Abstract
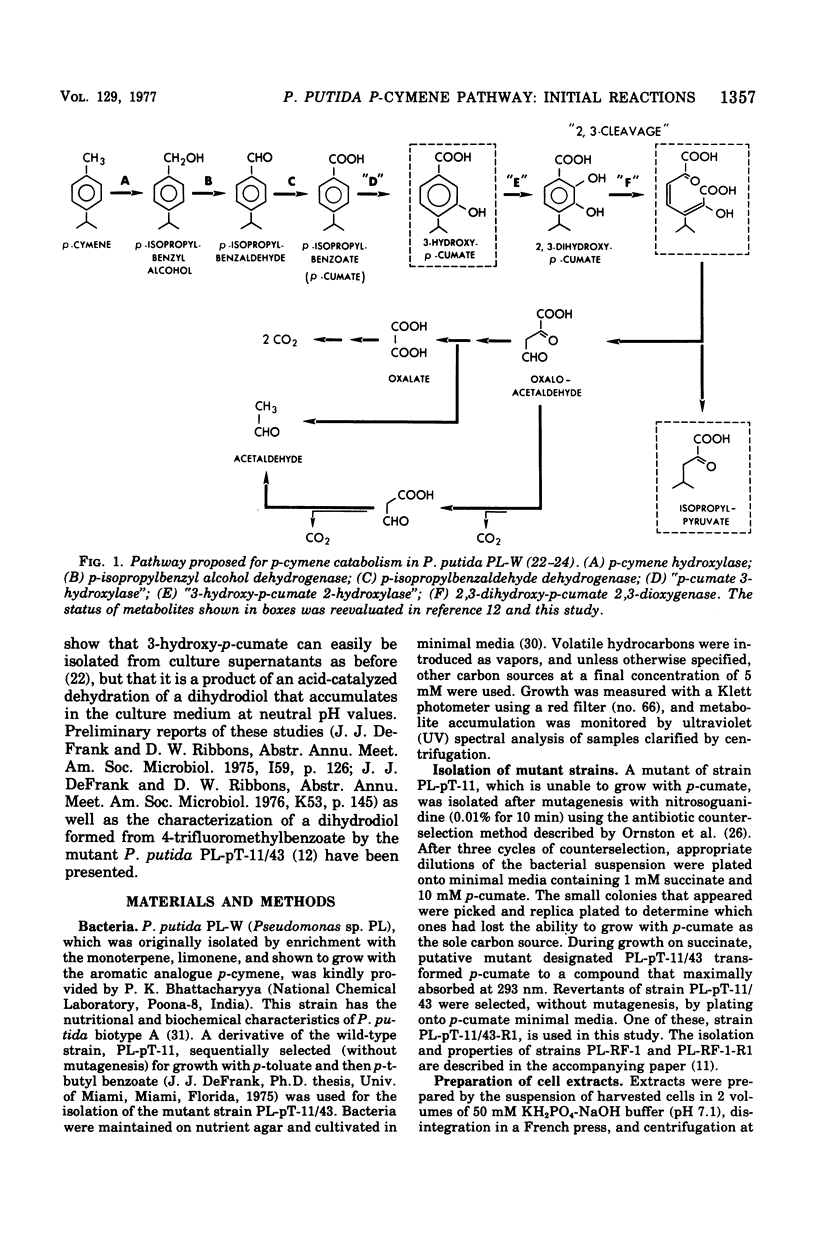
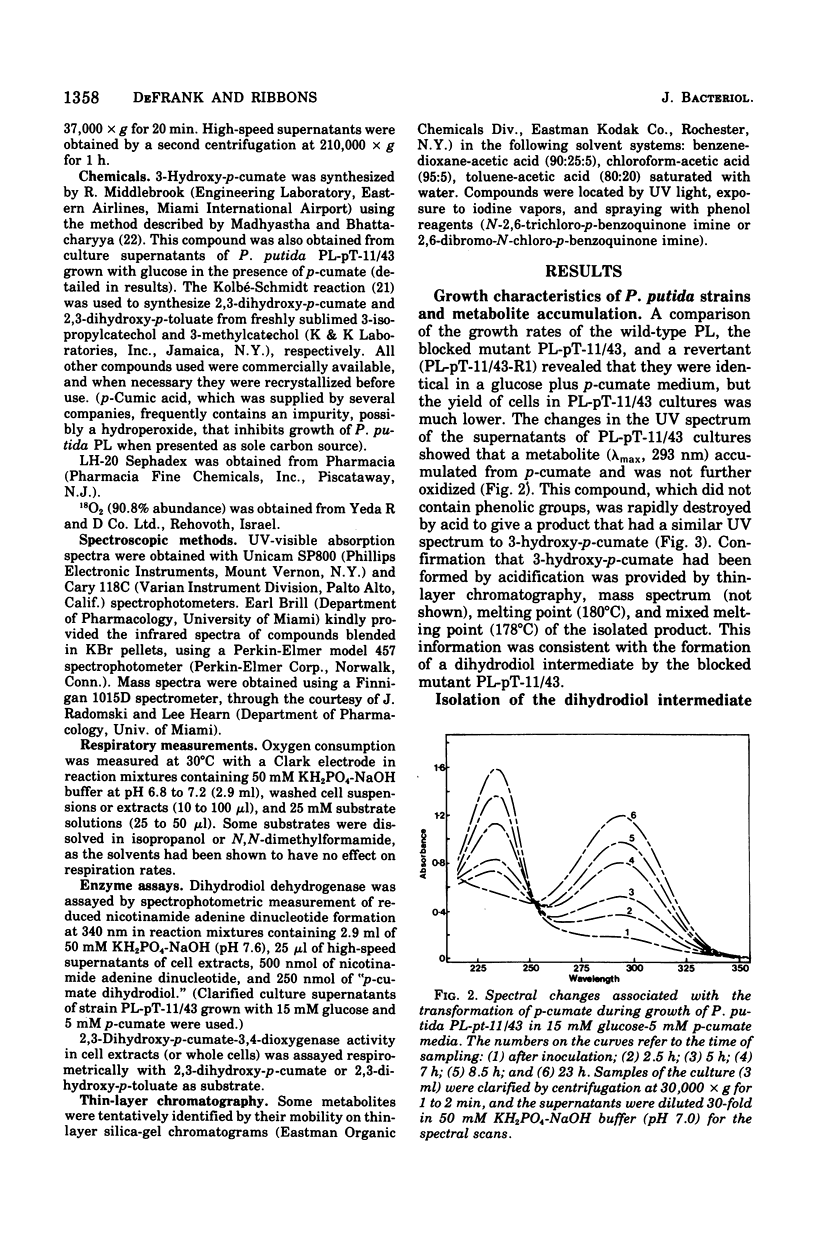
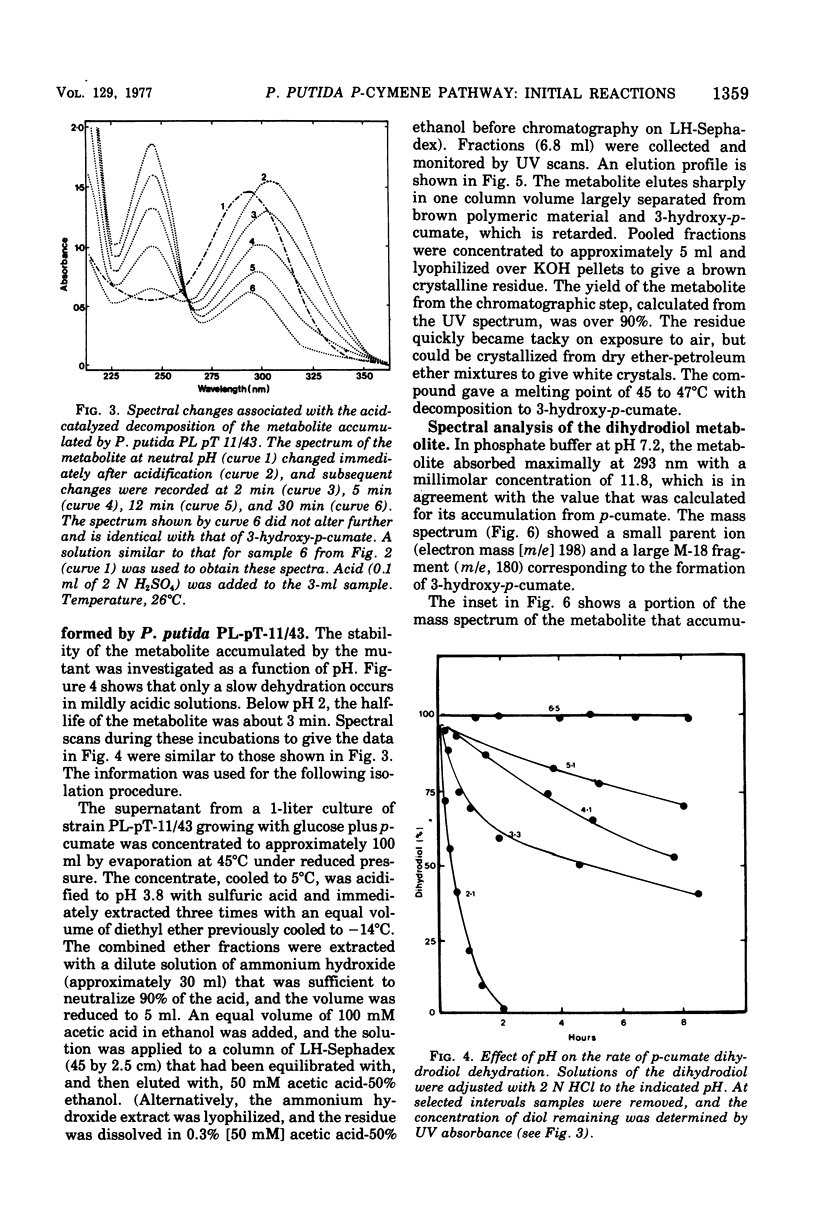
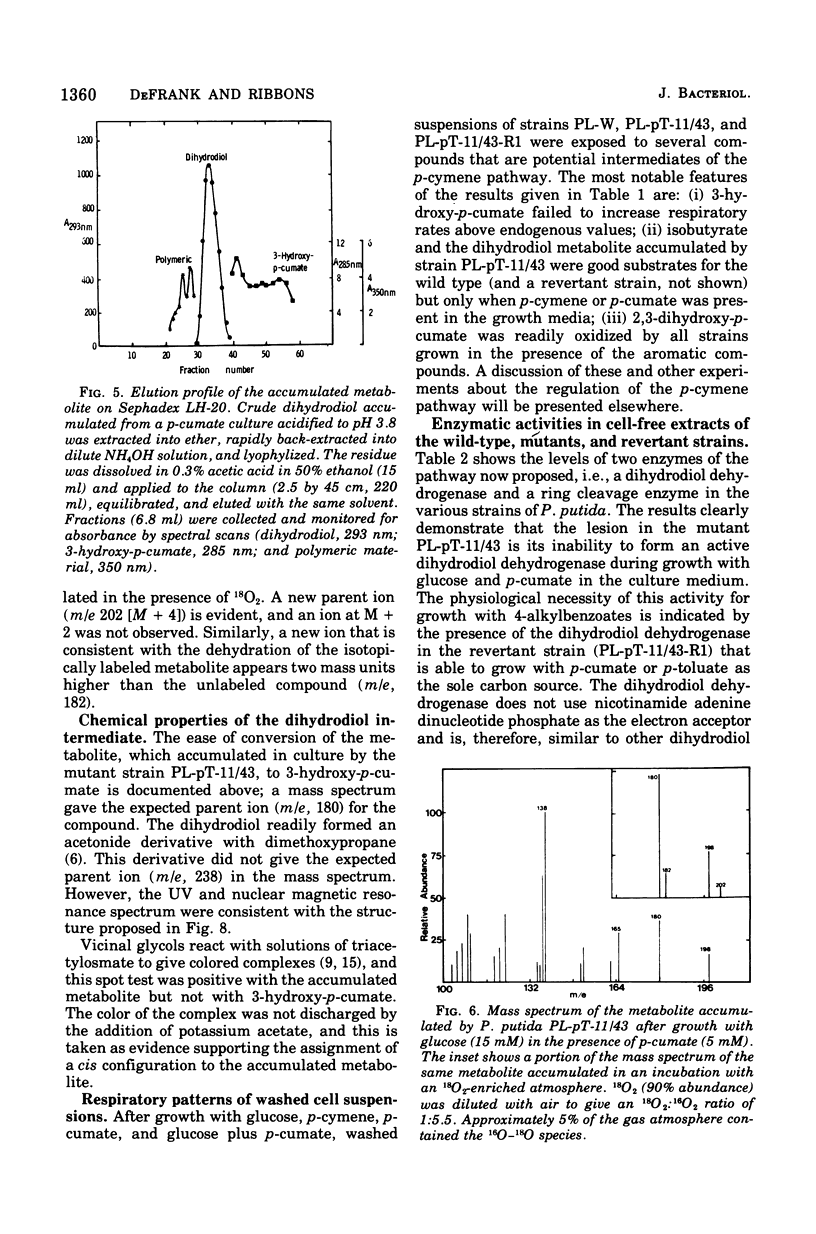
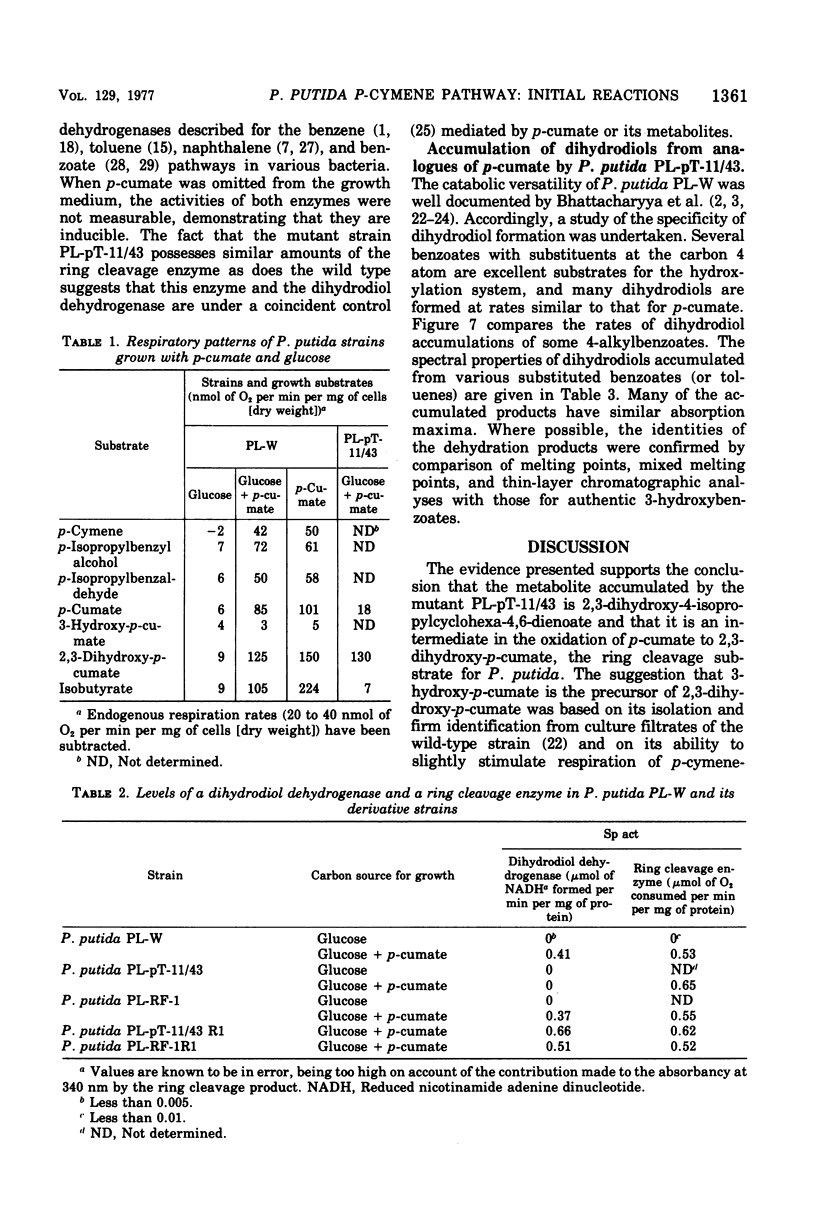
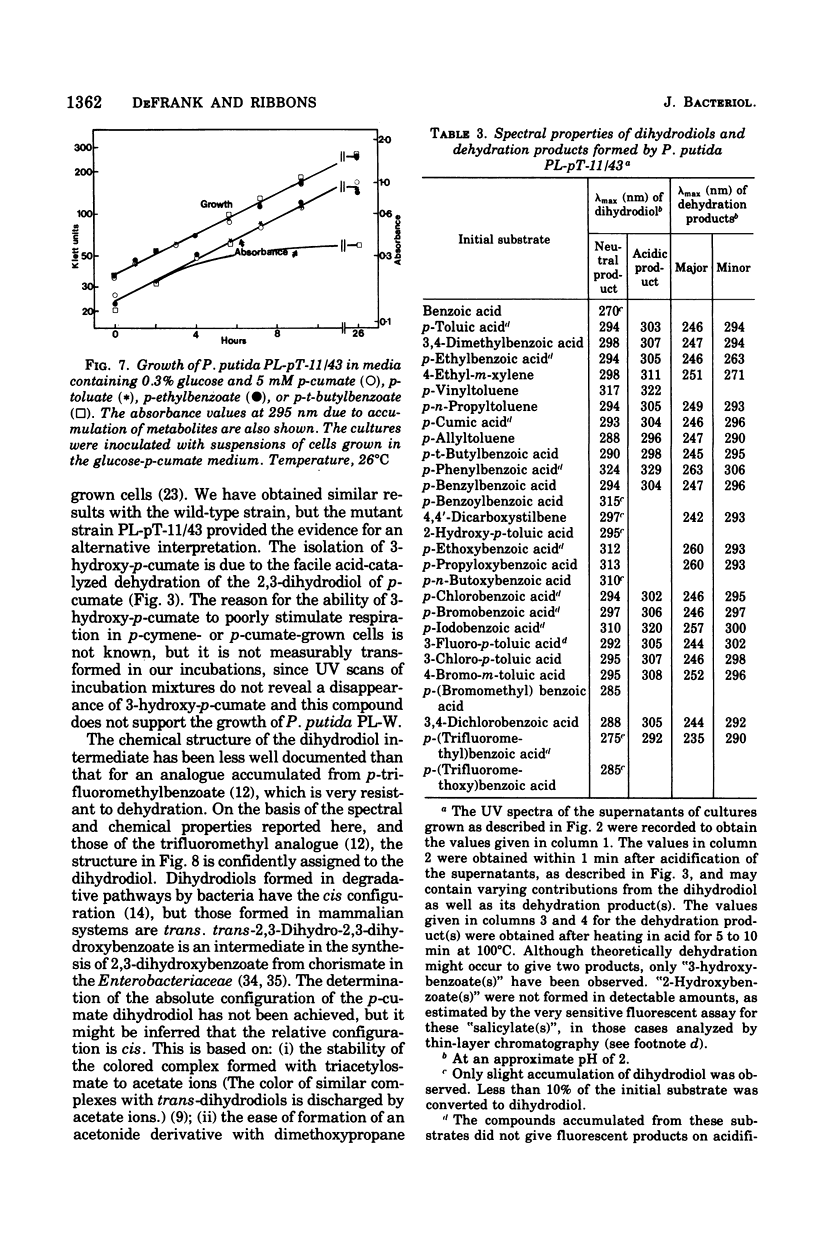
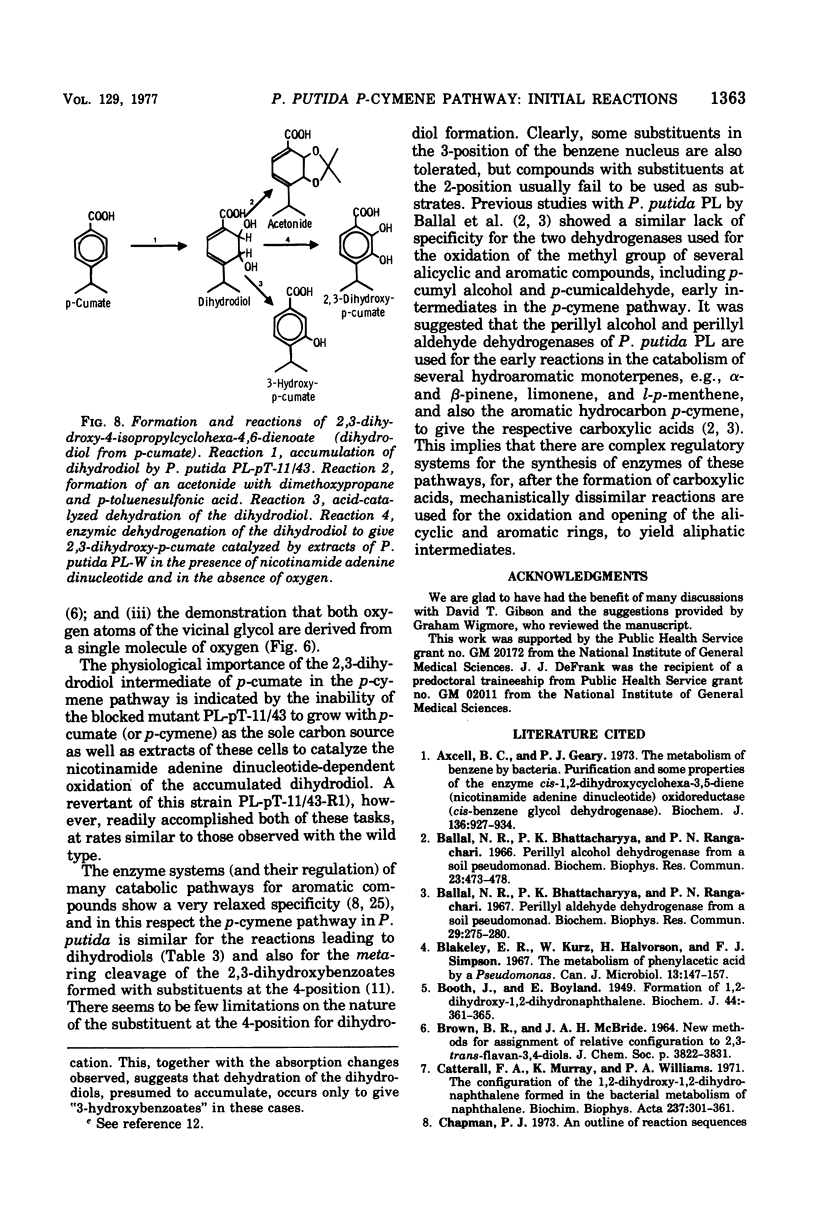
Initial reactions of the p-cymene pathway induced in Pseudomonas putida PL have been reinvestigated. Oxidation of the methyl group attached to the nucleus occurs in three steps to give p-cumic acid. The substrate for the ring cleavage of 2,3-dihydroxy-p-cumate is formed from p-cumate in two reactions via a dihydrodiol intermediate (2,3-dihydroxy-4-isopropylcyclohexa-4,6-dienoate) and not as previously postulated via 3-hydroxy-p-cumate. There are three pieces of evidence for the physiological role of the dihydrodiol intermediate. (i) a mutant of P. putida PL-pT-11/43, which is unable to grow with p-cumate, accumulates a compound from p-cumate, which was identified as 2,3-dihydroxy-4-isopropylcyclohexa-4,6-dienoate. (II) This metabolite is enzymically oxidized by a nicotinamide adenine dinucleotide-dependent dehydrogenase that is present in crude extracts of the wild type and a revertant strain (PL-pT-11/43-R1) but not in the mutant. (iii) 3-Hydroxy-p-cumate does not support growth of P . putida PL-W, and it is not oxidized by cells or extracts. 3-Hydroxy-p-cumate was readily isolated as before from culture supernatants, due to its ready formation from the dihydrodiol in acid solution. Mass spectral analysis of the dihydrodiol accumulated in 18O2-enriched atmospheres showed that both hydroxyl atoms are derived from the same molecule of O2. The formation and absorbance maxima of dihydrodiols that accumulated during the growth of the mutant PL-pT-11/43 in the presence of various benzoates (or toluenes) that have substituents at the carbon 4 atom also is reported.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axcell B. C., Geary P. J. The metabolism of benzene by bacteria. Purification and some properties of the enzyme cis-1,2-dihydroxycyclohexa-3,5-diene (nicotinamide adenine dinucleotide) oxidoreductase (cis-benzene glycol dehydrogenase). Biochem J. 1973 Dec;136(4):927–934. doi: 10.1042/bj1360927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballal N. R., Bhattacharyya P. K., Rangachari P. N. Perillyl alcohol dehydrogenase from a soil pseudomonad. Biochem Biophys Res Commun. 1966 May 25;23(4):473–478. doi: 10.1016/0006-291x(66)90752-2. [DOI] [PubMed] [Google Scholar]
- Ballal N. R., Bhattacharyya P. K., Rangachari P. N. Perillyl aldehyde dehydrogenase from a soil pseudomonad. Biochem Biophys Res Commun. 1967 Nov 17;29(3):275–280. doi: 10.1016/0006-291x(67)90448-2. [DOI] [PubMed] [Google Scholar]
- Blakley E. R., Kurz W., Halvorson H., Simpson F. J. The metabolism of phenylacetic acid by a Pseudomonas. Can J Microbiol. 1967 Feb;13(2):147–157. doi: 10.1139/m67-021. [DOI] [PubMed] [Google Scholar]
- Booth J., Boyland E. Metabolism of polycyclic compounds. 5. Formation of 1:2-dihydroxy-1:2-dihydronaphthalenes. Biochem J. 1949;44(3):361–365. [PMC free article] [PubMed] [Google Scholar]
- Catterall F. A., Murray K., Williams P. A. The configuration of the 1,2-dihydroxy-1,2-dihydronaphthalene formed in the bacterial metabolism of naphthalene. Biochim Biophys Acta. 1971 May 18;237(2):361–364. doi: 10.1016/0304-4165(71)90331-x. [DOI] [PubMed] [Google Scholar]
- DAVIS J. B., RAYMOND R. L. Oxidation of alkyl-substituted cyclic hydrocarbons by a Nocardia during growth on n-alkanes. Appl Microbiol. 1961 Sep;9:383–388. doi: 10.1128/am.9.5.383-388.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frenne E., Eberspächer J., Lingens F. The bacterial degradation of 5-amino-4-chloro-2-phenyl-3(2H)-pyridazinone. Eur J Biochem. 1973 Mar 1;33(2):357–363. doi: 10.1111/j.1432-1033.1973.tb02690.x. [DOI] [PubMed] [Google Scholar]
- DeFrank J. J., Ribbons D. W. p-Cymene pathway in Pseudomonas putida: ring cleavage of 2,3-dihydroxy-p-cumate and subsequent reactions. J Bacteriol. 1977 Mar;129(3):1365–1374. doi: 10.1128/jb.129.3.1365-1374.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrank J. J., Ribbons D. W. The p-cymene pathway in Pseudomonas putida PL: isolation of a dihydrodiol accumulated by a mutant. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1129–1135. doi: 10.1016/0006-291x(76)91020-2. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Hensley M., Yoshioka H., Mabry T. J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970 Mar 31;9(7):1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Jerina D. M., Yogi H., Yeh H. J. Oxidation of the carcinogens benzo [a] pyrene and benzo [a] anthracene to dihydrodiols by a bacterium. Science. 1975 Jul 25;189(4199):295–297. doi: 10.1126/science.1145203. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Roberts R. L., Wells M. C., Kobal V. M. Oxidation of biphenyl by a Beijerinckia species. Biochem Biophys Res Commun. 1973 Jan 23;50(2):211–219. doi: 10.1016/0006-291x(73)90828-0. [DOI] [PubMed] [Google Scholar]
- Högn T., Jaenicke L. Benzene metabolism of Moraxella species. Eur J Biochem. 1972 Oct;30(2):369–375. doi: 10.1111/j.1432-1033.1972.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Jerina D. M., Daly J. W., Jeffrey A. M., Gibson D. T. Cis-1,2-dihydroxy-1,2-dihydronaphthalene: a bacterial metabolite from naphthalene. Arch Biochem Biophys. 1971 Jan;142(1):394–396. doi: 10.1016/0003-9861(71)90298-0. [DOI] [PubMed] [Google Scholar]
- Madhyastha K. M., Bhattacharyya P. K. Microbiological transformations of terpenes. 13. Pathways for degradation of p-cymene in a soil pseudomonad (PL-strain). Indian J Biochem. 1968 Dec;5(4):161–167. [PubMed] [Google Scholar]
- Madhyastha K. M., Rangachari P. N., Raghabendra Rao M., Bhattacharyya P. K. Microbiological transformations of terpenes. XV. Enzyme systems in the catabolism of p-cymene in PL-strain. Indian J Biochem. 1968 Dec;5(4):167–173. [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K., Chou G. Isolation of spontaneous mutant strains of Pseudomonas putida. Biochem Biophys Res Commun. 1969 Jul 7;36(1):179–184. doi: 10.1016/0006-291x(69)90666-4. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T. R., Gibson D. T. Purification and propeties of (plus)-cis-naphthalene dihydrodiol dehydrogenase of Pseudomonas putida. J Bacteriol. 1974 Sep;119(3):879–888. doi: 10.1128/jb.119.3.879-888.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- Ribbons D. W. Metabolism of omicron-cresol by Pseudomonas aeruginosa strain T1. J Gen Microbiol. 1966 Aug;44(2):221–231. doi: 10.1099/00221287-44-2-221. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]
- Young I. G., Gibson F. The stereochemistry of intermediates involved in the biosynthesis of 2,3-dihydroxybenzoic acid. Biochim Biophys Acta. 1969 Apr 1;177(2):348–350. doi: 10.1016/0304-4165(69)90147-0. [DOI] [PubMed] [Google Scholar]
- Young I. G., Jackman L. M., Gibson F. The isolation, identification and properties of 2,3-dihydro-2,3-dihydroxybenzoic acid. An intermediate in the biosynthesis of 2,3-dihydroxybenzoic acid. Biochim Biophys Acta. 1969 May 6;177(3):381–388. doi: 10.1016/0304-4165(69)90300-6. [DOI] [PubMed] [Google Scholar]


