Abstract
Viral proteins are not naturally selected for high affinity major histocompatibility complex (MHC) binding sequences; indeed, if there is any selection, it is likely to be negative in nature. Thus, one should be able to increase viral peptide binding to MHC in the rational design of synthetic peptide vaccines. The T1 helper peptide from the HIV-1 envelope protein was made more immunogenic for inducing T cell proliferation to the native sequence by replacing a residue that exerts an adverse influence on peptide binding to an MHC class II molecule. Mice immunized with vaccine constructs combining the more potent Th helper (Th) epitope with a cytotoxic T lymphocyte (CTL) determinant developed greatly enhanced CTL responses. Use of class II MHC-congenic mice confirmed that the enhancement of CTL response was due to class II-restricted help. Thus, enhanced T cell help is key for optimal induction of CTL, and, by modification of the native immunogen to increase binding to MHC, it is possible to develop second generation vaccine constructs that enhance both Th cell activation and CTL induction.
The paradigm that the best vaccine is one that most closely mimics natural infection applies to viruses that produce acute infection leading to long-lasting protective immunity. However, HIV, which produces chronic infection that is usually ultimately fatal, may not fit that paradigm. Moreover, it would not be expected that natural selection would favor viral sequences that are highly immunogenic. Thus, it might be possible to alter viral sequences to enhance immunogenicity (1, 2). This approach is supported by the recent finding that alteration of a residue in an HIV pol epitope increases binding to HLA-A2.1 and in vivo immunogenicity for induction of CD8+ cytotoxic T lymphocytes (CTL) (3), as well as by related findings in other viral or tumor systems (4–7). Precedent also exists for increased CD4+ helper T cell recognition by altered peptides from our own studies of an HIV envelope peptide (8) as well as from studies in other systems (9–11). We have called this approach of increasing major histocompatibility complex (MHC) binding without loss of recognition by virus-specific T cells “epitope enhancement” (2, 12, 13).
Our previous studies in mice showed that covalent linkage between a helper and a CTL epitope was important for optimal in vivo induction of CTL, especially in the absence of physical entrapment as in an adjuvant emulsion (14). Although broadly recognized natural and artificial helper epitopes have been identified (10, 15, 16), including ones from tetanus toxoid, to which most people are already immune (16), there is an advantage in using epitopes from HIV itself in that natural boosting can occur early after exposure whereas this might not be expected to occur if the helper epitopes are not present in the virus. Moreover, Th1 helper T cell responses specific for HIV have been suggested to contribute to protection, perhaps through production of cytokines (17), so induction of appropriate CD4+ helper T cell responses to HIV epitopes may be important for a vaccine independent of the other sources of help for antibody or CTL responses. We have previously identified helper epitope-containing cluster peptides from the HIV envelope that are broadly presented by both murine and human class II MHC molecules (18) and have incorporated these into vaccine constructs, PCLUS 3–18MN and 6.1–18MN, (14, 19, 20) that are now in clinical trials. One of these helper sequences, PCLUS 3, encompasses the first helper epitope identified in an HIV protein, the T1 peptide (KQIINMWQEVGKAMYA, residues 428–443 in the HIV-IIIB gp160 envelope protein) (21–23), that also has been used in a peptide vaccine construct, T1-SP10A, now in human clinical trials as well (24–28). Thus, enhancement of the activity of this peptide might be valuable in developing second generation vaccines. Linkage of such an enhanced helper epitope to a CTL epitope might generate a more efficacious vaccine for induction of both helper T cells and CTL.
To show proof of principle in a murine model, we took advantage of the findings that residues other than motif anchors (29) may exert a negative or adverse influence on peptide–MHC binding affinity (8, 30) and that we could identify a substitution in the T1 peptide itself that increased its binding to a murine class II MHC molecule without loss of recognition by T cells specific for the HIV sequence (8). Replacement of residue 436 (glutamic acid) with either alanine or glutamine produced two substituted peptides that were several logs more potent than wild-type peptide in stimulating a Th cell hybridoma specific for T1 in vitro. The wild-type glutamic acid has a fairly large side chain with a negative charge. Because activity was enhanced when this was replaced by the small, uncharged side chain of alanine, or uncharged glutamine, which is the same size as glutamic acid but not the smaller, negatively charged aspartic acid, it is likely that the negative charge was responsible for the adverse reaction. Competition studies with an unrelated peptide, cytochrome c peptide, which also binds to the same class II molecule I-Ek, showed that the alanine-substituted peptide competes much more effectively than the wild-type T1 peptide (8), indicating that higher affinity binding to the MHC molecule can account for most, if not all, of its greater potency. Thus, we have seen that removing an adverse reaction in the natural peptide sequence can actually lead to a more potent antigen. We now have asked whether this greater in vitro potency would translate into greater immunogenicity in vivo for induction of helper T cells and whether this would allow us to construct a more effective vaccine for induction of CTL also.
MATERIALS AND METHODS
Peptide Synthesis.
Multideterminant cluster peptides containing discrete overlapping T helper cell epitopes that elicit help in mice and humans of multiple MHC types were colinearly synthesized with P18IIIB, the major neutralizing antibody epitope located at the tip of the V3 loop and an immunodominant CTL epitope in mice and humans, to form a synthetic peptide vaccine construct. The constructs used for immunization were PCLUS 3–18IIIB, KQIINMWQEVGKAMYAPPISGQIRRIQRGPGRAFVTIGK [comprised of Th determinants from HIV-1 IIIB envelope, amino acid residues 421–444 according to numbering in the Los Alamos sequence database (31) linked to P18IIIB shown in bold, amino acid residues 308–322], PCLUS 3-I13, KQIINMWQEVGKAMYAPPISGQIRRIQRGPGRAFVTI, PCLUS 3(A)-18IIIB, KQIINMWQAVGKAMYAPPISGQIRRIQRGPGRAFVTIGK, and PCLUS 3 (A)-I13, KQIINMWQAVGKAMYAPPISGQIRRIQRGPGRAFVTI. Peptides were synthesized on an automated peptide synthesizer (model 430A; Applied Biosystems) using t-boc chemistry as described (32). The peptides were cleaved from the resin with HF and initially purified by size exclusion chromatography (P4 Biogel; Bio-Rad). Purification to single peaks was achieved by reverse-phase HPLC on μbondapack reverse-phase C18 analytical and preparative columns (Waters). PCLUS 3–18IIIB and PCLUS 3(A)-I13 were synthesized and purified by Peninsula Laboratories and were single peaks on HPLC in two solvent systems as well as by mass spectrometry.
Adjuvants.
N-[1-(2, 3-dioleoyoxy) propyl]-N, N,N-trimethylammonium methyl sulfate (DOTAP; Boehringer Mannheim) was selected as the best adjuvant for eliciting CTL responses to vaccine constructs (20, 33) whereas complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant were used as adjuvants to examine lymph node proliferation to individual helper peptides and to develop a T1-CD4+ T helper cell line, respectively.
Generation of T Helper Line.
B10.BR congenic mice (H-2k) were immunized s.c. at the base of the tail with 20 nmol of T1 peptide in incomplete Freund’s adjuvant. After two immunizations, CD4+ spleen cells were positively selected using magnetic beads coated with anti-CD4 (Dynal, Great Neck, NY) and a CD4+ Th cell line maintained by stimulation with irradiated syngeneic spleen cells and 1 μM T1 peptide. Proliferation was assayed by measuring incorporation of [3H]thymidine in triplicate cultures of 3 × 104 Th cells and 5 × 105 irradiated syngeneic spleen cells/well in 96-well flat bottom plates stimulated with either T1 peptide or the modified peptide T1(A) 436 and harvested after 96 h of incubation.
Proliferation Assay.
B10.BR mice were immunized s.c. at the base of the tail with 0.6, 0.06, and 0.006 nmol of T1 or T1(A) 436 peptide in CFA. Ten days later, draining lymph nodes pooled from two mice were obtained and stimulated in vitro with 20 μM T1 peptide. Proliferation was assayed by measuring incorporation of [3H]thymidine in triplicate lymph node cultures of 5 × 105 per well in 96-well flat bottom plates. Results are presented as the percentage purified protein derivative of tuberculin (PPD; a component of CFA) response to normalize variability between animals.
Cytotoxicity Assay.
A.AL (I-Ek, Dd) and A.TH (I-As, Dd) mice were immunized s.c. at the base of the tail with vaccine constructs in the adjuvant DOTAP. Aqueous peptide solution was mixed with DOTAP (20 μg per mouse). Fourteen days later, spleen cell cultures pooled from two mice were stimulated in vitro with 0.5 μM peptide P18IIIB. 10% Rat T Stim (Collaborative Research) was added as a source of T cell growth factors 12 h poststimulation, and effectors were assayed for CTL 6–7 days later. Cytolytic activity of in vitro-stimulated secondary CTL was measured as described (14) using a 6-h assay with 51Cr-labeled peptide-pulsed targets. The percentage of specific 51Cr release was calculated as 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release). Spontaneous release was determined from target cells incubated without added effector cells and was <10% in all experiments. The transfected fibroblast line 18 neo (H-2d; class I MHC+, class II MHC− BALB/c 3T3 fibroblast transfected with the neomycin resistance gene, a gift of Ronald Germain, National Institute of Allergy and Infectious Diseases) was used as a target. The assay was performed in triplicate, with 5000 target cells/well. CTL data were found to be normally distributed based on the Shapiro–Wilk test, and means were plotted as arithmetic means ± SEM. Error bars smaller than the symbols could not be shown. Lysis of target cells without peptide was <10%. A two-way ANOVA was applied to the CTL lysis data, thereby averaging the respective values for each curve over all of the effector-to-target ratios. Means for each treatment were compared using a Tukey multiple comparison test as described (34). Alternatively, when data are represented as percentage-specific lysis vs. effector-to-target ratio in a titration of effector cell numbers, the number of lymphocytes required to achieve a given level of lysis can be determined. The ratio of these numbers for titration curves of two different effector populations gives the inverse ratio of frequencies of specific effectors in these populations. These values are referred to as relative numbers of lytic units at a given percentage of lysis. Statistical analysis was done using the jmp Statistical Software Program (SAS Institute, Cary, NC) on a Macintosh computer (Apple Computer, Cupertino, CA).
RESULTS AND DISCUSSION
To determine if the enhanced in vitro activity of the Ala-substituted T1 peptide would be effective with a polyclonal Th line specific for T1, a CD4+ T cell line was developed from B10.BR mice immunized with the wild-type peptide in incomplete Freund’s adjuvant. The response to the substituted peptide T1(A) 436 was equal to the native T1 sequence at the highest dose of peptide tested, but the substituted peptide was active at a >10-fold lower concentration of peptide than the native peptide for stimulating a comparable level of proliferation (using as a threshold for activity to elicit proliferation a response four times over the background of 250 cpm, i.e., 1000 cpm; Fig. 1A).
Figure 1.
Modification of a T cell helper epitope creates a more potent peptide than the native sequence. (A) Dose–response curves of CD4+ T cell line derived from B10.BR mice (expressing the class II molecule I-Ek) immunized with native T1 peptide and stimulated in vitro with native T1 peptide (○) and modified T1(A)436 (•). The background incorporation in the absence of antigen was 250 cpm. (B) Substituted peptide 436 Ala is immunogenic at lower doses than wild-type T1 peptide for induction of T cell responses to T1. Proliferation normalized to the PPD response of draining lymph node cell cultures from B10.BR mice immunized with T1(solid bars) and T1(A)436 (hatched bars) in CFA and stimulated in vitro with 20 μM native T1 peptide. The range of PPD responses was 32–50,000 cpm, the range of background incorporation was 5500–8000 cpm for the six immunization groups, and the PPD response was always 4- to 7-fold above the background. Comparable results were obtained in three (A) and two (B) independent experiments.
To determine whether this modification would make a more potent vaccine in vivo, i.e., whether we could immunize with the more potent substituted peptide and induce a more effective immune response against the natural HIV sequence in H-2k mice, which have the relevant class II molecule I-Ek, we immunized with different doses of either the wild-type peptide or the Ala-substituted peptide in CFA, and proliferative responses of draining lymph node T cells against the natural HIV T1 peptide were measured. Thymidine incorporation in response to peptide was normalized to PPD response (a mycobacterial antigen present in CFA) to control for mouse-to-mouse variability. At the highest dose used for immunization, the substituted peptide induced a higher response than T1 (Fig. 1B). At a 10-fold lower dose for immunization, the wild-type T1 peptide no longer appeared to be immunogenic, but the substituted peptide was still eliciting a response higher than that elicited by the wild-type peptide at a 10-fold higher dose. Even at a 100-fold lower dose, we still obtained some response to immunization with the substituted peptide. Thus, if we can immunize with a more potent peptide to induce the immune response, that immune response may now be effective against the wild-type viral sequence, even though the wild-type sequence itself is less effective at inducing an immune response. Indeed, subdominant epitopes could potentially be made dominant.
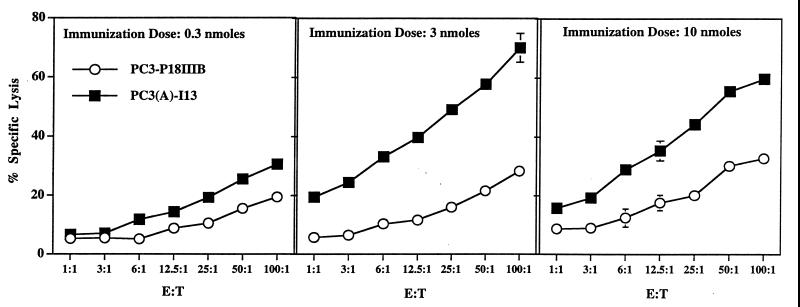
To see whether the enhanced Th determinant would provide greater help for induction of CTL, vaccine constructs were made in which the more potent helper peptide was linked to a CTL epitope, P18IIIB. As Th epitope, we used the slightly longer PCLUS 3 multideterminant cluster peptide containing T1 because this cluster peptide was used to broaden reactivity with diverse human and mouse MHC molecules for our peptide vaccine studies (14, 18–20), and this construct, PCLUS 3–18MN, is now in a phase I clinical trial. In addition, the CTL epitope used in this study, P18IIIB, RIQRGPGRAFVTIGK, was truncated by two residues to a 13-mer P18-I13, RIQRGPGRAFVTI, to eliminate a serum processing requirement found necessary to generate the minimal epitope (35–37). Previous work with multideterminant cluster–peptide vaccine constructs has shown a strict requirement for CD4+ cell help in production of neutralizing antibody and the induction of CTL (14, 19, 20). The second generation vaccine construct using the modified enhanced Th and CTL epitopes was more effective at inducing a CTL response than the native construct at all doses tested (Fig. 2). At the 3-nmol dose, the second generation construct induced >17-fold more lytic units (defined at 25% specific lysis). Furthermore, the modified construct was effective at the lowest dose tested whereas the native construct at this dose barely induced a CTL response.
Figure 2.
Sequence modification increases immunogenicity for CTL induction in vivo. CTL response of A.AL mice after a single immunization in the adjuvant DOTAP with increasing doses of native vaccine construct PCLUS 3–18IIIB (○) and second generation construct PCLUS 3 (A-436)-I13 (▪) to P18IIIB-pulsed BALB/c 3T3 fibroblast target cells. Mean specific 51Cr release of triplicate cultures ± SEM are plotted vs. effector-to-target cell ratio. Error bars not visible are smaller than the symbols. Lysis of fibroblast target cells without peptide was <15%. The CTL response of animals immunized with the second generation construct PC3(A)I13 was significantly different from that of animals immunized with the native construct P3–18IIIB at an immunization dose of 3 and 10 nmol (P < 0.05, Tukey–Kramer HSD). The second generation construct elicited >17-fold more lytic units at the 3-nmol dose when compared with the native construct at 25% specific lysis. Comparable results were obtained in three independent experiments.
Although we have seen in three previous studies (14, 20, 38) that covalent linkage of a helper epitope to the CTL epitope enhances immunogenicity for induction of CTL, compared with mixtures of the helper and CTL epitopes, we wanted to confirm the same was true in the case of the modified helper epitope. As shown in Fig. 3, the minimal CTL epitope peptide, I10, is not immunogenic at all in the absence of a helper epitope. Addition of the unmodified helper epitope T1 (not covalently linked) had only a marginal effect whereas admixture of the modified helper epitope T1(A)436 resulted in substantial CTL activity. For both the unmodified and the modified helper epitopes, covalent linkage to the CTL epitope resulted in significantly better CTL induction than just mixing the corresponding pairs of peptides. We conclude that the enhanced activity of the modified helper epitope is mediated more effectively if it is covalently linked to the CTL epitope but that the activity is so much greater that it is even effective to some extent when noncovalently mixed with the CTL epitope peptide.
Figure 3.
Covalent linkage of the Th and CTL epitopes is required for optimum CTL induction. A.AL mice were immunized with 20 nmol of peptide in the adjuvant DOTAP. Animals were immunized with either the minimum CTL epitope I10, RGPGRAFVTI, a mixture of helper and CTL epitopes T1 plus I10, a mixture of modified helper epitope T1(A) 436 plus I10, covalently linked helper and CTL epitope, PCLUS 3–18IIIB, or PCLUS 3 (A)436-I13. Fourteen days after a single s.c. immunization, bulk spleen cells were stimulated in vitro with 0.5 μM P18IIIB, and 10% Rat T Stim was added 24 h later as a source of interleukin 2. CTL activity was tested 7 days later in a 51Cr release assay at an effector-to-target ratio of 12.5:1 against 0.5 μM P18IIIB-pulsed 18 neo target cells.
Because a change in the CTL epitope was made in addition to modification of the helper epitope, it was necessary to determine the relative effects of the modified CTL epitope and modified Th epitope in enhancement of CTL induction. We addressed this question by two approaches, one using the genetics of MHC presentation and one involving the synthesis of additional peptides to cover all combinations of helper and CTL epitopes.
First, to test genetically whether the enhanced induction of CTL was linked to the MHC class II molecule to which the modified T1 peptide binds with higher affinity, we immunized congenic mice expressing the different class II molecules I-Ek and I-As, but with the same class I molecule Dd. Both I-Ek and I-As present T1 and PCLUS 3, but only I-Ek, not I-As, binds the Ala-substituted peptide with higher affinity (8, 21, 39). The CTL response to P18IIIB-pulsed targets was significantly better in A.AL mice expressing the relevant I-Ek class II allele (P < 0.05, Tukey–Kramer HSD test) whereas no difference in CTL induction was found in A.TH mice expressing I-As (Fig. 4). Thus, enhanced activity of the second generation vaccine construct depends primarily on more effective binding of the Th epitope to the class II MHC molecule. These results also emphasize the importance of strong class II MHC-restricted T cell help in optimally inducing class I MHC-restricted CTL.
Figure 4.
Enhancement of T cell help for CTL induction is MHC-linked. CTL response of A.AL (I-Ek, Dd) and A.TH (I-As, Dd) mice to P18IIIB-pulsed target cells. Mice were immunized with 16 nmol of native HIV-1 vaccine construct P3–18IIIB (○) and second generation construct PC3(A)I13 (•) in the adjuvant DOTAP. Spleen cells from two mice of each group were pooled 14 days after a single immunization and stimulated in vitro with 0.5 μM P18IIIB-pulsed spleen cells. Specific lysis of 51Cr-labeled 3T3 fibroblast target cells was measured 7 days after in vitro stimulation. Means of triplicate cultures at each effector-to-target cell ratio ± SEM are shown. Nonspecific lysis of unpulsed targets was <10%. The CTL response of animals immunized with the second generation construct PC3(A)I13 was significantly different from that of animals immunized with the native construct P3–18IIIB in A.AL mice expressing the class II molecule I-Ek (P < 0.05). Comparable results were obtained in three independent experiments.
Second, to examine all possible combinations of the original and modified helper and CTL epitopes in vaccine constructs, we made two new constructs, one containing the native helper epitope and truncated I13 CTL determinant and the other with the PC3(A) 436 substitution only and the full length 15-residue P18IIIB CTL determinant. All four combinations were then compared side-by-side to see which modification contributed the most to the overall enhancement. Enhancement for CTL induction was primarily due to modification of the helper epitope because both constructs containing PC3(A) 436 were significantly better than those using the native helper epitope PC3-P18IIIB and PC3-I13 (P < 0.05; Fig. 5). Induction of CTL did not decrease further upon decreasing the peptide dose 3-fold with the second generation constructs whereas both constructs containing the native T1 helper sequence elicited a lower CTL response at the lower dose. At the 5-nmol dose, PC3(A)-18IIIB elicited over 33-fold more lytic units than did PC3–18IIIB. Truncation of the CTL epitope alone (I13) did not produce a response significantly different from that of the construct containing the full length CTL epitope (P18IIIB) although we have frequently observed a slight enhancement of the CTL response with the truncated PC3-I13 construct compared with PC3-P18IIIB. Therefore, enhanced CTL are elicited at a lower immunization dose when the enhanced helper epitope is included in the construct, and this effect is observed over a 10-fold range in peptide concentration. We conclude from both the genetic and the peptide synthesis experiments that increasing class II MHC-restricted T cell help by modifying the helper epitope is the predominant mechanism by which the second generation vaccine constructs more effectively elicit CTL and, as a corollary, that the level of CD4+ T cell help is an important factor in the induction of CTL in vivo.
Figure 5.
Epitope enhancement for CTL induction is primarily due to modification of the T helper epitope. CTL response of A.AL mice immunized with 5 and 15 nmol of native construct P3–18IIIB (○), P3-I13 [comprised of the native helper epitope and the truncated CTL epitope I13 (□)], PC3(A)-18IIIB [comprised of the modified T helper epitope and full length CTL determinant P18IIIB (▴)], and PC3(A)-I13 [containing both the modified helper epitope and truncated CTL determinant I13 (⧫)]. Nonspecific lysis of fibroblast targets is indicated by dashed lines. Error bars representing SEM are visible only when greater than the size of the symbol. Animals immunized with constructs containing the modified multideterminant helper peptide PC3(A) had significantly greater specific lysis of P18IIIB-pulsed fibroblast target cells at both immunization doses than animals immunized with vaccine constructs containing the native multideterminant helper peptide (P < 0.05). Comparable results were obtained in two independent experiments.
In a previous publication (8), we showed that the modified T1 (A) 436 peptide had a higher affinity for binding to I-Ek than the wild-type T1 peptide. Here we have shown that the modified peptide was more immunogenic in vivo in mice expressing I-Ek for induction of T cell proliferation and that a vaccine construct containing this modified helper epitope was more immunogenic for induction of CTL than the corresponding wild-type construct. The correlation between increased affinity for MHC and increased immunogenicity in vivo does not in itself prove cause and effect. However, we also show here that the immunogenicity increase is genetically linked to the presence of the appropriate class II MHC molecule, implying that the increase depends on the interaction with the class II MHC molecule, rather than some nonspecific effect such as a difference in stability in vivo. In addition, although stability in vivo cannot be assessed directly, we have examined functional stability in undiluted mouse serum at 37°C using the proliferative response of a T1-specific CD4+ T cell line as a measure of functionally active peptide. For the duration of the experiment (13 h), T1 was remarkably stable, and T1 (A) 436 was, if anything, slightly less stable. Thus, the greater activity of the T1 (A) 436 peptide cannot be attributed to greater stability and, based on the genetic experiments, must involve interaction with the class II MHC molecule. Therefore, although more complicated explanations might be devised, the most straightforward interpretation of all of the data is that the increase in immunogenicity is a direct result of the measured increase in affinity for the class II MHC molecule.
A single amino acid change in a peptide residue involved in binding the MHC molecule, i.e., an agretopic residue, may lead to enhanced binding and increased immunogenicity (3, 5). Indeed, heteroclitic T cell responses have been demonstrated by modification of peptide antigens that result in an increased affinity for MHC molecules or for the T cell receptor (4, 9, 11). Non agretopic residues, not involved in binding MHC, may also influence binding by directly inhibiting interactions with the MHC or by imposing a conformational constraint on the peptide’s ability to bind MHC. In addition, flanking residues, outside the minimal epitope presented by the MHC, may adversely influence processing and underscore the importance of defining minimal epitopes in peptide-based vaccine design (40, 41). Mutations in the MHC molecule that affect amino acid residues lining the peptide binding groove may also influence peptide binding in an allele-specific manner or the interaction of T cell antigen receptor with the peptide–MHC complex (42). Also, we have shown that the potential to elicit more cross-reactive CTL and increase the breadth of the CTL response may be achieved by stimulating CTL primed in vivo with a peptide that contains a single amino acid substitution that differs in chemical structure from the key native T cell antigen receptor contact residue (43). Conversely, substitutions made in an amino acid residue that can positively interact with the T cell antigen receptor, i.e., epitopic residues, may lead to nonrecognition by the T cell, such as seen in escape mutants, inhibition of the T cell response (antagonism), or an altered pattern of cytokine secretion (44–48). Thus, antagonism, which can also result from other alterations in the peptide-MHC complex (49, 50), must be avoided in making modified immunogens.
Induction of CTL has been shown to require a higher density of peptide–MHC complexes on stimulatory cells than is required for susceptibility of a target to lysis (51). Such higher density could be achieved by enhanced affinity for MHC, using sequence modifications such as that illustrated here. Thus, this epitope enhancement approach might allow induction of CTL responses to naturally subdominant epitopes, which may not have elicited escape mutants. Furthermore, in the case of tumor vaccines, this approach could make immunogenic epitopes that, in native form, would be too weak to be tolerogenic. Thus, tolerogenicity of more dominant tumor antigens in bulky tumors could be circumvented.
Although these precise second generation vaccine constructs were designed to be more effective in mice, not humans, they demonstrate proof of principle that this sequence modification approach to epitope enhancement can lead to more potent peptide vaccines. Other modifications that enhance binding to human HLA molecules must now be determined to apply this concept to human vaccines. The unmodified helper peptides PCLUS 3 and PCLUS 6.1 have been found to be immunogenic in humans, based on preliminary results of a clinical trial (52), but they could potentially be made even more effective by this approach. In addition, we would like to test this type of approach in nonhuman primates, in which protective efficacy could be assessed. Furthermore, the approach is not limited to peptide vaccines because similar modifications that enhance MHC binding without altering T cell recognition could in principle be applied to recombinant viral or bacterial subunit vaccines or recombinant viral or bacterial vectors expressing such recombinant proteins. Even live attenuated virus vaccines could be similarly enhanced.
Acknowledgments
We thank Drs. Ronald Germain and Jonathan Yewdell for critical reading of the manuscript and very helpful suggestions. Dr. Ronald Germain contributed to the early planning of these studies.
ABBREVIATIONS
- CTL
cytotoxic T lymphocyte
- MHC
major histocompatibility complex
- DOTAP
N-[1-(2, 3-dioleoyoxy) propyl]-N, N,N-trimethylammonium methyl sulfate
- PPD
purified protein derivative of tuberculin
- Th
T helper cell
- CFA
complete Freund’s adjuvant
References
- 1.Berzofsky J A. Ann NY Acad Sci. 1993;690:256–264. doi: 10.1111/j.1749-6632.1993.tb44014.x. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky J A. Ann NY Acad Sci. 1995;754:161–168. doi: 10.1111/j.1749-6632.1995.tb44449.x. [DOI] [PubMed] [Google Scholar]
- 3.Pogue R R, Eron J, Frelinger J A, Matsui M. Proc Natl Acad Sci USA. 1995;92:8166–8170. doi: 10.1073/pnas.92.18.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodmer H C, Pemberton R M, Rothbard J B, Askonas B A. Cell. 1988;52:253–258. doi: 10.1016/0092-8674(88)90514-4. [DOI] [PubMed] [Google Scholar]
- 5.Lipford G B, Bauer S, Wagner H, Heeg K. Vaccine. 1995;13:313–320. doi: 10.1016/0264-410x(95)93320-9. [DOI] [PubMed] [Google Scholar]
- 6.Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast W M, Melief C J M, Oseroff C, Yuan L, Ruppert J, Sidney J, del Guercio M-F, Southwood S, Kubo R T, Chesnut R W, Grey H M, Chisari F V. J Immunol. 1994;153:5586–5592. [PubMed] [Google Scholar]
- 7.Parkhurst M R, Salgaller M L, Southwood S, Robbins P F, Sette A, Rosenberg S A, Kawakami Y. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 8.Boehncke W-H, Takeshita T, Pendleton C D, Sadegh-Nasseri S, Racioppi L, Houghten R A, Berzofsky J A, Germain R N. J Immunol. 1993;150:331–341. [PubMed] [Google Scholar]
- 9.Schwartz R H, Fox B S, Fraga E, Chen C, Singh B. J Immunol. 1985;135:2598–2608. [PubMed] [Google Scholar]
- 10.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra H M, Kubo R T, Sette A, Grey H M. Immunity. 1994;1:751–761. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 11.England R E, Kullberg M C, Cornette J L, Berzofsky J A. J Immunol. 1995;155:4295–4306. [PubMed] [Google Scholar]
- 12.Cease K B, Berzofsky J A. Annu Rev Immunol. 1994;12:923–989. doi: 10.1146/annurev.iy.12.040194.004423. [DOI] [PubMed] [Google Scholar]
- 13.Berzofsky, J. A. & Berkower, I. J. (1995) AIDS 9, Suppl., S143–S157. [PubMed]
- 14.Shirai M, Pendleton C D, Ahlers J, Takeshita T, Newman M, Berzofsky J A. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 15.Sinigaglia F, Guttinger M, Kilgus J, Doran D M, Matile H, Etlinger H, Trzeciak A, Gillessen D, Pink J R L. Nature (London) 1988;336:778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- 16.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Eur J Immunol. 1989;19:2237–2242. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 17.Clerici M, Shearer G M. Immunol Today. 1994;15:575–581. doi: 10.1016/0167-5699(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 18.Berzofsky J A, Pendleton C D, Clerici M, Ahlers J, Lucey D R, Putney S D, Shearer G M. J Clin Invest. 1991;88:876–884. doi: 10.1172/JCI115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlers J D, Pendleton C D, Dunlop N, Minassian A, Nara P L, Berzofsky J A. J Immunol. 1993;150:5647–5665. [PubMed] [Google Scholar]
- 20.Ahlers J D, Dunlop N, Pendleton C D, Newman M, Nara P L, Berzofsky J A. AIDS Res Hum Retroviruses. 1996;12:259–272. doi: 10.1089/aid.1996.12.259. [DOI] [PubMed] [Google Scholar]
- 21.Cease K B, Margalit H, Cornette J L, Putney S D, Robey W G, Ouyang C, Streicher H Z, Fischinger P J, Gallo R C, DeLisi C, Berzofsky J A. Proc Natl Acad Sci USA. 1987;84:4249–4253. doi: 10.1073/pnas.84.12.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berzofsky J A, Bensussan A, Cease K B, Bourge J F, Cheynier R, Lurhuma Z, Salaün J-J, Gallo R C, Shearer G M, Zagury D. Nature (London) 1988;334:706–708. doi: 10.1038/334706a0. [DOI] [PubMed] [Google Scholar]
- 23.Clerici M, Stocks N I, Zajac R A, Boswell R N, Bernstein D C, Mann D L, Shearer G M, Berzofsky J A. Nature (London) 1989;339:383–385. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- 24.Palker T J, Matthews T J, Langlois A, Tanner M E, Martin M E, Scearce R M, Kim J E, Berzofsky J A, Bolognesi D P, Haynes B F. J Immunol. 1989;142:3612–3619. [PubMed] [Google Scholar]
- 25.Haynes B F, Torres J V, Langlois A J, Bolognesi D P, Gardner M B, Palker T J, Scearce R M, Jones D M, Moody M A, McDanal C, Matthews T J. J Immunol. 1993;151:1646–1653. [PubMed] [Google Scholar]
- 26.Hart M K, Weinhold K J, Scearce R M, Washburn E M, Clark C A, Palker T J, Haynes B F. Proc Natl Acad Sci USA. 1991;88:9448–9452. doi: 10.1073/pnas.88.21.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasutomi Y, Palker T J, Gardner M B, Haynes B F, Letvin N L. J Immunol. 1993;151:5096–5105. [PubMed] [Google Scholar]
- 28.Haynes B F, Moody M A, Heinley C S, Korber B, Millard W A, Scearce R M. AIDS Res Hum Retroviruses. 1995;11:211–221. doi: 10.1089/aid.1995.11.211. [DOI] [PubMed] [Google Scholar]
- 29.Rammensee H-G, Friede T, Stevanovíc S. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 30.Ruppert J, Sidney J, Celis E, Kubo R T, Grey H M, Sette A. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 31.Myers G, Josephs S F, Berzofsky J A, Rabson A B, Smith T F, Wong-Staal F. Human Retroviruses and AIDS, 1989. Los Alamos, NM: Los Alamos National Laboratory; 1989. [Google Scholar]
- 32.Stewart J M, Young J D. Solid Phase Peptide Synthesis. Rockford, IL: Pierce Chemical Co.; 1984. [Google Scholar]
- 33.Walker C, Selby M, Erickson A, Cataldo D, Valensi J-P, Van Nest G. Proc Natl Acad Sci USA. 1992;89:7915–7918. doi: 10.1073/pnas.89.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahlers J D, Dunlop N, Alling D W, Nara P L, Berzofsky J A. J Immunol. 1997;158:3947–3958. [PubMed] [Google Scholar]
- 35.Kozlowski S, Corr M, Takeshita T, Boyd L F, Pendleton C D, Germain R N, Berzofsky J A, Margulies D H. J Exp Med. 1992;175:1417–1422. doi: 10.1084/jem.175.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirai M, Pendleton C D, Berzofsky J A. J Immunol. 1992;148:1657–1667. [PubMed] [Google Scholar]
- 37.Takeshita T, Takahashi H, Kozlowski S, Ahlers J D, Pendleton C D, Moore R L, Nakagawa Y, Yokomuro K, Fox B S, Margulies D H, Berzofsky J A. J Immunol. 1995;154:1973–1986. [PubMed] [Google Scholar]
- 38.Shirai M, Chen M, Arichi T, Nishioka M, Newman M, Nakazawa T, Feinstone S M, Berzofsky J A. J Infect Dis. 1996;173:24–31. doi: 10.1093/infdis/173.1.24. [DOI] [PubMed] [Google Scholar]
- 39.Hale P M, Cease K B, Houghten R A, Ouyang C, Putney S, Javaherian K, Margalit H, Cornette J L, Spouge J L, DeLisi C, Berzofsky J A. Int Immunol. 1989;1:409–415. doi: 10.1093/intimm/1.4.409. [DOI] [PubMed] [Google Scholar]
- 40.Manca F, Habeshaw J A, Dalgleish A G, Fenoglio D, Li Pira G, Sercarz E E. Eur J Immunol. 1993;23:269–274. doi: 10.1002/eji.1830230142. [DOI] [PubMed] [Google Scholar]
- 41.Eisenlohr L C, Yewdell J W, Bennink J R. J Exp Med. 1992;175:481–487. doi: 10.1084/jem.175.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller D, Pederson K, Murray R, Frelinger J A. J Immunol. 1991;147:1392–1397. [PubMed] [Google Scholar]
- 43.Takahashi H, Nakagawa Y, Pendleton C D, Houghten R A, Yokomuro K, Germain R N, Berzofsky J A. Science. 1992;255:333–336. doi: 10.1126/science.1372448. [DOI] [PubMed] [Google Scholar]
- 44.Klenerman P, Rowland-Jones S, McAdam S, Edwards J, Daenke S, Lalloo D, Köppe B, Rosenberg W, Boyd D, Edwards A, Giangrande P, Phillips R E, McMichael A J. Nature (London) 1994;369:403–407. doi: 10.1038/369403a0. [DOI] [PubMed] [Google Scholar]
- 45.Sloan-Lancaster J, Evavold B D, Allen P M. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 46.Sloan-Lancaster J, Shaw A S, Rothbard J B, Allen P M. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Evavold B D, Allen P M. Science. 1991;252:1308–1310. [PubMed] [Google Scholar]
- 49.Racioppi L, Ronchese F, Matis L A, Germain R N. J Exp Med. 1993;177:1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madrenas J, Wange R L, Wang J L, Isakov N, Samelson L E, Germain R N. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 51.Alexander M A, Damico C A, Wieties K M, Hansen T H, Connolly J M. J Exp Med. 1991;173:849–858. doi: 10.1084/jem.173.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Humphrey, R. W., Pinto, L. A., Fowke, K., Merced-Galindez, F., Ahlers, J. D., Little, R., Dunlop, N., Pendleton, C. D., Kohler, D., Nara, P., Shearer, G. M., Berzofsky, J. A. & Yarchoan, R. (1997) AIDS Res. Hum. Retroviruses, in press (abstr.). [DOI] [PubMed]