Abstract
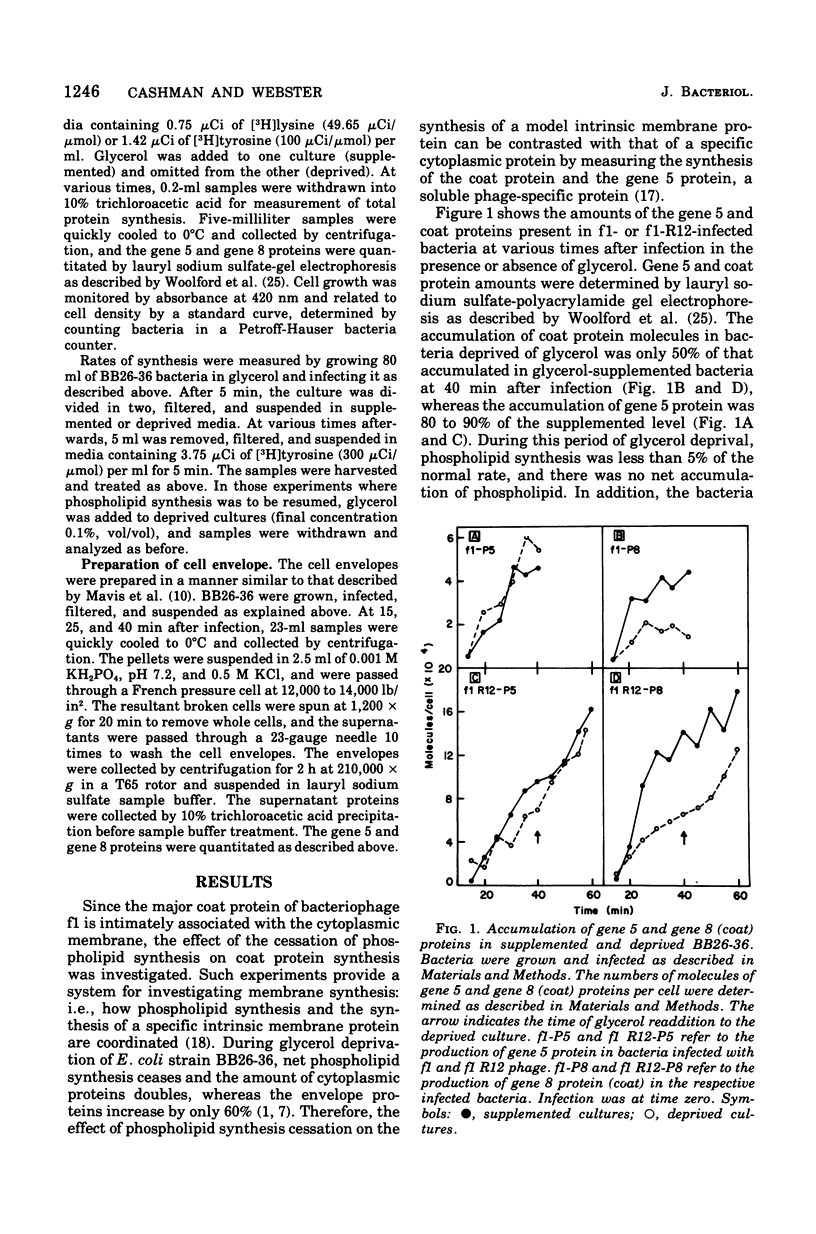
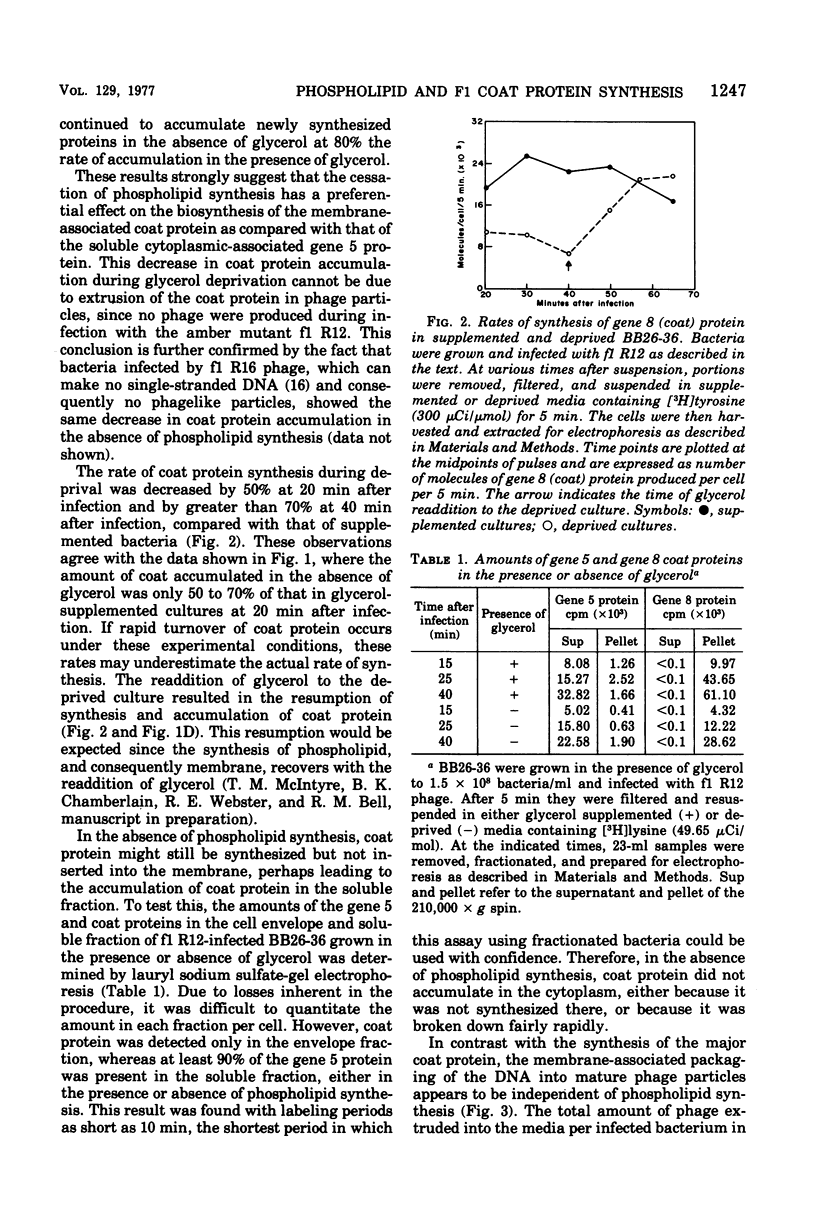
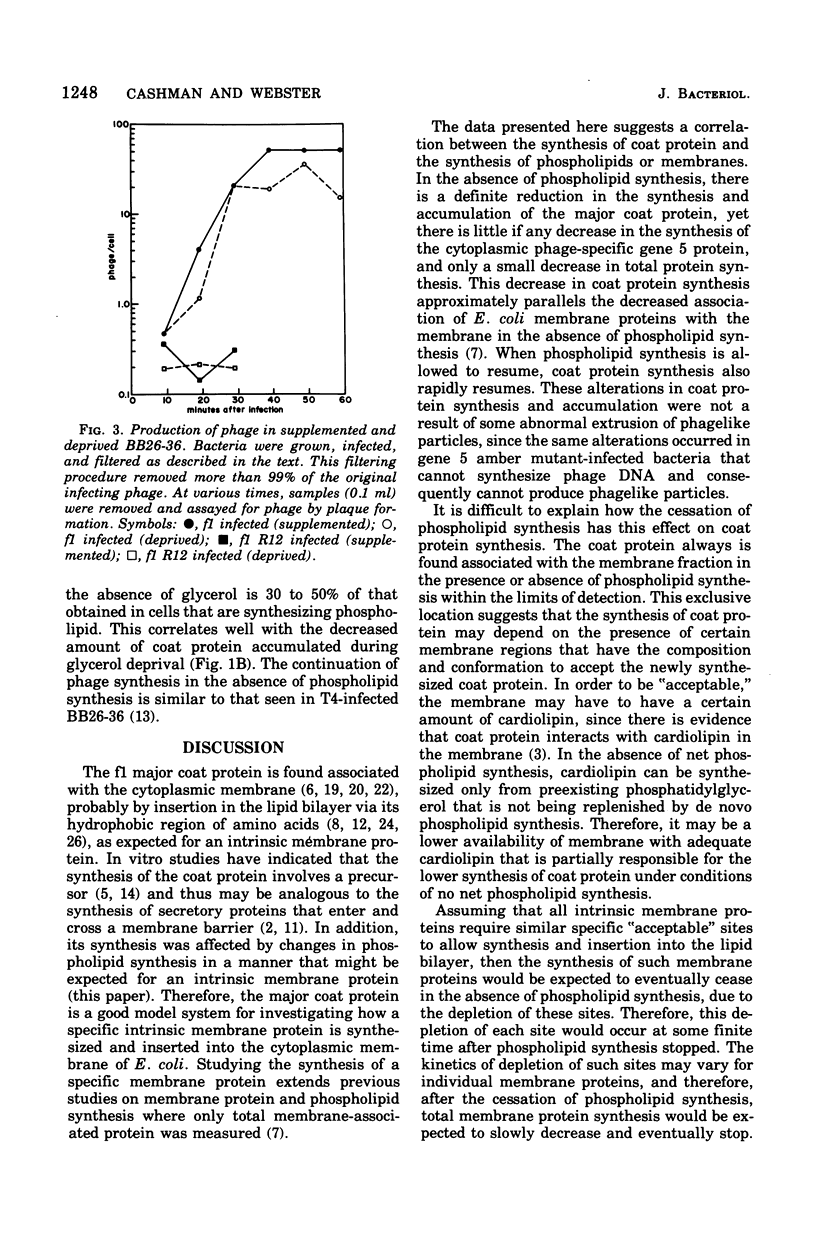
The major coat protein of the bacteriophage f1 is synthesized during infection of Escherichia coli and becomes tightly associated with the host membrane. This synthesis was studied in conjunction with the strain BB26-36, a mutant defective in phospholipid synthesis, to investigate basic questions concerning membrane protein and phospholipid synthesis. Coat protein synthesis is decreased in the absence of net phospholipid synthesis. The coat protein produced under these conditions is still found tightly associated with the membrane. Resumption of phospholipid synthesis leads to an increase in the synthesis and accumulation of the coat protein. Therefore, a correlation between coat protein and phospholipid synthesis seems to exist. However, the packaging of phage deoxyribonucleic acid into phage particles proceeds in the absence of phospholipid synthesis, and the number of phage particles produced appears to depend only on the amount of coat protein in the membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis: macromolecular synthesis in an sn-glycerol 3-phosphate acyltransferase Km mutant. J Bacteriol. 1974 Mar;117(3):1065–1076. doi: 10.1128/jb.117.3.1065-1076.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain B. K., Webster R. E. Lipid-protein interactions in Escherichia coli. Membrane-associated f1 bacteriophage coat protein and phospholipid metabolism. J Biol Chem. 1976 Dec 25;251(24):7739–7745. [PubMed] [Google Scholar]
- Denhardt D. T. The single-stranded DNA phages. CRC Crit Rev Microbiol. 1975 Dec;4(2):161–223. doi: 10.3109/10408417509111575. [DOI] [PubMed] [Google Scholar]
- Konings R. N., Hulsebos T., Van den Hondel C. A. Identification and characterization of the in vitro synthesized gene products of bacteriophage M13. J Virol. 1975 Mar;15(3):570–584. doi: 10.1128/jvi.15.3.570-584.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N. S., Pratt D. Bacteriophage M 13 gene 2 protein: increasing its yield in infected cells, and identification and localization. Virology. 1974 Oct;61(2):334–342. doi: 10.1016/0042-6822(74)90271-2. [DOI] [PubMed] [Google Scholar]
- Makino S., Woolford J. L., Jr, Tanford C., Webster R. E. Interaction of deoxycholate and of detergents with the coat protein of bacteriophage f1. J Biol Chem. 1975 Jun 10;250(11):4327–4332. [PubMed] [Google Scholar]
- Marvin D. A., Hohn B. Filamentous bacterial viruses. Bacteriol Rev. 1969 Jun;33(2):172–209. doi: 10.1128/br.33.2.172-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Bell R. M., Vagelos P. R. Effect of phospholipase C hydrolysis of membrane phospholipids on membranous enzymes. J Biol Chem. 1972 May 10;247(9):2835–2841. [PubMed] [Google Scholar]
- McIntyre T. M., Bell R. M. Mutants of Escherichia coli defective in membrane phospholipid synthesis. Effect of cessation of net phospholipid synthesis on cytoplasmic and outer membranes. J Biol Chem. 1975 Dec 10;250(23):9053–9059. [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Chamberlain B. K., Webster R. E., Tanford C. Evidence for a major conformational change of coat protein in assembly of fl bacteriophage. Nature. 1976 Jan 29;259(5541):335–337. doi: 10.1038/259335a0. [DOI] [PubMed] [Google Scholar]
- Nunn W. D., Cronan J. E., Jr Host membrane lipid synthesis is not required for successful phage T4 infection. Virology. 1976 Jan;69(1):332–335. doi: 10.1016/0042-6822(76)90221-x. [DOI] [PubMed] [Google Scholar]
- Pieczenik G., Model P., Robertson H. D. Sequence and symmetry in ribosome binding sites of bacteriophage f1 RNA. J Mol Biol. 1974 Dec 5;90(2):191–124. doi: 10.1016/0022-2836(74)90368-4. [DOI] [PubMed] [Google Scholar]
- Pratt D., Erdahl W. S. Genetic control of bacteriophage M13 DNA synthesis. J Mol Biol. 1968 Oct 14;37(1):181–200. doi: 10.1016/0022-2836(68)90082-x. [DOI] [PubMed] [Google Scholar]
- Pratt D., Laws P., Griffith J. Complex of bacteriophage M13 single-stranded DNA and gene 5 protein. J Mol Biol. 1974 Feb 5;82(4):425–439. doi: 10.1016/0022-2836(74)90239-3. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Trenkner E. Pool sizes of fd bacteriophage components in infected bacterial cells. Virology. 1970 Jan;40(1):18–22. doi: 10.1016/0042-6822(70)90374-0. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Ochoa S. Synthesis of virus-specific proteins in Escherichia coli infected with the RNA bacteriophage MS2. Eur J Biochem. 1967 Mar;1(1):3–11. doi: 10.1007/978-3-662-25813-2_2. [DOI] [PubMed] [Google Scholar]
- WEIGERT M. G., GAREN A. AMINO ACID SUBSTITUTIONS RESULTING FROM SUPPRESSION OF NONSENSE MUTATIONS. I. SERINE INSERTION BY THE SU-1 SUPPRESSOR GENE. J Mol Biol. 1965 Jun;12:448–455. doi: 10.1016/s0022-2836(65)80267-4. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Cashman J. S. Abortive infection of Escherichia coli with the bacteriophage f1: cytoplasmic membrane proteins and the f1 DNA-gene 5 protein complex. Virology. 1973 Sep;55(1):20–38. doi: 10.1016/s0042-6822(73)81005-0. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Cashman J. S., Webster R. E. F1 Coat protein synthesis and altered phospholipid metabolism in f1 infected Escherichia coli. Virology. 1974 Apr;58(2):544–560. doi: 10.1016/0042-6822(74)90088-9. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Webster R. E. Proteolytic digestion of the micellar complex of f1 coat protein and deoxycholate. J Biol Chem. 1975 Jun 10;250(11):4333–4339. [PubMed] [Google Scholar]


