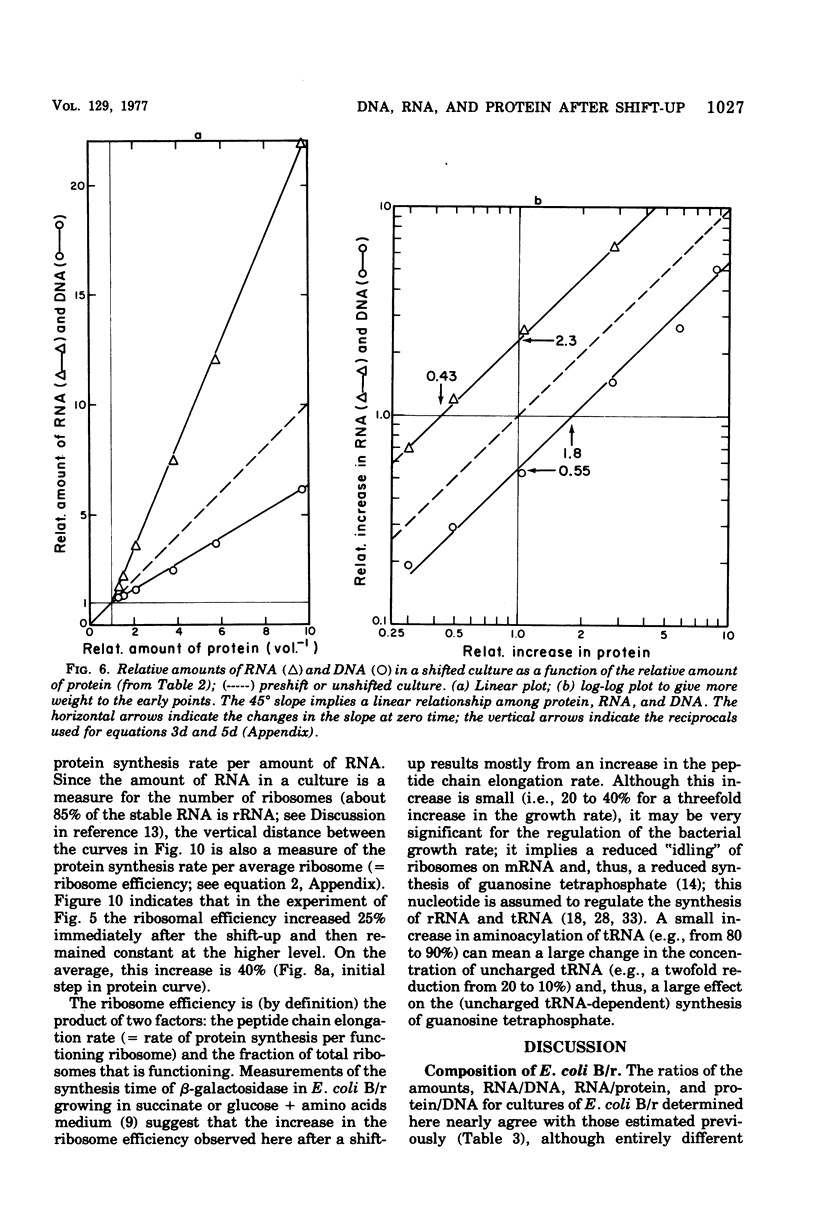
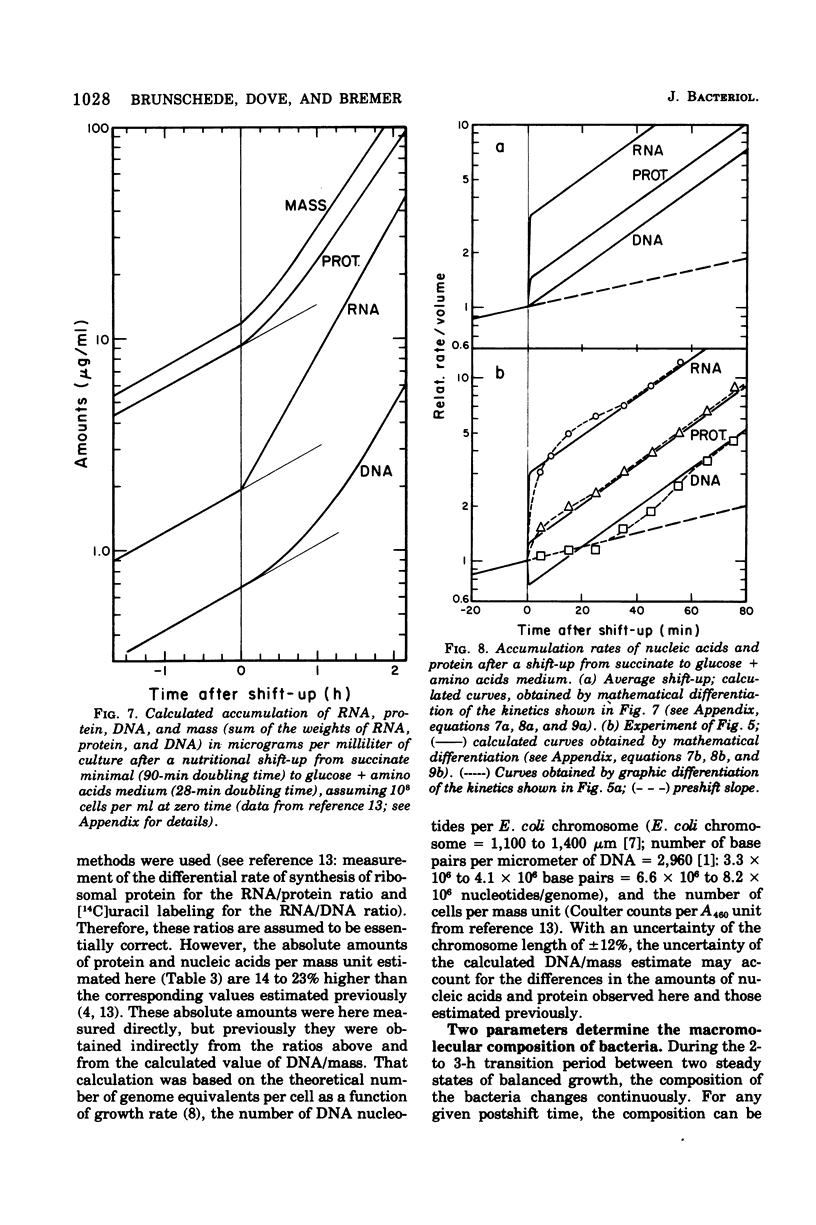
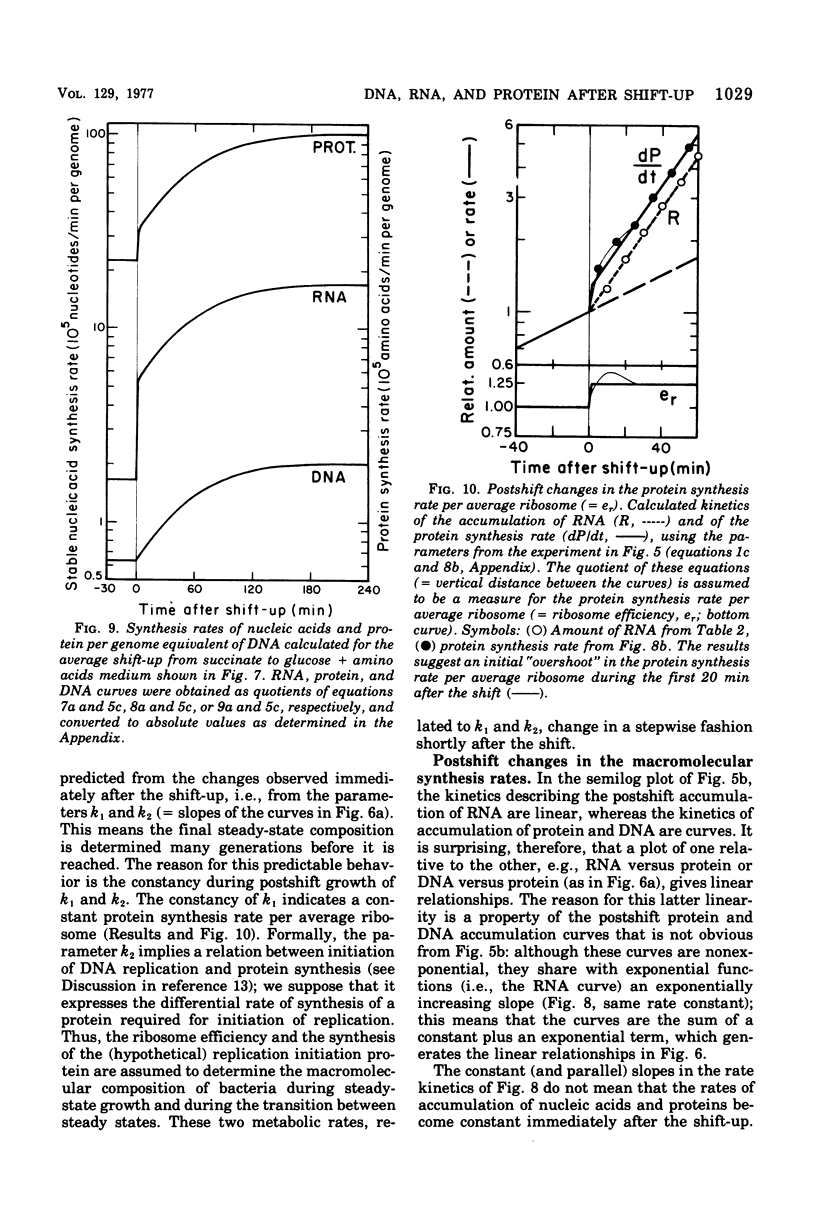
The accumulation of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), and protein was followed in cultures of Escherichia coli B/r during exponential growth in different media and for 2 h after a nutritional shift-up from succinate minimal medium (growth rate [mu1] = 0.67 doublings per h) to glucose plus amino acids medium (mu2 = 3.14 doublings per h). During postshift growth of the culture, the amounts of RNA (R), DNA (D), and protein (P) increased such that the ratios of the increments (delta R/delta P; delta D/delta P) were constants (k1, k2). This implies that the rates of accumulation of nuclei1:k2:1. These constants change from their preshift value to their final postshift value (i.e., k1 and k2) within a few minutes after the shift. k1 is a function of the activity of ribosomes, whereas k2 is related to the initiation of rounds of DNA replication. These parameters and the observed change in the doubling time of RNA (= mu2/mu1) were used to derive kinetic equations that describe the accumulation of DNA, RNA, protein, and cell mass during the 2- to 3-h transition period after a shift-up. The calculated kinetics agree closely with the observed kinetics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Dennis P. P. Gene activities for ribosomal components in Escherichia coli B/r. Biochem J. 1975 Sep;150(3):469–475. doi: 10.1042/bj1500469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Dennis P. P. Transition period following a nutritional shift-up in the bacterium Escherichia coli B/r: stable RNA and protein synthesis. J Theor Biol. 1975 Aug;52(2):365–382. doi: 10.1016/0022-5193(75)90007-7. [DOI] [PubMed] [Google Scholar]
- Bremer H. Parameters affecting the rate of synthesis of ribosomes and RNA polymerase in bacteria. J Theor Biol. 1975 Sep;53(1):115–124. doi: 10.1016/0022-5193(75)90106-x. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dalbow D. G., Young R. Synthesis time of beta-galactosidase in Escherichia coli B/r as a function of growth rate. Biochem J. 1975 Jul;150(1):13–20. doi: 10.1042/bj1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Differential rate of ribosomal protein synthesis in Escherichia coli B/r. J Mol Biol. 1974 Apr 15;84(3):407–422. doi: 10.1016/0022-2836(74)90449-5. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Bremer H. Macromolecular composition during steady-state growth of Escherichia coli B-r. J Bacteriol. 1974 Jul;119(1):270–281. doi: 10.1128/jb.119.1.270-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis P. P., Nomura M. Regulation of the expression of ribosomal protein genes in Escherichia coli. J Mol Biol. 1975 Sep 5;97(1):61–76. doi: 10.1016/s0022-2836(75)80022-2. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Block R. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged transfer ribonucleic acid in the acceptor site of ribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1564–1568. doi: 10.1073/pnas.70.5.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakura Y., Ishihama A. Biosynthesis of RNA polymerase in Escherichia coli. II. control of RNA polymerase synthesis during nutritional shift up and down. Mol Gen Genet. 1975 Dec 23;142(1):67–84. [PubMed] [Google Scholar]
- Koch A. L. Overall controls on the biosynthesis of ribosomes in growing bacteria. J Theor Biol. 1970 Aug;28(2):201–231. doi: 10.1016/0022-5193(70)90053-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazzarini R. A., Cashel M., Gallant J. On the regulation of guanosine tetraphosphate levels in stringent and relaxed strains of Escherichia coli. J Biol Chem. 1971 Jul 25;246(14):4381–4385. [PubMed] [Google Scholar]
- Matzura H., Hansen B. S., Zeuthen J. Biosynthesis of the beta and beta' subunits of RNA polymerase in Escherichia coli. J Mol Biol. 1973 Feb 15;74(1):9–20. doi: 10.1016/0022-2836(73)90350-1. [DOI] [PubMed] [Google Scholar]
- Meijs W. H., Schilperoort R. A. Determination of the amount of DNA on nitrocellulose mebrane filters. FEBS Lett. 1971 Jan 12;12(3):166–168. doi: 10.1016/0014-5793(71)80059-5. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of ribonucleic acid synthesis in growing bacterial cells. I. Control over the total rate of RNA synthesis. J Mol Biol. 1972 Dec 30;72(3):751–764. doi: 10.1016/0022-2836(72)90189-1. [DOI] [PubMed] [Google Scholar]
- Nierlich D. P. Regulation of ribonucleic acid synthesis in growing bacterial cells. II. Control over the composition of the newly made RNA. J Mol Biol. 1972 Dec 30;72(3):765–777. doi: 10.1016/0022-2836(72)90190-8. [DOI] [PubMed] [Google Scholar]
- Norris T. E., Koch A. L. Effect of growth rate on the relative rates of synthesis of messenger, ribosomal and transfer RNA in Escherichia coli. J Mol Biol. 1972 Mar 14;64(3):633–649. doi: 10.1016/0022-2836(72)90088-5. [DOI] [PubMed] [Google Scholar]
- Rosset R., Julien J., Monier R. Ribonucleic acid composition of bacteria as a function of growth rate. J Mol Biol. 1966 Jul;18(2):308–320. doi: 10.1016/s0022-2836(66)80248-6. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SPAHR P. F. Amino acid composition of ribosomes from Escherichia coli. J Mol Biol. 1962 May;4:395–406. doi: 10.1016/s0022-2836(62)80020-5. [DOI] [PubMed] [Google Scholar]
- Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967 Jul 14;27(1):41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- Yuan D., Shen V. Stability of ribosomal and transfer ribonucleic acid in Escherichia coli B/r after treatment with ethylenedinitrilotetraacetic acid and rifampicin. J Bacteriol. 1975 May;122(2):425–432. doi: 10.1128/jb.122.2.425-432.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A. J., Gruber M., Jorgensen P. The mechanism of action of ppGpp on rRNA synthesis in vitro. Cell. 1976 May;8(1):123–128. doi: 10.1016/0092-8674(76)90193-8. [DOI] [PubMed] [Google Scholar]


