Abstract
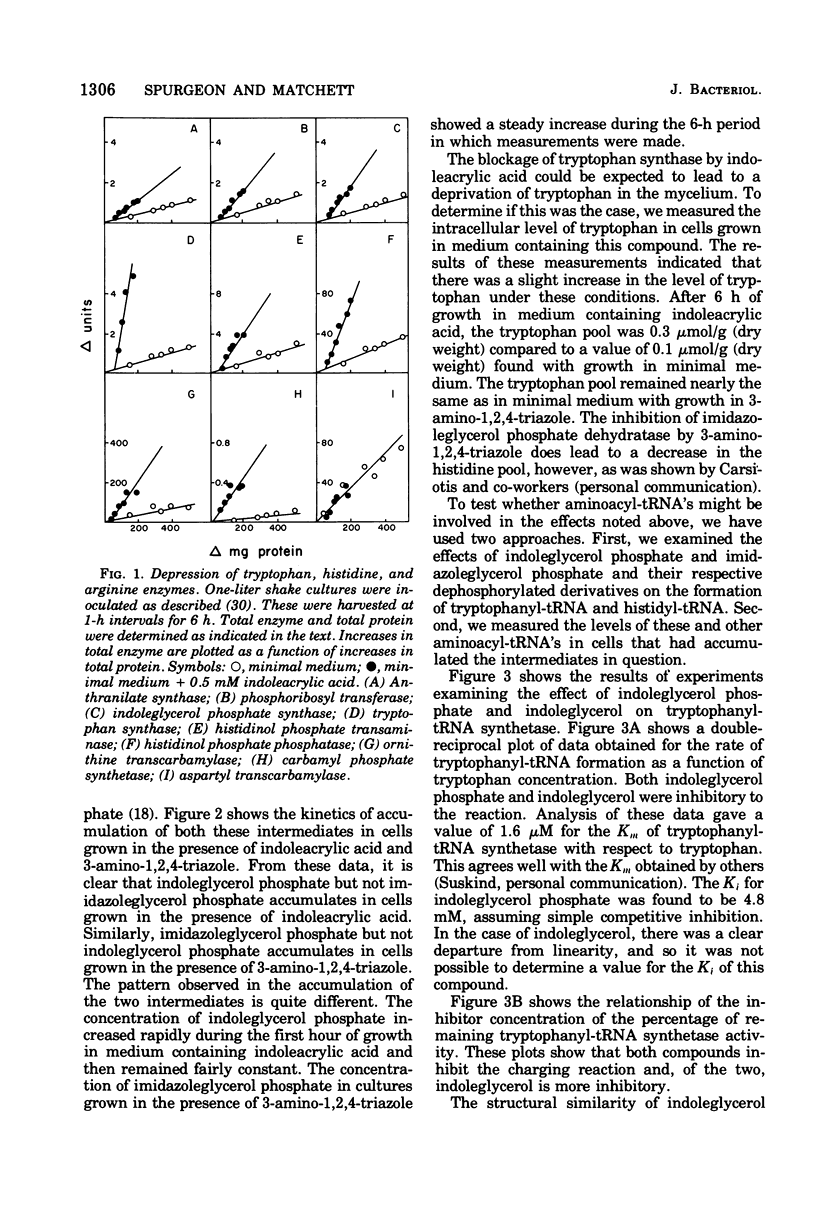
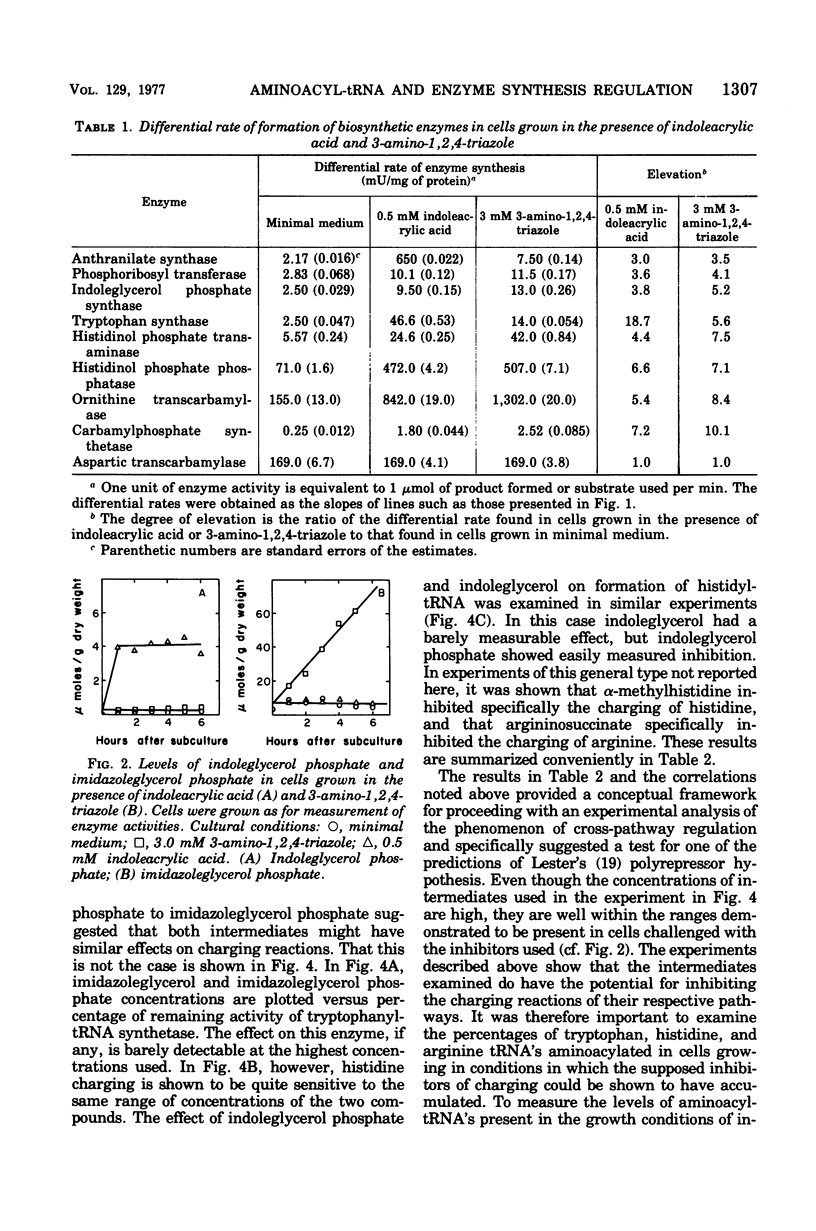
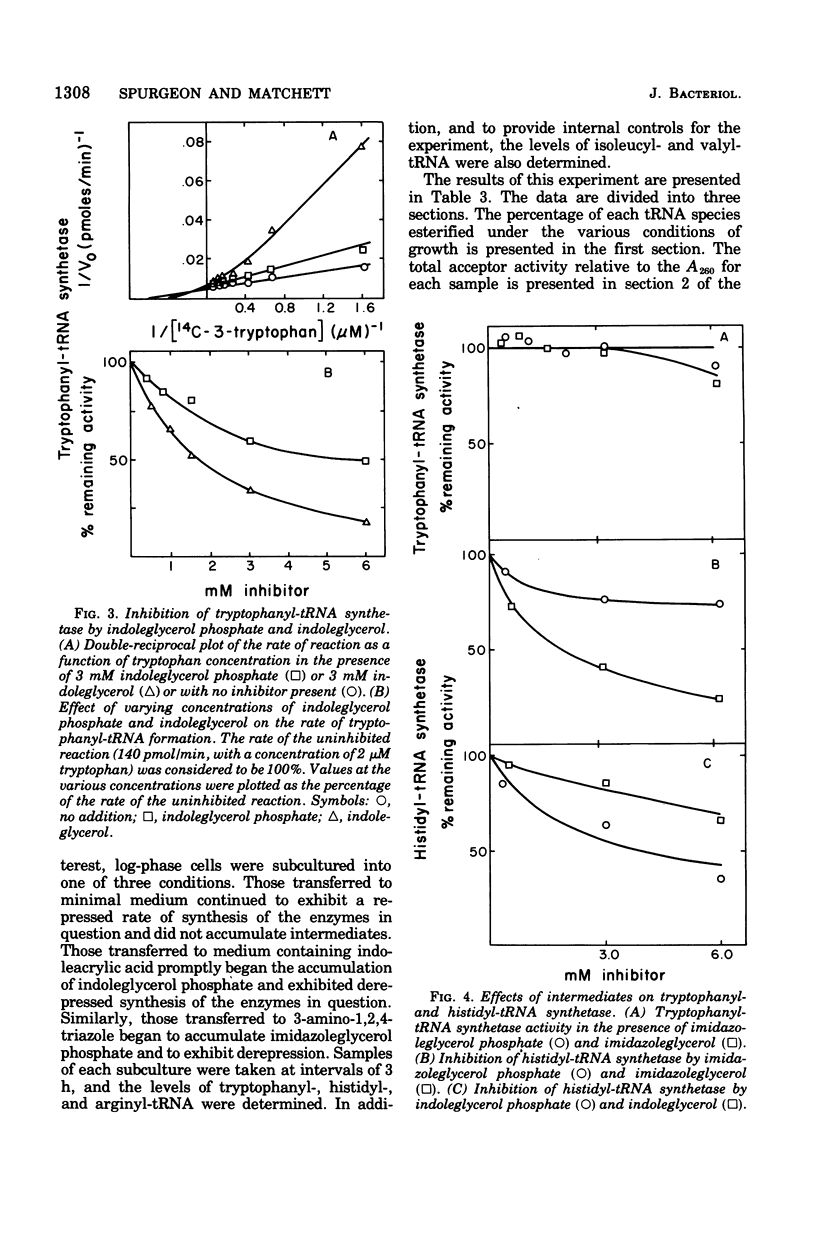
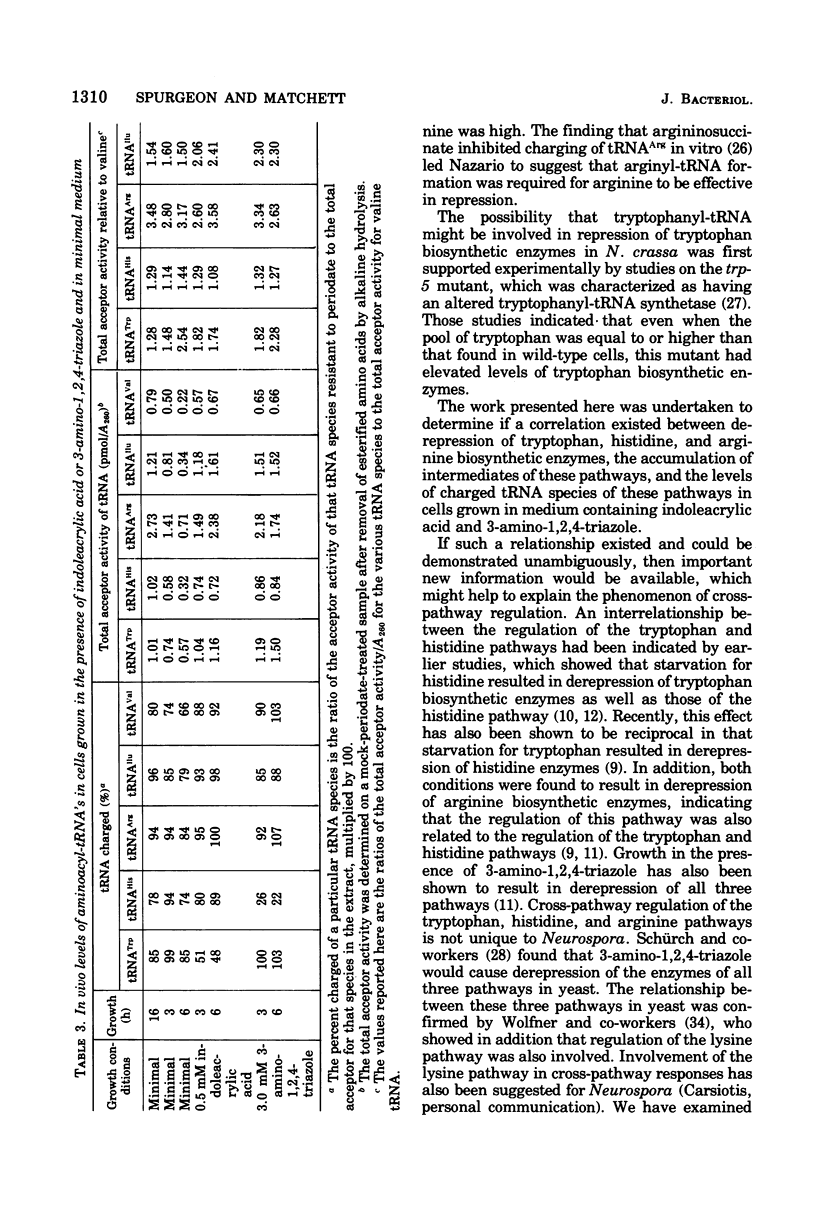
Growth conditions that result in the accumulation of the tryptophan intermediate indoleglycerol phosphate or of the histidine intermediate imidazoleglycerol phosphate cause mycelia of Neurospora crassa to exhibit an immediate and sustained increase in the differential rate at which the biosynthetic enzymes of the tryptophan, histidine, and arginine pathways are synthesized. These accumulated intermediates are shown to be inhibitors of the activity of aminoacyltransfer ribonucleic acid (tRNA) synthetases, as judged by an in vitro esterification assay. The tryptophan intermediate is shown to inhibit the charging of tryptophan, and the histidine intermediate is shown to inhibit charging of histidine. The inhibitions noted are consistent with the finding that the level of charged tRNATrp is decreased significantly in cells that have accumulated indoleglycerol phosphate and that of tRNAHis is decreased significantly in cells that have accumulated imidazoleglycerol phosphate. These results are interpreted as support for the involvement of aminoacyl-tRNA species in mediating cross-pathway regulation of the tryptophan, histidine, and arginine biosynthetic pathways as proposed in Lester's polyrepressor hypothesis (G. Lester, 1971). the correlations noted lead to the conclusion that Neurospora utilizes regulatory mechanisms that have the ability to react to changes in the level of charging of tRNA species.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HORECKER B. L. The biosynthesis of histidine: imidazoleacetol phosphate transaminase. J Biol Chem. 1956 May;220(1):113–128. [PubMed] [Google Scholar]
- AMES B. N. The biosynthesis of histidine; D-erythro-imidazoleglycerol phosphate dehydrase. J Biol Chem. 1957 Sep;228(1):131–143. [PubMed] [Google Scholar]
- AMES B. N. The biosynthesis of histidine; L-histidinol phosphate phosphatase. J Biol Chem. 1957 Jun;226(2):583–593. [PubMed] [Google Scholar]
- Barnett W. E. Interspecies aminoacyl-sRNA formation: fractionation of Neurospora enzymes involved in anomalous aminoacylation. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1462–1467. doi: 10.1073/pnas.53.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Hatfield G. W. Autoregulation: a role for a biosynthetic enzyme in the control of gene expression. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2757–2761. doi: 10.1073/pnas.70.10.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Lacy A. M., Cleary T. J., Fankhauser D. B. Histidine-mediated control of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1970 Oct;104(1):98–106. doi: 10.1128/jb.104.1.98-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMOSS J. A. Studies on the mechanism of the tryptophan synthetase reaction. Biochim Biophys Acta. 1962 Aug 13;62:279–293. doi: 10.1016/0006-3002(62)90041-0. [DOI] [PubMed] [Google Scholar]
- DEMOSS J. A. THE CONVERSION OF SHIKIMIC ACID TO ANTHRANILIC ACID BY EXTRACTS OF NEUROSPORA CRASSA. J Biol Chem. 1965 Mar;240:1231–1235. [PubMed] [Google Scholar]
- Davis R. H. Carbamyl phosphate synthesis in Neurospora crassa. II. Genetics, metabolic position, and regulation of arginine-specific carbamyl phosphokinase. Biochim Biophys Acta. 1965 Aug 24;107(1):54–68. doi: 10.1016/0304-4165(65)90388-0. [DOI] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Characterization of altered forms of glycyl transfer ribonucleic acid synthetase and the effects of such alterations on aminoacyl transfer ribonucleic acid synthesis in vivo. J Bacteriol. 1970 Apr;102(1):204–212. doi: 10.1128/jb.102.1.204-212.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton J. L., Kearney P. C., Ames B. N. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys. 1965 Dec;112(3):544–547. doi: 10.1016/0003-9861(65)90093-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. Regulation of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1971 Jul;107(1):193–202. doi: 10.1128/jb.107.1.193-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Matchett W. H. Inhibition of tryptophan synthetase by indoleacrylic acid. J Bacteriol. 1972 Apr;110(1):146–154. doi: 10.1128/jb.110.1.146-154.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett W. H., Turner J. R., Wiley W. R. The role of tryptophan in the physiology of Neurospora. Yale J Biol Med. 1968 Feb;40(4):257–283. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Magee P. T., Hartwell L. H. Role of isoleucyl-transfer ribonucleic acid synthetase in ribonucleic acid synthesis and enzyme repression in yeast. J Bacteriol. 1969 Nov;100(2):579–584. doi: 10.1128/jb.100.2.579-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario M., Kinsey J. A., Ahmad M. Neurospora mutant deficient in tryptophanyl-transfer ribonucleic acid synthetase activity. J Bacteriol. 1971 Jan;105(1):121–126. doi: 10.1128/jb.105.1.121-126.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario M. The accumulation of argininosuccinate in Neurospora crassa. II. Inhibition of arginyl-tRNA synthesis by argininosuccinate. Biochim Biophys Acta. 1967 Aug 22;145(1):146–152. doi: 10.1016/0005-2787(67)90663-6. [DOI] [PubMed] [Google Scholar]
- Schürch A., Miozzari J., Hütter R. Regulation of tryptophan biosynthesis in Saccharomyces cerevisiae: mode of action of 5-methyl-tryptophan and 5-methyl-tryptophan-sensitive mutants. J Bacteriol. 1974 Mar;117(3):1131–1140. doi: 10.1128/jb.117.3.1131-1140.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn A., Horowitz N. H. A study of transfer ribonucleic acid in Neurospora. I. The attachment of amino acids and amino acid analogs. Biochemistry. 1969 Jan;8(1):295–303. doi: 10.1021/bi00829a042. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegman J., DeMoss J. A. The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa. J Biol Chem. 1965 Oct;240(10):3781–3788. [PubMed] [Google Scholar]
- Wolfner M., Yep D., Messenguy F., Fink G. R. Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol. 1975 Aug 5;96(2):273–290. doi: 10.1016/0022-2836(75)90348-4. [DOI] [PubMed] [Google Scholar]
- YANOFSKY C. The enzymatic conversion of anthranilic acid to indole. J Biol Chem. 1956 Nov;223(1):171–184. [PubMed] [Google Scholar]


