Abstract
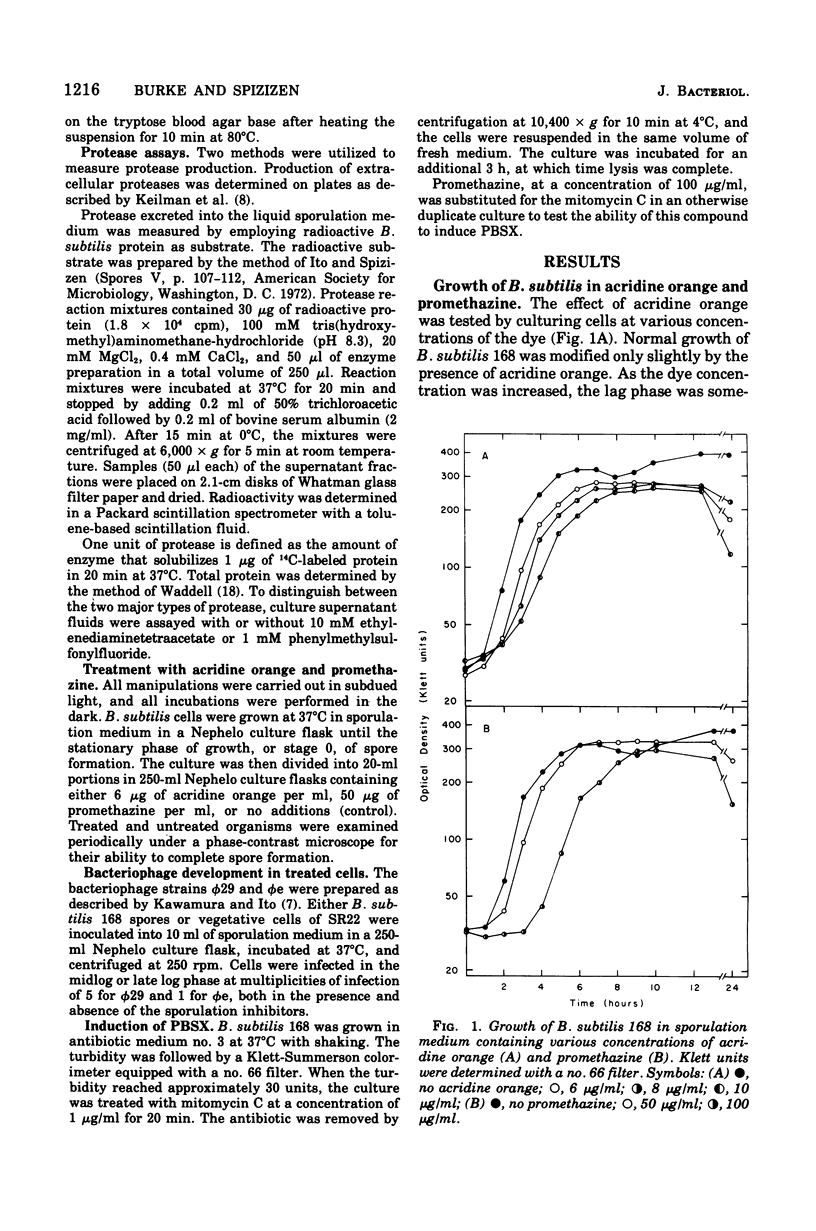
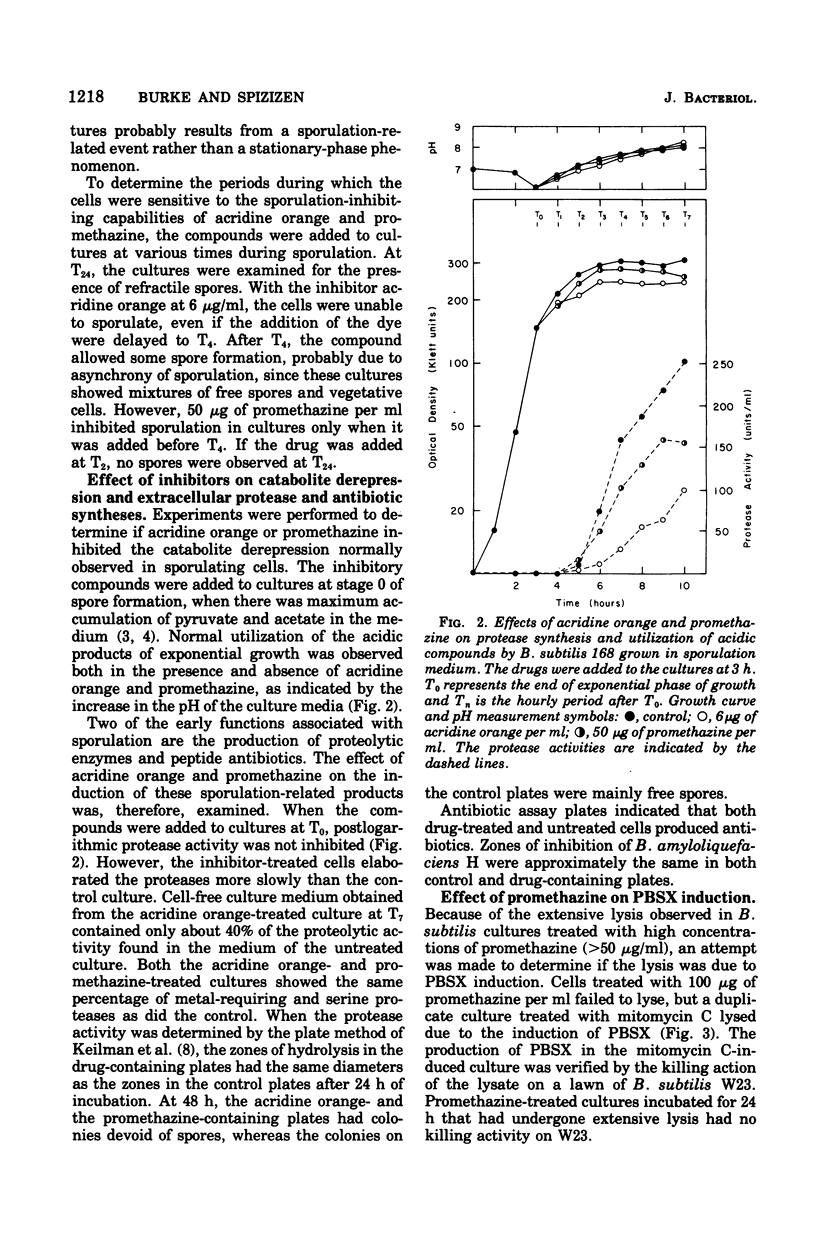
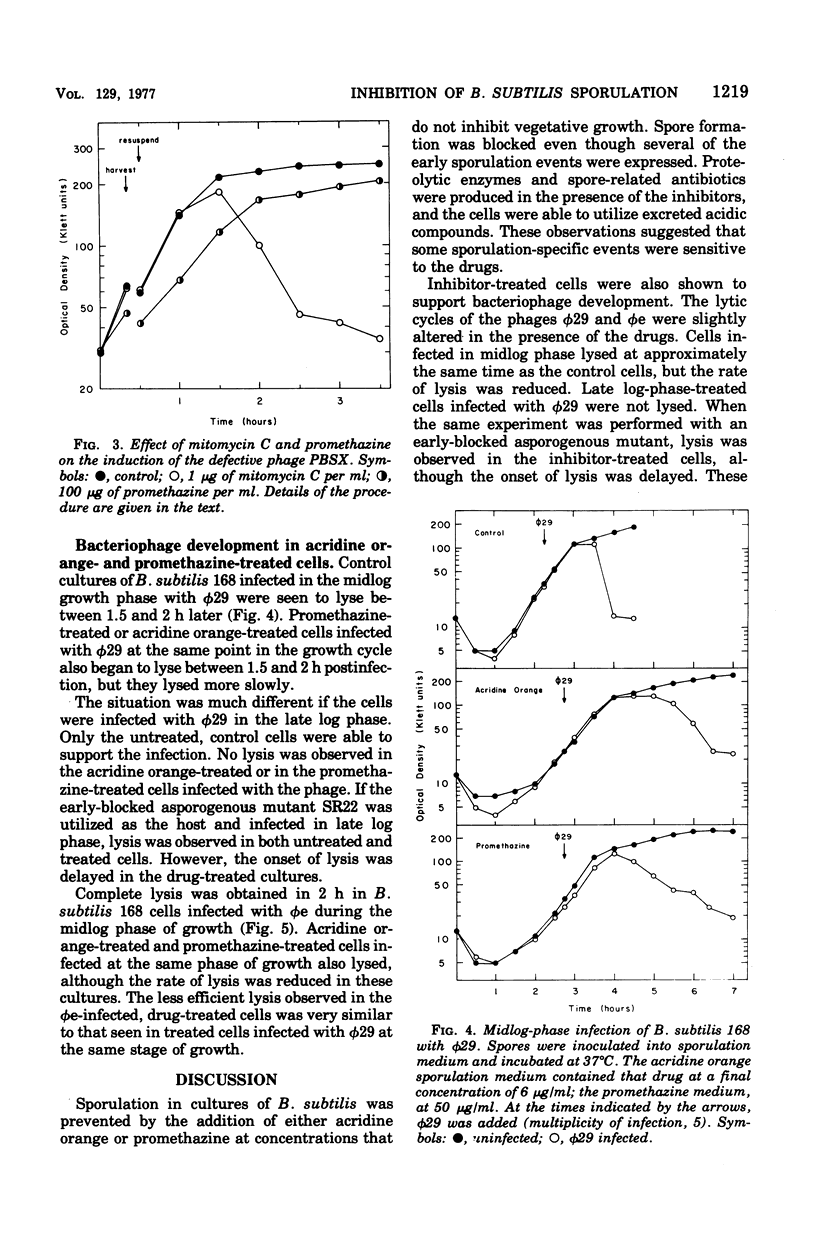
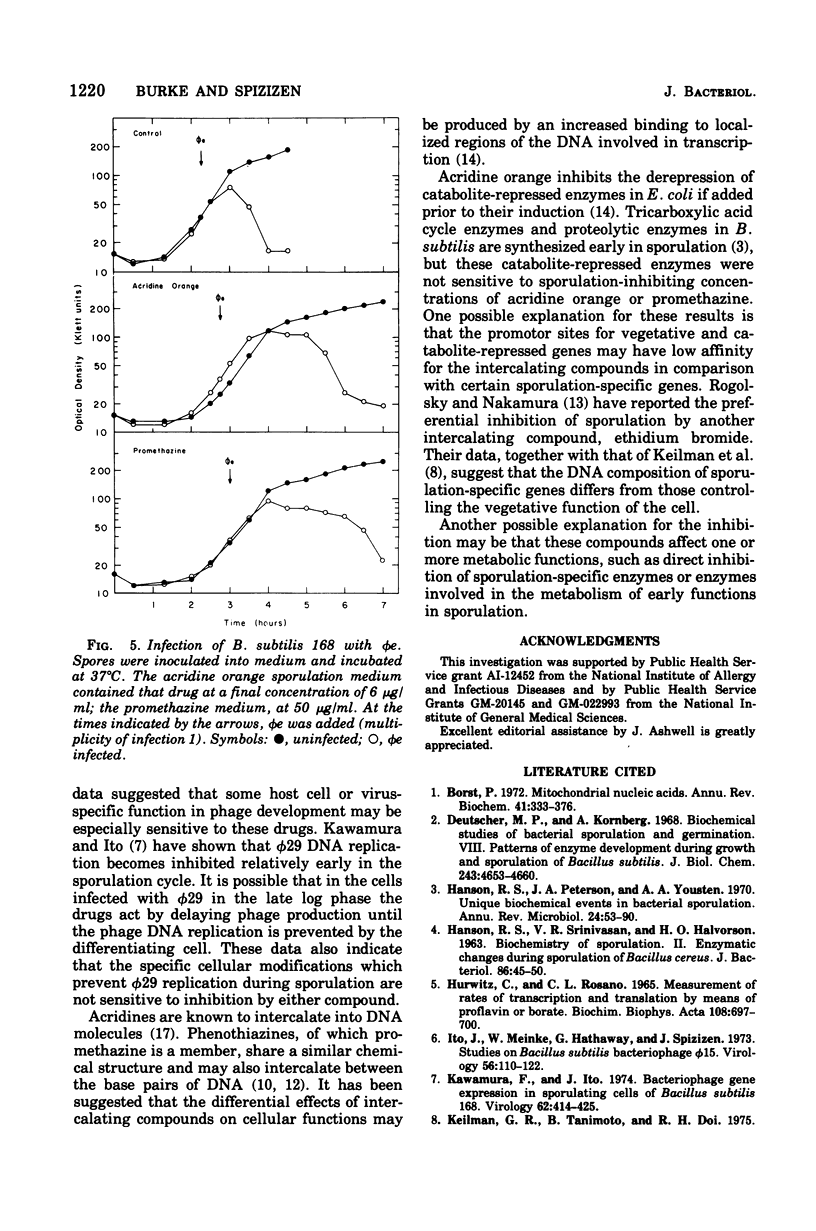
Two structurally similar compounds were found to inhibit sporulation in Bacillus subtilis 168. A dye, acridine orange, and an antischizophrenic drug, promethazine, blocked spore formation at concentrations subinhibitory to vegetative growth, while allowing synthesis of serine protease, antibiotic, and certain catabolite-repressed enzymes. The sporulation process was sensitive to promethazine through T2, whereas acridine orange was inhibitory until T4. The drug-treated cells were able to support the replication of phages phie and phi29, although the lytic cycles were altered slightly. The selective inhibition of sporulation by these compounds may be related to the affinity of some sporulation-specific genes to intercalating compounds.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst P. Mitochondrial nucleic acids. Annu Rev Biochem. 1972;41:333–376. doi: 10.1146/annurev.bi.41.070172.002001. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 8. Patterns of enzyme development during growth and sporulation of Baccillus subtilis. J Biol Chem. 1968 Sep 25;243(18):4653–4660. [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Peterson J. A., Yousten A. A. Unique biochemical events in bacterial sporulation. Annu Rev Microbiol. 1970;24:53–90. doi: 10.1146/annurev.mi.24.100170.000413. [DOI] [PubMed] [Google Scholar]
- Hurwitz C., Rosano C. L. Measurement of rates of transcription and translation by means of proflavine or borate. Biochim Biophys Acta. 1965 Dec 9;108(4):697–700. doi: 10.1016/0005-2787(65)90065-1. [DOI] [PubMed] [Google Scholar]
- Ito J., Meinke W., Hathaway G., Spizizen J. Studies on Bacillus subtilis bacteriophage phi 15. Virology. 1973 Nov;56(1):110–122. doi: 10.1016/0042-6822(73)90291-2. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Ito J. Bacteriophage gene expression in sporulating cells of Bacillus subtilis 168. Virology. 1974 Dec;62(2):414–425. doi: 10.1016/0042-6822(74)90403-6. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Molnár J., Király J., Mándi Y. The antibacterial action and R-factor-inhibiting activity by chlorpromazine. Experientia. 1975 Apr 15;31(4):444–445. doi: 10.1007/BF02026369. [DOI] [PubMed] [Google Scholar]
- Mándi T. Y., molnár J., Holland I. B., Béládi I. Efficient curing of an Escherichia coli F-prime plasmid by phenothiazines. Genet Res. 1975 Aug;26(1):109–111. doi: 10.1017/s0016672300015895. [DOI] [PubMed] [Google Scholar]
- Porumb T., Slade E. F. Electron-spin-resonance studies of a chlorpromazine derivative bound to DNA fibres. Eur J Biochem. 1976 May 17;65(1):21–24. doi: 10.1111/j.1432-1033.1976.tb10384.x. [DOI] [PubMed] [Google Scholar]
- Rogolsky M., Nakamura H. T. Sensitivity of an early step in the sporulation of Bacillus subtilis to selective inhibition by ethidium bromide. J Bacteriol. 1974 Jul;119(1):57–61. doi: 10.1128/jb.119.1.57-61.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran L., Pogell B. M. Differential inhibition of catabolite-sensitive enzyme induction by intercalating dyes. Nat New Biol. 1973 Oct 31;245(148):257–260. doi: 10.1038/newbio245257a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. Sporulation and the production of antibiotics, exoenzymes, and exotonins. Bacteriol Rev. 1969 Mar;33(1):48–71. doi: 10.1128/br.33.1.48-71.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber J. P., Duane M. P. Fluorescence of complexes of acridine dye with synthetic polydeoxyribonucleotides: a physical model of frameshift mutation. J Mol Biol. 1974 Mar 15;83(4):487–501. doi: 10.1016/0022-2836(74)90509-9. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]



