Abstract
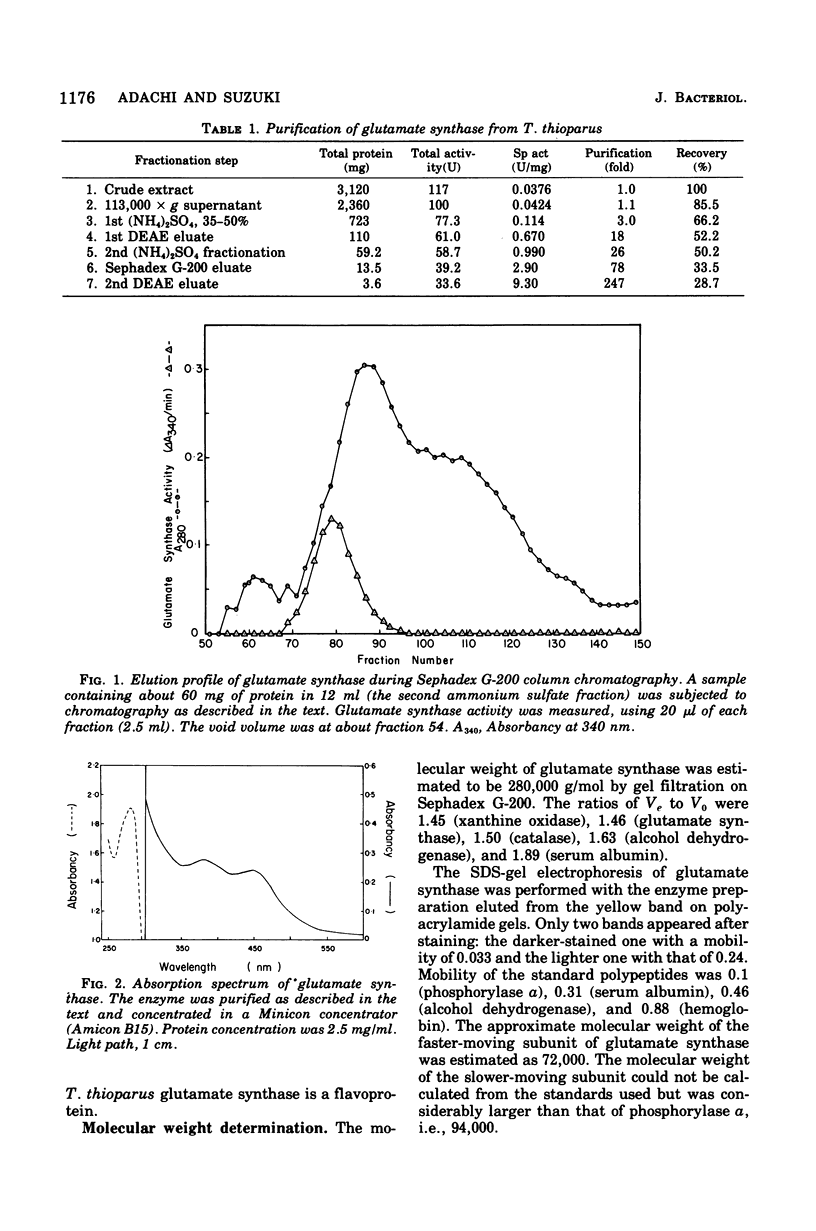
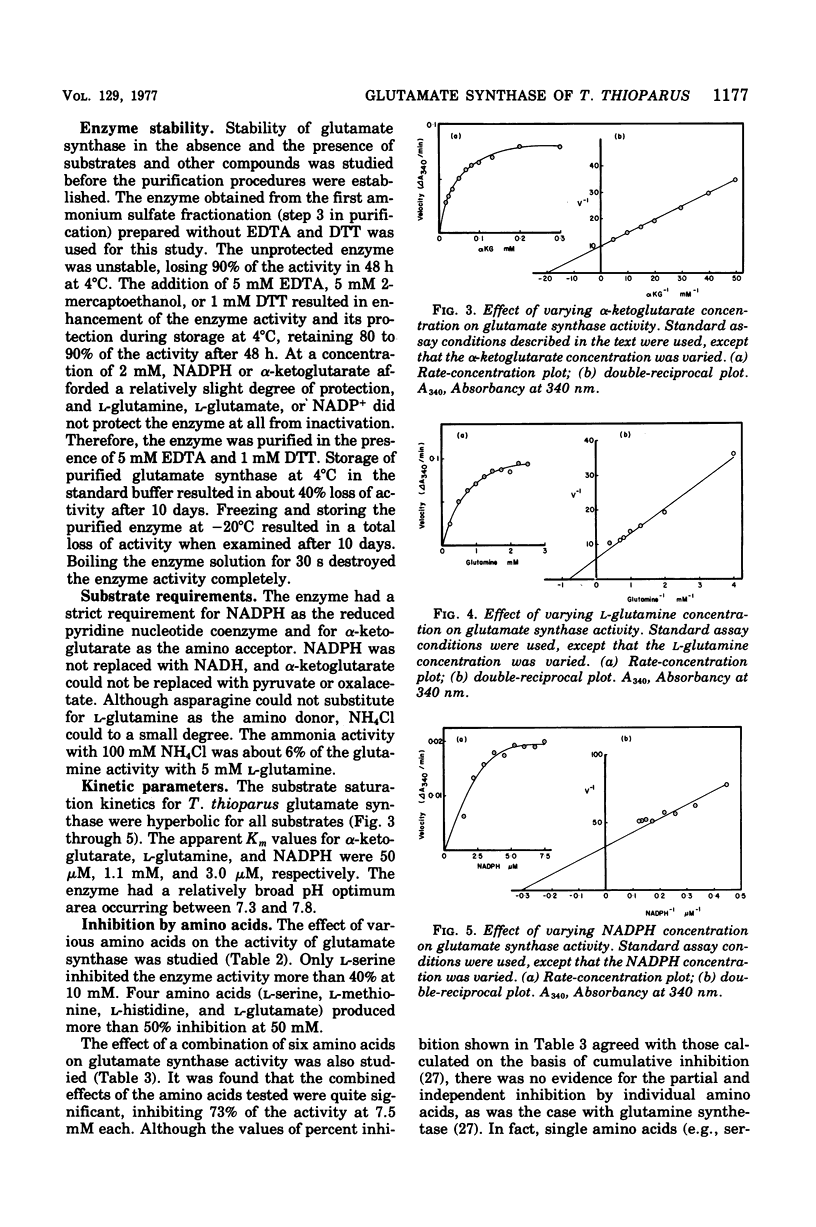
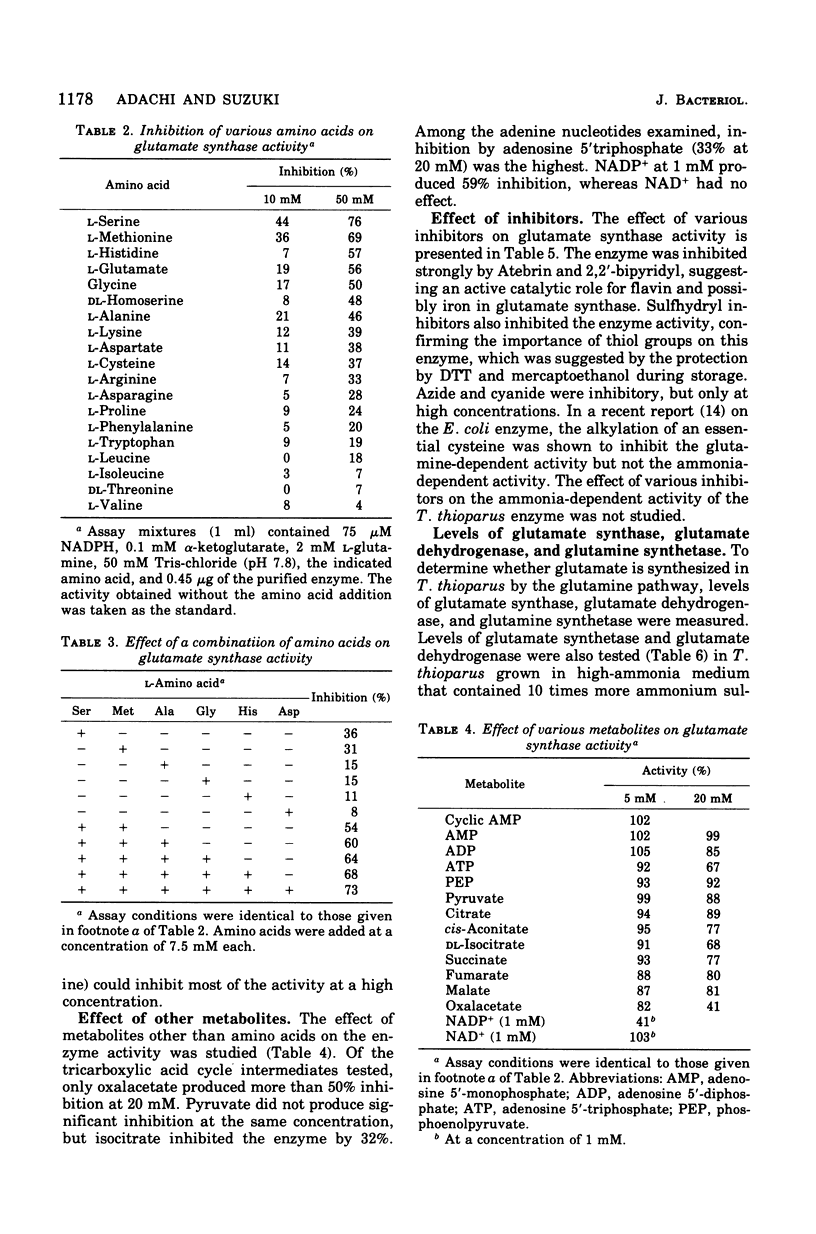
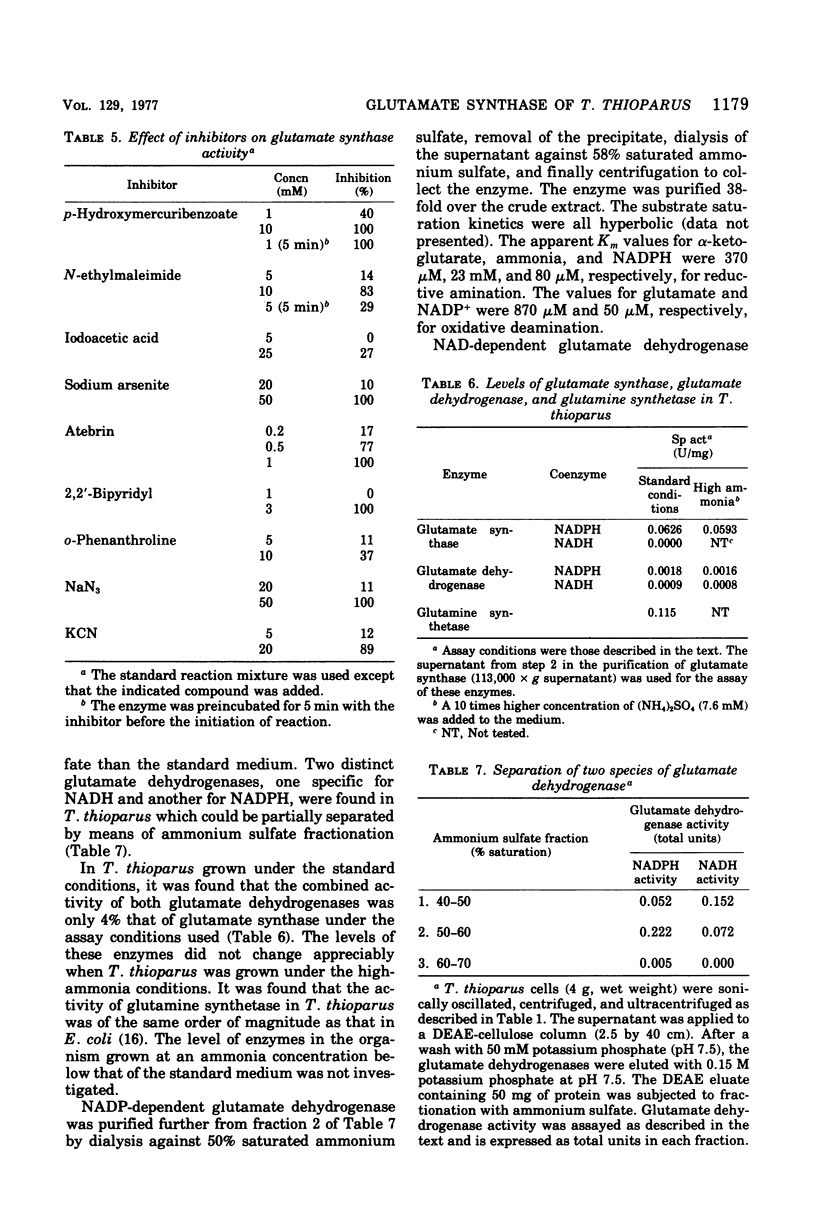
Glutamate synthase was purified about 250-fold from Thiobacillus thioparus and was characterized. The molecular weight was estimated as 280,000 g/mol. The enzyme showed absorption maxima at 280, 380, and 450 nm and was inhibited by Atebrin, suggesting that T. thioparus glutamate synthase is a flavoprotein. The enzyme activity was also inhibited by iron chelators and thiolbinding agents. The enzyme was specific for reduced nicotinamide adenine dinucleotide phosphate (NADPH) and alpha-ketoglutarate, but L-glutamine was partially replaced by ammonia as the amino donor. The Km values of glutamate synthase for NADPH, alpha-ketoglutarate, and glutamine were 3.0 muM, 50 muM, and 1.1 mM, respectively. The enzyme had a pH optimum between 7.3 and 7.8. Glutamate synthase from T. thioparus was relatively insensitive to feedback inhibition by single amino acids but was sensitive to the combined effects of several amino acids. Enzymes involved in glutamate synthesis in T. thioparus were studied. Glutamine synthetase and glutamate synthase, as well as two glutamate dehydrogenases (NADH and NADPH dependent), were present in this organism. This levels of glutamate synthase and glutamate dehydrogenase were similar in T. thioparus grown on 0.7 or 7.0 mM ammonium sulfate. The sum of the activities of both glutamate dehydrogenases was only 1/25 of that of glutamate synthase under the assay conditions. It was concluded that the glutamine pathway is important for ammonia assimilation in this autotrophic bacterium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. J., Jeng I., Barker H. A. Purification and properties of L-erythro-3,5-diaminohexanoate dehydrogenase from a lysine-fermenting Clostridium. J Biol Chem. 1972 Dec 10;247(23):7724–7734. [PubMed] [Google Scholar]
- Brenchley J. E., Baker C. A., Patil L. G. Regulation of the ammonia assimilatory enzymes in Salmonella typhimurium. J Bacteriol. 1975 Oct;124(1):182–189. doi: 10.1128/jb.124.1.182-189.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casselton P. J. Concurrent regulation of two enzymes in fungi. Sci Prog. 1969 Summer;57(226):207–227. [PubMed] [Google Scholar]
- Dennen D. W., Niederpruem D. J. Regulation of glutamate dehydrogenases during morphogenesis of Schizophyllum commune. J Bacteriol. 1967 Mar;93(3):904–913. doi: 10.1128/jb.93.3.904-913.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. I. Survey of enzymes and properties of sulfite: cytochrome c oxidoreductase. Can J Biochem. 1970 Mar;48(3):334–343. doi: 10.1139/o70-056. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B. Enzyme regulation, lysine pathways and cell wall structures as indicators of major lines of evolution in fungi. Nature. 1971 May 21;231(5299):164–168. doi: 10.1038/231164a0. [DOI] [PubMed] [Google Scholar]
- LéJohn H. B., McCrea B. E. Evidence for two species of glutamate dehydrogenases in Thiobacillus novellus. J Bacteriol. 1968 Jan;95(1):87–94. doi: 10.1128/jb.95.1.87-94.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LéJohn H. B., Stevenson R. M. Multiple regulatory processes in nicotinamide adenine dinucleotide-specific glutamic dehydrogenases. Catabolite repression; nicotinamide adenine dinucleotide phosphate, reduced nicotinamide adenine dinucleotide phosphate, and phosphoenolpyruvate as activators; allosteric inhibition by substrates. J Biol Chem. 1970 Aug 10;245(15):3890–3900. [PubMed] [Google Scholar]
- Mantsala P., Zalkin H. Active subunits of Escherichia coli glutamate synthase. J Bacteriol. 1976 Apr;126(1):539–541. doi: 10.1128/jb.126.1.539-541.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W., Brown C. M. 'Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP); an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970 Dec;64(2):187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Mäntsälä P., Zalkin H. Glutamate synthase. Properties of the glutamine-dependent activity. J Biol Chem. 1976 Jun 10;251(11):3294–3299. [PubMed] [Google Scholar]
- Nagatani H., Shimizu M., Valentine R. C. The mechanism of ammonia assimilation in nitrogen fixing Bacteria. Arch Mikrobiol. 1971;79(2):164–175. doi: 10.1007/BF00424923. [DOI] [PubMed] [Google Scholar]
- Roon R. J., Even H. L., Larimore F. Glutamate synthase: properties of the reduced nicotinamide adenine dinucleotide-dependent enzyme from Saccharomyces cerevisiae. J Bacteriol. 1974 Apr;118(1):89–95. doi: 10.1128/jb.118.1.89-95.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. The occurrence of two different glutamic dehydrogenases in Neurospora. Can J Microbiol. 1961 Jun;7:319–328. doi: 10.1139/m61-039. [DOI] [PubMed] [Google Scholar]
- Senior P. J. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol. 1975 Aug;123(2):407–418. doi: 10.1128/jb.123.2.407-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey R. L. Cultivation of Organisms Concerned in the Oxidation of Thiosulfate. J Bacteriol. 1934 Oct;28(4):365–386. doi: 10.1128/jb.28.4.365-386.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem J. 1970 Apr;117(2):405–407. doi: 10.1042/bj1170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta P. P., Platzer K. E., Haschemeyer R. H., Meister A. Glutamine-binding subunit of glutamate synthase and partial reactions catalyzed by this glutamine amidotransferase. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4607–4611. doi: 10.1073/pnas.71.11.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Woolfolk C. A., Stadtman E. R. Regulation of glutamine synthetase. 3. Cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1967 Mar 20;118(3):736–755. doi: 10.1016/0003-9861(67)90412-2. [DOI] [PubMed] [Google Scholar]



