Abstract
Clinical and experimental evidence indicates that intestinal inflammatory conditions can be exacerbated by behavioral conditions such as depression. The recent demonstration of a tonic counterinflammatory influence mediated by the vagus nerve in experimental colitis provides a potential link between behavior and gut inflammation. Here we show that experimental conditions that induced depressive-like behaviors in mice increased susceptibility to intestinal inflammation by interfering with the tonic vagal inhibition of proinflammatory macrophages and that tricyclic antidepressants restored vagal function and reduced intestinal inflammation. These results show that reserpine-induced monoamine depletion and maternal separation, 2 models for depression, produced a vulnerability to colitis by a mechanism involving parasympathetic transmission and the presence of gut macrophages. The tricyclic antidepressant desmethylimipramine protected against this vulnerability by a vagal-dependent mechanism. Together these results illustrate the critical role of the vagus in both the vulnerability to inflammation induced by depressive-like conditions and the protection afforded by tricyclic antidepressants and rationalize a clinical evaluation of both parasympathomimetics and tricyclic antidepressants in treatment of inflammatory bowel disease.
Introduction
Depression may coexist with Crohn disease more often than would be expected by chance (1). In some studies, depression correlated well with disease activity, suggesting that it is secondary to the disability imposed by Crohn disease (2–4). In other studies, depression was unrelated to disease activity (5) and, in some cases, actually predated the onset of Crohn disease (6). It is therefore unclear whether depression is an epiphenomenon that occurs as a result of the disease, or whether depression may play a role in facilitating the expression of inflammatory bowel disease (IBD). In a recent study, we showed that mice subjected to maternal deprivation develop a behavioral pattern reminiscent of depression and are more susceptible to inflammation (7). However, the underlying mechanism is unknown.
Linkages between major affective disorders and a variety of conditions have been suggested, and prominent in this literature is a relationship between depression and susceptibility to ischemic heart disease, particularly in men (8, 9). There are 2 broad hypotheses to explain this relationship, and they relate to inflammation or to autonomic imbalance. Major depression is strongly associated with increased levels of C-reactive protein (CRP) among men (10). Depression is also associated with increases in inflammation and coagulation factors in individuals who are free of cardiovascular disease, offering a stronger link between depression per se and susceptibility to inflammation (11). Indeed, some depressed patients exhibit increased TNF-α levels, which normalize upon treatment with antidepressants (12). It remains unclear as to whether this relationship reflects a causal role for depression in susceptibility to inflammatory stimuli or a common inflammation-based etiopathology (13, 14).
The second broad hypothesis is that depression results in autonomic imbalance, with impaired parasympathomimetic functions (15) and a dominant sympathetic drive, contributing to cardiac pathophysiology (16). The notion that depression is associated with parasympathetic dysfunction has led to the exploitation of vagal electrical stimulation as a novel treatment for refractory depression (17–19).
We recently provided proof of concept that depressive-like behavior is associated with an exaggerated response to inflammatory stimuli in the gut, using a murine model of maternal separation (MS) (7). We have also demonstrated that the vagus nerve provides tonic inhibition of acute inflammation in a murine model of colitis (20). The inhibitory effect of the vagus is mediated via macrophages (20) and likely involves the activation of nicotinic acetylcholine receptors on the macrophage, resulting in selective suppression of proinflammatory cytokine secretion (20–22). Taken together, these observations have prompted the hypotheses that the susceptibility to gut inflammation induced by depression results from impaired vagal function and that the protection afforded by tricyclic antidepressants (7) is due to restoration of normal parasympathetic input to the gut.
In this study, we test these hypotheses using 2 models of depression that include a well-established model: the MS model (7, 23) and the i.c.v. administration of reserpine. The therapeutic use of reserpine for hypertension resulted in the realization that it could induce severe depression (24). Reserpine acts by depleting biogenic amines. In rodents, acute administration of a large dose of reserpine i.p. depleted concentrations of noradrenalin, adrenaline, dopamine, and serotonin (5-HT) in the brain for more than a week, but depression persisted for only 72 hours (25). Therefore, in our study we induced a sustained depression by administering a low dose of reserpine by i.c.v. injection for 14 days. Studies were performed on mice with or without vagotomy and pyloroplasty (VXP) in C57BL/6 or in macrophage-depleted mice, and colitis was induced by either dextran sulfate sodium (DSS) or dinitrobenzene sulfonic acid (DNBS). Desmethylimipramine (DMI) was used as the antidepressant. We show that reserpine induced depressive behavior and that this was accompanied by impaired parasympathetic function and severe colitis. DMI protected against this vulnerability to colitis in depressed mice, but protection was entirely dependent on vagal integrity. We also demonstrate the same profile of results using the MS model of depressive behavior, indicating the broader applicability of our findings.
Results
Effect of procedure of VXP and/or i.c.v. cannulation.
VXP, i.c.v. cannulation, and MS caused no changes in weight gain, colonic appearance or histology, CRP, myeloperoxidase (MPO), or cytokine levels in C57BL/6 or M-CSF–deficient mice without colitis. TNF-α, IL-1β, and IL-6 colonic tissue levels were below the lowest standard of the assay in these mice (data not shown).
Characterization of models of depression.
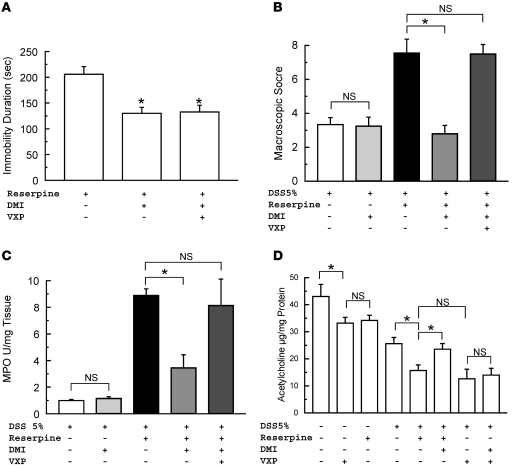
When mice were tested in the tail suspension test, chronic i.c.v. infusion of reserpine (1 and 100 μg/d) significantly increased the duration of immobility and reduced the latency to immobility in a dose-dependent manner (Figure 1, A and B). MS significantly increased the duration of immobility and decreased the latency to immobility compared with the unseparated (US) control group. Immobility duration and latency duration were 178.2 ± 23.1 and 61.1 ± 2.1 seconds in the MS group (P < 0.05) and 118.3 ± 9.8 and 71.3 ± 3.58 seconds in the US group (P < 0.05), respectively. The 5-HT concentration was determined in the brain sections (excluding the brainstem). A significant reduction in 5-HT levels in the brain was seen in reserpinized mice (RM) and also in MS mice compared with control mice (Table 1). Reserpine at 1 μg/d reduced significantly 5-HT content of the brain, but the concentration of this neurotransmitter was reduced significantly further in RM infused with 100 μg/d compared with control mice (Table 1). To avoid total depletion of 5-HT and to maintain a response to DMI, a 1-μg/d dose was used for the reserpine infusion experiments.
Figure 1. Effect of reserpine (1 and 100 μg/d) and VXP treatment on immobility duration and immobility latency of tail suspension test.
(A) Immobility duration. (B) Immobility latency. *P < 0.05 compared with control group, n = 12; #P < 0.05 compared with reserpine 100 μg/d, n = 4. The values are shown as means ± SEM.
Table 1 .
Influence of experimental depression and DMI treatment on 5-HT level in the brain
In contrast, a single i.p. injection of reserpine (14 μg, the full dose given to RM) had no effect on these behavioral tests (data not shown). Furthermore, VXP did not alter the effect of reserpine on MS behavior (Figure 1).
The effect of chronic injection of reserpine on DSS-induced colitis.
DSS induced a colitis that was characterized by weight loss and frequent stools; this was evident by day 3 in sham-operated mice (see Figure 2A). In RM, the onset of colitis was evident within 2 days of DSS, and these mice showed a significantly higher disease activity index (DAI) for all 5 days (Figure 2A). As illustrated in Figure 2, B–D, the increased severity of colitis in RM compared with untreated mice with colitis was evidenced by the 2.25-fold increase in the macroscopic damage score (P < 0.05) (Figure 2B), by the 9-fold increase in MPO activity (P < 0.05) (Figure 2C), and by the 1.38-fold increase in CRP levels (P < 0.05) (Figure 2D). The increased severity of DSS colitis in RM was also evident histologically, as shown in Figure 3, A and B. The histological damage score increased from 1.5 ± 0.28 in untreated mice with DSS colitis to 2.7 ± 0.25 in RM with colitis (P < 0.05). Similarly, changes in cytokine concentrations in the colon were also increased in RM with colitis. The concentrations of TNF-α, IL-1β, and IL-6 in control mice with colitis were 33.7 ± 3.8, 54.2 ± 8.4, and 67.3 ± 2.7 pg/mg protein, respectively. The increase in TNF-α was 1.97 ± 0.1-fold higher (P < 0.05) in RM compared with untreated mice with DSS colitis, and respective values for IL-1β and IL-6 were 1.87 ± 0.16-fold (P < 0.05) and 2.26 ± 0.14-fold (P < 0.05). To determine whether the antiinflammatory cytokines IL-10 and TGF-β played a role in this response, we studied the levels of both cytokines in colonic tissue. The concentrations of IL-10 and TGF-β in control mice with colitis were 6.25 ± 0.8 and 85.1 ± 2.8 pg/mg protein, respectively. As already demonstrated after vagotomy (26), no significant differences were found in IL-10, but the increase in TGF-β was 1.6 ± 0.2-fold higher in RM compared with untreated mice with DSS colitis (P < 0.05).
Figure 2. Influence of experimental design on colitis.
(A) Effects of reserpine (1 μg/d for 14 days, ICV-R) treatment versus vehicle (ICV-C) on the DAI during the development of DSS-induced colitis in mice over 5 days. Mice were given 5% DSS solution in the drinking water to induce colitis 9 days after the beginning of the reserpine treatment. Reserpine increased the DAI (n = 12). (B) Macroscopic scores were higher in RM (n = 12). (C) MPO activity was higher in RM (n = 12). (D) CRP, an acute inflammatory marker, was measured by ELISA. A significant increase of CRP was detected in the RM group compared with the sham-operated group (ICV-C) (n = 12). The values are shown as means ± SEM. *P < 0.05 compared with ICV-C group.
Figure 3. Effect of experimental depression on H&E staining.
(A) Appearance of a colon in a DSS-treated nonreserpinized mouse. (B) Appearance of a colon in a DSS-treated RM; DSS treatment was associated with areas of greater erosion and with more inflammatory cell infiltration. (C) DMI treatment significantly reduced infiltration. (D) Following VXP, DMI treatment did not appear to result in any significant improvement. Images were taken after 5 days DSS induction of colitis on RM (1 μg/d for 14 days) and nonreserpinized mice. DMI treatment (15 mg/kg) was initiated 2 days after the beginning of the reserpine (1 μg/d) treatment. H&E staining. Original magnification, ×100.
The effect of chronic injection of reserpine on DNBS-induced colitis.
Chronic reserpine treatment increased the severity of DNBS colitis. As shown in Table 2, there was a significant increase in all parameters of inflammation, and this profile was similar to that seen with DSS colitis.
Table 2 .
Influence of reserpine and DMI treatment on DNBS-induced colitis
The role of parasympathetic nerves in colitis in RM.
DSS colitis was characterized by mild inflammation in the proximal colon and a more pronounced inflammation in the mid and distal parts of the colon. In keeping with our previous findings (20), VXP increased the severity of colitis in DSS-treated mice without reserpine, and this was evident throughout the colon (data not shown). As illustrated in Figure 4, A–C, the increased severity of colitis in VXP mice compared to sham-operated DSS mice was evident in the 2.25-fold increase in the macroscopic damage score (P < 0.05) (Figure 4A), in the 7.9-fold increase in MPO activity (P < 0.05) (Figure 4B), and in the 1.51-fold increase in CRP levels (P < 0.05) (Figure 4C). As already shown, reserpine caused a substantial increase in all parameters of inflammation in mice with DSS colitis, and no further deterioration was evident in VXP mice who were subsequently reserpinized prior to the induction of DSS colitis (Figure 4). However, the parasympathomimetic agent nicotine, in the presence or absence of VXP, ameliorated the inflammation in RM with DSS colitis. This was evident in all parameters of inflammation, including macroscopic damage score (P < 0.05) (Figure 4A), MPO activity (P < 0.05) (Figure 4B), CRP levels (P < 0.05) (Figure 4C), and IL-1β (P < 0.05) (Figure 4D). Fold increases in IL-6 and TNF-α were 0.94 ± 0.21 and 1.18 ± 0.26 compared with non-VXP mice with DSS colitis and 0.99 ± 0.1 and 1.01 ± 0.2, respectively, compared with the VXP group with DSS colitis. Altered parasympathetic function in RM was also reflected in the colonic concentrations of acetylcholine, the primary neurotransmitter of the parasympathetic system. As shown in Figure 5, vagotomy in control mice without colitis caused a 23% decrease in acetylcholine concentration (P < 0.05), illustrating the vagal contribution to the concentration of this neurotransmitter in the colon. This concentration of acetylcholine was also reduced in RM without colitis. Colitis per se also reduced the acetylcholine content of the colon (P < 0.05), but the concentration of this neurotransmitter was reduced significantly further in RM and in VXP mice with colitis (P < 0.05) (Figure 5). Taken together, these findings indicate that reserpine treatment is accompanied by impaired parasympathomimetic function in the colon.
Figure 4. Influence of nicotine treatment on experimental depression and VXP.
Influence of 10 days of nicotine (20 μg/ml in drinking water) treatments, starting 4 days after the beginning of reserpine (1 μg/d) treatment, on (A) macroscopic score, (B) MPO activity, (C) CRP levels, and (D) IL-1β cytokine levels in colonic tissue after 5 days DSS-induced colitis on RM (1 μg/d for 14 days) and nonreserpinized mice. Fold change compared with DSS 5% nonreserpinized mice. In presence or in absence of VXP, nicotine treatment significantly decreased all 4 markers. *P < 0.05, n ≥ 8. The values are shown as means ± SEM.
Figure 5. Influence of reserpine treatment (1 μg/d for 14 days) and VXP on acetylcholine level in colonic tissue in group without colitis and group with 5 days DSS-induced colitis.
VXP or colitis alone significantly decreased the level of acetylcholine in mice without colitis (n = 10). A greater decrease was found in the colitic group with reserpine or VXP treatment (n = 12). *P < 0.05, n ≥ 8. The values are shown as means ± SEM.
The role of macrophages in colitis in RM.
We (20) and others (21, 22) have identified macrophages as the critical target cells for vagal modulation of inflammation. In the next experiment, we examined the role of M-CSF–dependent macrophages in op/op mice deficient in these cells. There was no difference between M-CSF–C57BL/6–deficient (op/op) and nonhomozygous (+/?) mice without colitis receiving reserpine in either the macroscopic scores (0.1 ± 0.11 and 0.9 ± 0.72, respectively) or MPO activity (0.1 ± 0.62 and 0.6 ± 0.31 U/mg of tissue, respectively). As shown in Figure 6, reserpine worsened the colitis in +/? mice, as reflected by macroscopic damage (P < 0.05) (Figure 6A), MPO activity (P < 0.05) (Figure 6B), and CRP levels (P < 0.05) (Figure 6C). Increases were also seen in IL-1β (P < 0.05) (Figure 6D), TNF-α (4.8 ± 0.17-fold; P < 0.05), and IL-6 (2.3 ± 0.2-fold; P < 0.05) compared with nonreserpinized +/? mice receiving DSS. In contrast, no further increase for any DSS-induced inflammatory parameter was evident following reserpine treatment in op/op mice lacking M-CSF–derived macrophages (see Figure 6). The fold changes in TNF-α and IL-6 were 1.15 ± 0.34 and 1.24 ± 0.24, respectively, with no significant change compared with nonreserpinized op/op mice receiving DSS. No differences were seen for IL-10, but the level of TGF-β was associated with a 1.9 ± 0.14-fold increase (P < 0.05) in +/? RM.
Figure 6. Influence of reserpine treatments (1 μg/d for 14 days) on MCSF-deficient mice.
(A) Macroscopic score, (B) MPO activity, (C) CRP level, and (D) cytokine IL-1β level in colonic tissue after 5 days of DSS-induced colitis. Reserpine treatment (black bars) increased all 4 markers in +/? mouse but did not affect the op/op mice. Fold change is relative to op/op or +/? DSS 5% RM. *P < 0.05, n ≥ 8; #P < 0.05 versus +/? DSS 5% control mice. The values are shown as means ± SEM.
The effect of DMI on colitis in RM.
As shown in Figure 7A, i.p. administration of DMI attenuated the duration of immobility (P < 0.05) associated with depressive behavior. This action was not altered by VXP. DMI administration in RM significantly increased the level of 5-HT in the brain (P < 0.05) (Table 1). A similar profile was seen with the immobility latency test, in which DMI showed an increase of the latency from 56.21 ± 3.1 to 67.4 ± 2.9 seconds (P < 0.05), whereas VXP and nicotine did not affect this test (53.77 ± 2.56 and 47.8 ± 3.85 seconds, respectively).
Figure 7. Effect of VXP on DMI treatment of colitis.
(A) Influence of 12 days of DMI (15 mg/d, i.p.) treatment, starting 2 days after the beginning of reserpine (1 μg/d) treatment and/or VXP treatment on behavior. DMI effect was not affected by VXP. (B) Macroscopic score, (C) MPO activity, and (D) acetylcholine levels in colonic tissue after 5 days of DSS 5% regime. *P < 0.05. The values are shown as means ± SEM.
We next examined the effect of DMI on inflammatory indices in mice with DSS colitis. DMI had no effect on any parameter of inflammation in mice with DSS colitis that had not received reserpine. As shown in Figure 7, B and C, DMI did not lower the macroscopic score or attenuate MPO activity in these mice. Similarly, values for CRP were 25.3 ± 0.1 and 26.7 ± 0.8 ng/ml in untreated and DMI-treated mice with colitis. IL-1β, IL-6, and TNF-α cytokine levels were also similar (1.2 ± 0.3, 0.97 ± 0.4, and 1.2 ± 0.4-fold increases, respectively) compared with untreated mice with colitis. These results indicate that DMI has no direct antiinflammatory effect in this model of colitis.
As already demonstrated, reserpine treatment increased the severity of DSS colitis. As shown in Figure 7, B and C, this reserpine-induced deterioration was abolished by DMI. Indeed, all parameters of inflammation were improved in RM treated with DMI. Values for histological score were decreased from 2.7 ± 0.25 to 1.12 ± 0.21 (P < 0.05) (see also Figure 3C), CRP levels decreased from 34.1 ± 0.7 to 25.1 ± 2.3 ng/ml (P < 0.05), and cytokine fold increases were 0.31 ± 0.22 (P < 0.05), 0.5 ± 0.33 (P < 0.05), and 1.02 ± 0.2 (P < 0.05) for IL-1β, IL-6, and TNF-α, respectively, compared with control. The counterinflammatory effect of DMI was dependent on the integrity of the vagus, as no improvement was seen in VXP RM with colitis following DMI treatment (Figure 3D and Figure 7, B and C). Moreover, no further increase for TGF-β was evident following DMI treatment in RM. The fold change in TGF-β was 1.1 ± 0.2, with no significant change compared with nonreserpinized mice receiving DSS, and no differences were seen for IL-10.
To further examine the dependency of DMI on parasympathetic function, we examined changes in colonic acetylcholine levels following administration of the antidepressant, and the results are shown in Figure 7D. As previously shown, reserpine treatment was associated with a significant reduction in acetylcholine levels in the colitis following DSS. This reduction was attenuated by DMI, as shown in Figure 7D. The acetylcholine level in DMI-treated RM was similar to the level seen in mice with DSS colitis in the absence of either reserpine (23.55 ± 2.07 μg/mg protein) or DMI treatments (25.59 ± 2.28 μg/mg protein). In DMI-treated RM and vagotomized mice, the level of acetylcholine was similar to that seen in mice with DSS colitis in the presence of reserpine (14.95 ± 1.04 and 13.419 ± 1.78 μg/mg protein, respectively). Taken together, these findings indicate that the counterinflammatory effect of DMI in this model is mediated by a restoration of parasympathetic function in the gut.
The role of parasympathetic nerves and DMI in colitis in MS mice.
To determine whether the above described changes were restricted to the reserpine-based model, we performed studies in the MS mouse model, which also exhibits depressive-like behavior. As illustrated in Table 3, the increased severity of colitis in MS mice, compared with US DSS mice, was evidenced by the 1.65-fold increase (P < 0.05) in the macroscopic damage score, by the 1.56-fold increase in MPO activity (P < 0.05), and by the 1.47-fold increase in CRP levels (P < 0.05). The increased severity of DSS colitis in MS mice was also evident histologically (P < 0.05), and changes in cytokine concentrations in the colon were also seen in MS mice with colitis. The concentrations of TNF-α, IL-1β, and IL-6 in control with colitis mice were 21.7 ± 8.1, 81.7 ± 20.2, and 51.7 ± 2.3 pg/mg protein, respectively. The fold increase in TNF-α was 4.0 ± 0.8 higher in MS mice compared with US mice with DSS colitis (P < 0.05), and respective values for IL-1β and IL-6 were 2.3 ± 0.4 (P < 0.05) and 1.6 ± 0.3 (P < 0.05).
Table 3 .
Influence of MS and DMI treatment on DSS-induced colitis
Administration of DMI attenuated the duration of immobility associated with depressive behavior (P < 0.05) (Table 3) and significantly increased the level of 5-HT in the brain in MS mice (P < 0.05) (Table 1). This action was not altered by VXP (data not shown). DMI had no effect on any parameter of inflammation in US mice with DSS colitis. As already demonstrated, MS increased the severity of DSS colitis, and this deterioration was abolished by DMI. Indeed, all parameters of inflammation were improved in MS mice treated with DMI, and the counterinflammatory effect of DMI was dependent on the integrity of the vagus, as no improvement was seen in vagotomized MS mice with colitis following DMI treatment.
To examine the dependency of DMI on parasympathetic function, we next examined changes in colonic acetylcholine levels following administration of the antidepressant. In MS mice, DSS treatment was accompanied by a significant reduction in acetylcholine levels in the colon (P < 0.05), and this reduction was attenuated by DMI (P < 0.05), as shown in Table 3. The acetylcholine level in DMI-treated MS mice was similar to the level seen in mice with DSS colitis in US. In DMI-treated MS and vagotomized mice, the level of acetylcholine was similar to the level seen in MS mice with DSS colitis. In keeping with our previous findings, these results support the notion that the counterinflammatory effect of DMI in this model is also mediated by a restoration of parasympathetic function in the gut.
Discussion
The results of this study are consistent with the hypotheses that impaired parasympathetic function in mice with reserpine-induced depression results in an increased susceptibility to experimental colitis and that the protective effect of DMI is due to restoration of vagal parasympathetic function.
The evidence in favor of impaired parasympathetic function as a basis for the vulnerability of RS to colitis is based on the observation of a protective role of the vagus against acute colitis in animal models (27, 28). In reserpine-treated mice with colitis, colonic levels of the parasympathetic neurotransmitter acetylcholine were reduced to a level similar to that seen in vagotomized mice that had not been reserpinized. The implication is that a substantial amount of the acetylcholine in the colon is of vagal origin. We interpret the reduction in acetylcholine to this level to reflect vagal impairment secondary to reserpine-induced depression.
In addition, we interpret the ability of nicotine to reduce the severity of colitis in reserpine-treated mice to be in keeping with underlying parasympathetic dysfunction. The fact that vagotomy did not worsen colitis in RM does not mitigate against this hypothesis. We have shown that vagotomy per se substantially increased the severity of DSS colitis, and we argue that the deleterious effect of subsequent reserpine treatment on colitis would be masked by the effect of the vagotomy. We acknowledge that vagal innervation is restricted to the proximal colon. However, it is possible that inflammatory changes modulated in the vagally innervated proximal colon result in changes in the enteric nervous system in the remainder of the colon (29), resulting in a reduction in acetylcholine release. This may account for the effect of vagotomy on colitis seen in this and other studies (20, 26, 27, 30). We also demonstrate the same profile of results using the maternal deprivation model. No deterioration in colitis was seen in macrophage-deficient op/op RM in this study. This observation provides indirect evidence of the importance of the vagus in these models, as studies from our group (20) and others (21, 22, 28, 31) have identified the macrophage as the target cell for the vagal-mediated counterinflammatory mechanism. In this study we use nicotine as a parasympathomimetic in the gut. While it is known that nicotine has antidepressive effects (32), we found no evidence of a central effect of nicotine on behavior in our study.
Our results are consistent with the hypothesis that the tricyclic antidepressant DMI attenuates the inflammatory response in RM by enhancing parasympathetic function. This conclusion is based on the following observations: first, the beneficial effect of DMI was absent in vagotomized mice with colitis. Second, DMI therapy increased colonic acetylcholine levels in RM, and this effect was not seen in vagotomized mice. We were unable to determine whether DMI would confer protection in macrophage-deficient mice, as no increase in severity of colitis was seen in the op/op mouse. We are aware of the possibility that the tricyclic antidepressant may have direct antiinflammatory effects (33–36), but these were not evident in our experiments. DMI had no effect on any parameter of inflammation in DSS-treated mice without depression.
It is conceivable that other factors contributed to the deleterious effect of depression on colitis in our study. Parasympathetic impairment results in a dominant sympathetic drive, and it is known that this enhances colonic inflammation (37). In addition, vagotomy alters lymphocyte trafficking (38) and mast cell numbers in the gut (39) and influences gut physiology (40, 41), and these factors could contribute to the changes in severity of colitis seen in our study.
The results of this study have clinical relevance. First, they prompt close consideration of the relationship between depression and disease activity in patients with IBD. Second, if there is indeed a correlation, then depressed patients with IBD might be selected for novel treatment strategies. These include not only the use of tricyclic antidepressants but also the use of highly selective nicotinic receptor agonists (22, 42–44) or, in appropriate patients, vagal stimulation, which is now being evaluated for refractory depression and for epilepsy (45, 46).
Methods
Animals.
Male C57BL/6 (7–9 weeks old) and C57BL/6 female mice on gestational days 15–16 were purchased from Taconic Farms Suppliers and maintained in the animal care facility at McMaster University under specific pathogen–free conditions. Dams were housed individually. M-CSF–C57BL/6–deficient (op/op) as well as nonhomozygotic +/? breeding pairs were purchased from The Jackson Laboratory. Nonhomozygous mice (op/+ or +/+) are phenotypically indistinguishable from each other (therefore designated +/?). Op/op mice have osteopetrosis and lack teeth; they were therefore fed a powdered diet, while +/? mice received conventional food. No differences in food intake or body weight were observed between these groups. Mice were housed under standard conditions for a minimum of 1 week before experimentation. All experiments were approved by the McMaster University animal ethics committee and conducted under the Canadian guidelines for animal research.
Maternal deprivation.
The MS protocol used in the present study was a slight modification of one previously published (23). Dams and their litters were assigned at random to the control (US) group or the MS group. MS pups were removed from their home cages and dams from P2 to P21 for 180 minutes daily by placing them as a litter in a new isolation cage. The isolation cages were lined with chip bedding and kept at 37°C ± 5°C by using a heating pad placed under the cages. Dams of the MS group also were removed from the home cages and transferred to separate holding cages during the MS procedure. The operator’s gloved hands were rubbed in the bedding of each litter before handling of pups to prevent rejection by dams on reunion. US litters were left undisturbed, except for routine cage care by the technician. At weaning (P22), male offspring were identified and used as subjects in the study. Subjects were weighed at P60.
Vagotomy.
On P68 and P78, MS and US mice were anesthetized using ketamine (150 mg/kg, i.p.; Bimeda-MTC) and xylazine (10 mg/kg, i.p.; Bayer HealthCare), and ventral and dorsal truncal branches of the subdiaphragmatic vagus were cut (1 cm above gastroesophageal junction). Preliminary studies showed marked gastric dilation in vagotomized mice, and a surgical pyloroplasty was therefore incorporated into the protocol. VXP were subsequently performed under the same anesthesia. No gastric dilation was observed in mice undergoing this procedure. In sham-operated mice, vagal trunks were similarly exposed but not cut, but a pyloroplasty was performed. All mice were maintained on a normal diet (except for the op/op mice, which received a powdered diet).
Validation of vagotomy.
Due to a short protocol used, the integrity of vagotomy in our study was not measured by the cholecystokinin octapeptide test. The completeness of vagotomy was verified during postmortem inspection of vagal nerve endings using microscopic inspection associated with a Bielschowsky silver staining (47). Briefly, the nerve fibers were sensitized with a silver solution. The sections were treated with ammoniacal silver and then reduced to a visible metallic silver (data not shown).
Chronic i.c.v. infusion and i.p. injection.
Micro-osmotic pumps (Alzet; Durect Corp.) were filled with vehicle (acetic acid 0.2%) or reserpine solution (1 or 100 μg/d for 14 days). These doses were based on methods previously developed in another laboratory for chronic i.c.v. infusion in mice (48). The micro-osmotic pumps were connected by a 1.5- to 2-cm length of PE-60 tubing to a 3-mm-long cannula (Plastics One) and sterilized. For pump implantation, mice were anesthetized with ketamine (150 mg/kg, i.p.; Bimeda-MTC) and xylazine (10 mg/kg, i.p.; Bayer HealthCare). The scalp was shaved, an incision was made along the midline, and the skull was scraped clean of periosteum. Hemostats were used to make a pocket under the skin between the shoulder blades. A microdrill was used to drill a hole approximately 1.1 mm lateral and 1 mm caudal to bregma. The minipump was placed in the pocket between the shoulder blades, the cannula was inserted through the drilled hole into the lateral ventricle, and the cannula pedestal was affixed to the skull with cyanoacrylate glue (Loctite). The animals were allowed to recover on a heating pad. Another group of mice was injected i.p. with the full dose of reserpine.
Tail test.
The immobility latency and the total duration of immobility induced by tail suspension was measured according to the method described by Steru et al. (49) as a facile means of evaluating potential antidepressants. Mice were suspended from a stick 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was recorded during a 6-min period (50). The animal was considered to be immobile when it did not show any movement of body and hanged passively. Behavior was assessed 2 days before surgery and 8 days after surgery or 2 days before and 6 hours after the acute injection of reserpine.
Induction of DSS and DNBS colitis.
DSS (MW, 40 kDa; ICN Biomedicals Inc.) was added to the drinking water in a final concentration of 5% (wt/vol) for 5 days (20). Controls were all time matched and consisted of mice that received normal drinking water only. Mean DSS consumption was noted per cage each day. For the DNBS study, mice were anesthetized with Isoflurane (Abbott). A 10-cm-long PE-90 tubing (ClayAdam) attached to a tuberculin syringe was inserted 3.5 cm into the colon. Colitis was induced by administration of 4 mg DNBS in 100 μl solution (ICN Biomedicals Inc.) in 30% ethanol and left for 3 days (51). Control mice (without colitis) received saline administration. Mice with colitis were supplied with 6% sucrose in drinking water to prevent dehydration.
Experimental protocol.
The tail test was performed 2 days before surgery. VXP and placement of the micro-osmotic pump were performed on the day of surgery. Mice were tested for depression-like behavior on day 8 after surgery. Exposure to DSS (5%) commenced on the ninth day following surgery and continued for 5 days. In separate experiments, nicotine (20 μg/ml) was added to the drinking water on day 4 after surgery and for 5 days after the induction of colitis in vagotomized mice (52). As previously described (7) DMI was administrated i.p. at the dose of 15 mg/kg for 12 days starting 2 days after surgery. Exposure to DNBS (4 mg) commenced on the eleventh day following surgery for 3 days.
Assessment of the severity of colitis — DAI.
DAI scores have historically correlated well with the pathological findings in a DSS–induced model of IBD (53). DAI is the combined score of weight loss, stool consistency, and bleeding. Scores were defined as follows: body weight loss, 0, no loss; 1, 5%–10%; 2, 10%–15%; 3, 15%–20%; 4, 20%; stool consistency, 0, normal; 2, loose stool; 4, diarrhea; bleeding, 0, no blood; 2, presence of bleeding; and 4, gross bleeding (Hemoccult II; Beckman Coulter). DAI was scored from day 0 to day 5 during DSS treatment.
CRP assay in serum.
Blood was collected 5 or 3 days after the beginning of the DSS or DNBS treatment, respectively, by intracardiac puncture in anesthetized (isoflurane) mice. CRP levels were determined using ELISA commercial kit (R&D Systems).
Macroscopic scores.
Five days after the beginning of the DSS or 3 days after the beginning of the DNBS treatment, mice were killed and the abdominal cavity was opened, the colon was located, and observations on distension, fluid content, hyperemia, and erythema were recorded. The colon was removed and opened longitudinally, and macroscopic damage was immediately assessed on the full section of the colon. Macroscopic scores were performed using a previously described scoring system for DSS colitis (53) and for DNBS (54).
Colonic histology and MPO activity.
Formalin-fixed colon segments coming from the splenic flexure were paraffin embedded, and 3-μm sections were stained with H&E. Colonic damage was scored based on a published scoring system that considers architectural derangements, goblet cell depletion, edema/ulceration, and degree of inflammatory cell infiltrate (53). MPO activity was determined following an established protocol (55). Briefly, MPO activity, used as a marker of granulocyte infiltration, was measured using a modified version of the method described by Bradley et al. (56). Tissue samples were homogenized (50 mg/ml) in 50 mM ice-cold potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyl trimethyl ammonium bromide (Sigma-Aldrich). The homogenate was freeze thawed 3 times, briefly sonicated, and then centrifuged at 12,000 rpm for 12 min at 4°C. The supernatant was then added to a solution of O-dianisidine (Sigma-Aldrich) and hydrogen peroxide. The absorbance of the colorimetric reaction was measured by a spectrophotometer. MPO is expressed in units per milligram of wet tissue, 1 unit being the quantity of enzyme able to convert 1 μmol of hydrogen peroxide to water in 1 minute at room temperature.
Cytokines and acetylcholine tissue levels.
Colonic sample was homogenized in 700 μl of Tris HCl buffer containing protease inhibitors (Sigma-Aldrich). Samples were centrifuged for 30 min, and the supernatant was frozen at –80°C until assay. Cytokine levels (IL-1β, IL-6, TNF-α, TGF-β, and IL-10), were determined using ELISA commercial kit (R&D Systems). The amount of acetylcholine was measured using the acetylcholine assay kit (Invitrogen).
Determination of brain 5-HT content.
The brain was separated from the brainstem and homogenized in 1 ml of 0.2 M perchloric acid, then centrifuged at 10,000 g for 5 min. The supernatants were neutralized with 0.5 ml of 1.0 M borate buffer (pH 9.25) and centrifuged at 10,000 g for 1 min. The 5-HT content in the supernatant was analyzed by enzyme immunoassay using a commercially available kit (Beckman Coulter). The 5-HT content of the tissue was expressed as a function of wet weight (ng/g tissue). The number of brains used per group was n ≥ 5.
Statistics.
Results are presented as means ± SEM. Statistical analysis was performed using 1-way ANOVA followed by the Student-Newman-Keuls multiple comparisons post-hoc analysis, and a P value of less than 0.05 was considered significant. The number of mice per group was n ≥ 8 except for reserpine infusion 100 μg/d, which was n = 4.
Acknowledgments
This study was supported by grants from the Crohn’s and Colitis Foundation of Canada to S.M. Collins. J.-E. Ghia was supported by a Canadian Association of Gastroenterology fellowship and by l’Institut de France. The authors thank W. Jackson for her expertise and help.
Footnotes
Nonstandard abbreviations used: CRP, C-reactive protein; DAI, disease activity index; DMI, desmethylimipramine; DNBS, dinitrobenzene sulfonic acid; DSS, dextran sulfate sodium; 5-HT, serotonin; IBD, inflammatory bowel disease; MPO, myeloperoxidase; MS, maternal separation; RM, reserpinized mice; US, unseparated; VXP, vagotomy and pyloroplasty.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:2209–2218 (2008). doi:10.1172/JCI32849
References
- 1.Helzer J.E., Chammas S., Norland C.C., Stillings W.A., Alpers D.H. A study of the association between Crohn’s disease and psychiatric illness. Gastroenterology. 1984;86:324–330. [PubMed] [Google Scholar]
- 2.Mardini H.E., Kip K.E., Wilson J.W. Crohn’s disease: a two-year prospective study of the association between psychological distress and disease activity. Dig. Dis. Sci. 2004;49:492–497. doi: 10.1023/B:DDAS.0000020509.23162.cc. [DOI] [PubMed] [Google Scholar]
- 3.Porcelli P., Leoci C., Guerra V. A prospective study of the relationship between disease activity and psychologic distress in patients with inflammatory bowel disease. Scand. J. Gastroenterol. 1996;31:792–796. doi: 10.3109/00365529609010354. [DOI] [PubMed] [Google Scholar]
- 4.Walker E.A., Gelfand M.D., Gelfand A.N., Creed F., Katon W.J. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. Gen. Hosp. Psychiatry. 1996;18:220–229. doi: 10.1016/0163-8343(96)00036-9. [DOI] [PubMed] [Google Scholar]
- 5.Addolorato G., Capristo E., Stefanini G.F., Gasbarrini G. Inflammatory bowel disease: a study of the association between anxiety and depression, physical morbidity, and nutritional status. Scand. J. Gastroenterol. 1997;32:1013–1021. doi: 10.3109/00365529709011218. [DOI] [PubMed] [Google Scholar]
- 6.Tarter R.E., Switala J., Carra J., Edwards K.L., Van Thiel D.H. Inflammatory bowel disease: psychiatric status of patients before and after disease onset. Int. J. Psychiatry Med. 1987;17:173–181. doi: 10.2190/cb02-2v8b-y624-r781. [DOI] [PubMed] [Google Scholar]
- 7.Varghese A.K., et al. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743–1753. doi: 10.1053/j.gastro.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W., Krishnan R.R., O’Connor C.M. Depression and heart disease: evidence of a link, and its therapeutic implications. CNS Drugs. 2002;16:111–127. doi: 10.2165/00023210-200216020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rudisch B., Nemeroff C.B. Epidemiology of comorbid coronary artery disease and depression. Biol. Psychiatry. 2003;54:227–240. doi: 10.1016/S0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 10.Ford D.E., Erlinger T.P. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 11.Panagiotakos D.B., et al. Inflammation, coagulation, and depressive symptomatology in cardiovascular disease-free people; the ATTICA study. Eur. Heart J. 2004;25:492–499. doi: 10.1016/j.ehj.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 12.Tuglu C., Kara S.H., Caliyurt O., Vardar E., Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology. (Berl.) 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- 13.Anisman H., Merali Z. Cytokines, stress and depressive illness: brain-immune interactions. Ann. Med. 2003;35:2–11. doi: 10.1080/07853890310004075. [DOI] [PubMed] [Google Scholar]
- 14.Capuron L., Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav. Immun. 2003;17(Suppl. 1):S119–S124. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 15.Nahas Z., et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 16.Gorman J.M., Sloan R.P. Heart rate variability in depressive and anxiety disorders. Am. Heart J. 2000;140:77–83. doi: 10.1067/mhj.2000.109981. [DOI] [PubMed] [Google Scholar]
- 17.Goodnick P.J., Rush A.J., George M.S., Marangell L.B., Sackeim H.A. Vagus nerve stimulation in depression. Expert Opin. Pharmacother. 2001;2:1061–1063. doi: 10.1517/14656566.2.7.1061. [DOI] [PubMed] [Google Scholar]
- 18.Groves D.A., Brown V.J. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Shafique S., Dalsing M.C. Vagus nerve stimulation therapy for treatment of drug-resistant epilepsy and depression. Perspect. Vasc. Surg. Endovasc. Ther. 2006;18:323–327. doi: 10.1177/1531003506297200. [DOI] [PubMed] [Google Scholar]
- 20.Ghia J.E., et al. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Borovikova L.V., et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 22.de Jonge W.J., et al. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 2005;6:844–851. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 23.MacQueen G.M., Ramakrishnan K., Ratnasingan R., Chen B., Young L.T. Desipramine treatment reduces the long-term behavioural and neurochemical sequelae of early-life maternal separation. Int. J. Neuropsychopharmacol. 2003;6:391–396. doi: 10.1017/S1461145703003729. [DOI] [PubMed] [Google Scholar]
- 24.Leith N.J., Barrett R.J. Effects of chronic amphetamine or reserpine on self-stimulation responding: animal model of depression? Psychopharmacology. (Berl.) 1980;72:9–15. doi: 10.1007/BF00433801. [DOI] [PubMed] [Google Scholar]
- 25.Gaylord D., Curzon G. Test of emotional behavior in rats following depletion of norepinephrine, of serotonin, or both. Psychopharmacologie. 1972;34:275–288. doi: 10.1007/BF00422552. [DOI] [PubMed] [Google Scholar]
- 26.Ghia J.E., Blennerhassett P., Collins S.M. Vagus nerve integrity and experimental colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;293:G560–G567. doi: 10.1152/ajpgi.00098.2007. [DOI] [PubMed] [Google Scholar]
- 27.Mazelin L., Theodorou V., More J., Fioramonti J., Bueno L. Protective role of vagal afferents in experimentally-induced colitis in rats. J. Auton. Nerv. Syst. 1998;73:38–45. doi: 10.1016/S0165-1838(98)00113-1. [DOI] [PubMed] [Google Scholar]
- 28.van Westerloo D.J., et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology. 2006;130:1822–1830. doi: 10.1053/j.gastro.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson K., McHugh K., Collins S.M. Experimental colitis alters myenteric nerve function at inflamed and noninflamed sites in the rat. Gastroenterology. 1995;109:718–722. doi: 10.1016/0016-5085(95)90378-X. [DOI] [PubMed] [Google Scholar]
- 30.Gschossmann J.M., Mayer E.A., Miller J.C., Raybould H.E. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterol. Motil. 2002;14:403–408. doi: 10.1046/j.1365-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- 31.Pavlov V., Wang H., Czura C., Friedman S., Tracey K. The cholinergic anti-inflammatory pathway: a missing link in neuroimmunomodulation. Mol. Med. 2003;9:125–134. [PMC free article] [PubMed] [Google Scholar]
- 32.McClernon F.J., Hiott F.B., Westman E.C., Rose J.E., Levin E.D. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology. (Berl.) 2006;189:125–133. doi: 10.1007/s00213-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 33.Berlin G., Enerback L. Non-differential inhibition of histamine and serotonin release from mast cells by amitriptyline. Agents Actions. 1986;18:89–91. doi: 10.1007/BF01987991. [DOI] [PubMed] [Google Scholar]
- 34.Ferjan I., Erjavec F. Characteristics of the inhibitory effect of tricyclic antidepressants on histamine release from rat peritoneal mast cells. Inflamm. Res. 1996;45(Suppl. 1):S17–S18. doi: 10.1007/BF03354068. [DOI] [PubMed] [Google Scholar]
- 35.Gripenberg J. Incorporation of imipramine into isolated rat peritoneal mast cells in vitro. Acta. Physiol. Scand. 1974;92:56–65. doi: 10.1111/j.1748-1716.1974.tb05722.x. [DOI] [PubMed] [Google Scholar]
- 36.Sacerdote P., Bianchi M., Panerai A.E. In vivo and in vitro clomipramine treatment decreases the migration of macrophages in the rat. Eur. J. Pharmacol. 1997;319:287–290. doi: 10.1016/S0014-2999(96)00984-3. [DOI] [PubMed] [Google Scholar]
- 37.McCafferty D.M., Wallace J.L., Sharkey K.A. Effects of chemical sympathectomy and sensory nerve ablation on experimental colitis in the rat. Am. J. Physiol. 1997;272:G272–G280. doi: 10.1152/ajpgi.1997.272.2.G272. [DOI] [PubMed] [Google Scholar]
- 38.Antonica A., Ayroldi E., Magni F., Paolocci N. Lymphocyte traffic changes induced by monolateral vagal denervation in mouse thymus and peripheral lymphoid organs. J. Neuroimmunol. 1996;64:115–122. doi: 10.1016/0165-5728(95)00157-3. [DOI] [PubMed] [Google Scholar]
- 39.Gottwald T.P., Lhotak S., Stead R.H. Effects of subdiaphragmatic vagotomy on mucosal mast cell densities in stomach and jejunum of rats. Adv. Exp. Med. Biol. 1995;371A:303–306. doi: 10.1007/978-1-4615-1941-6_63. [DOI] [PubMed] [Google Scholar]
- 40.Dapoigny M., Cowles V.E., Zhu Y.R., Condon R.E. Vagal influence on colonic motor activity in conscious nonhuman primates. Am. J. Physiol. 1992;262:G231–G236. doi: 10.1152/ajpgi.1992.262.2.G231. [DOI] [PubMed] [Google Scholar]
- 41.Pahlin P.E., Kewenter J. The vagal control of the ileo-cecal sphincter in the cat. Acta. Physiol. Scand. 1976;96:433–442. doi: 10.1111/j.1748-1716.1976.tb10213.x. [DOI] [PubMed] [Google Scholar]
- 42.Gallowitsch-Puerta M., Pavlov V.A. Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 2007;80:2325–2329. doi: 10.1016/j.lfs.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang H., et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 44.Wang H., et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 45.Diamond A., Kenney C., Jankovic J. Effect of vagal nerve stimulation in a case of Tourette’s syndrome and complex partial epilepsy. Mov. Disord. 2006;21:1273–1275. doi: 10.1002/mds.20949. [DOI] [PubMed] [Google Scholar]
- 46.Rychlicki F., et al. Vagus nerve stimulation: clinical experience in drug-resistant pediatric epileptic patients. Seizure. 2006;15:483–490. doi: 10.1016/j.seizure.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Rosenwald A., Reusche E., Ogomori K., Teichert H.M. Comparison of silver stainings and immunohistology for the detection of neurofibrillary tangles and extracellular cerebral amyloid in paraffin sections. Acta. Neuropathol. (Berl.) 1993;86:182–186. doi: 10.1007/BF00334887. [DOI] [PubMed] [Google Scholar]
- 48.Lenard N.R., Roerig S.C. Development of antinociceptive tolerance and physical dependence following morphine i.c.v. infusion in mice. Eur. J. Pharmacol. 2005;527:71–76. doi: 10.1016/j.ejphar.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 49.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. (Berl.) 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigues A.L., et al. Involvement of monoaminergic system in the antidepressant-like effect of the hydroalcoholic extract of Siphocampylus verticillatus. Life Sci. 2002;70:1347–1358. doi: 10.1016/S0024-3205(01)01498-9. [DOI] [PubMed] [Google Scholar]
- 51.Sturiale S., et al. Neutral endopeptidase (EC 3.4.24.11) terminates colitis by degrading substance P. Proc. Natl. Acad. Sci. U. S. A. 1999;96:11653–11658. doi: 10.1073/pnas.96.20.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eliakim R., Karmeli F., Rachmilewitz D., Cohen P., Fich A. Effect of chronic nicotine administration on trinitrobenzene sulphonic acid-induced colitis. Eur. J. Gastroenterol. Hepatol. 1998;10:1013–1019. [PubMed] [Google Scholar]
- 53.Cooper H.S., Murthy S.N., Shah R.S., Sedergran D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 54.Appleyard C.B., Wallace J.L. Reactivation of hapten-induced colitis and its prevention by anti-inflammatory drugs. Am. J. Physiol. 1995;269:G119–G125. doi: 10.1152/ajpgi.1995.269.1.G119. [DOI] [PubMed] [Google Scholar]
- 55.Boughton-Smith N.K., Wallace J.L., Whittle B.J. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1988;25:115–123. doi: 10.1007/BF01969102. [DOI] [PubMed] [Google Scholar]
- 56.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]