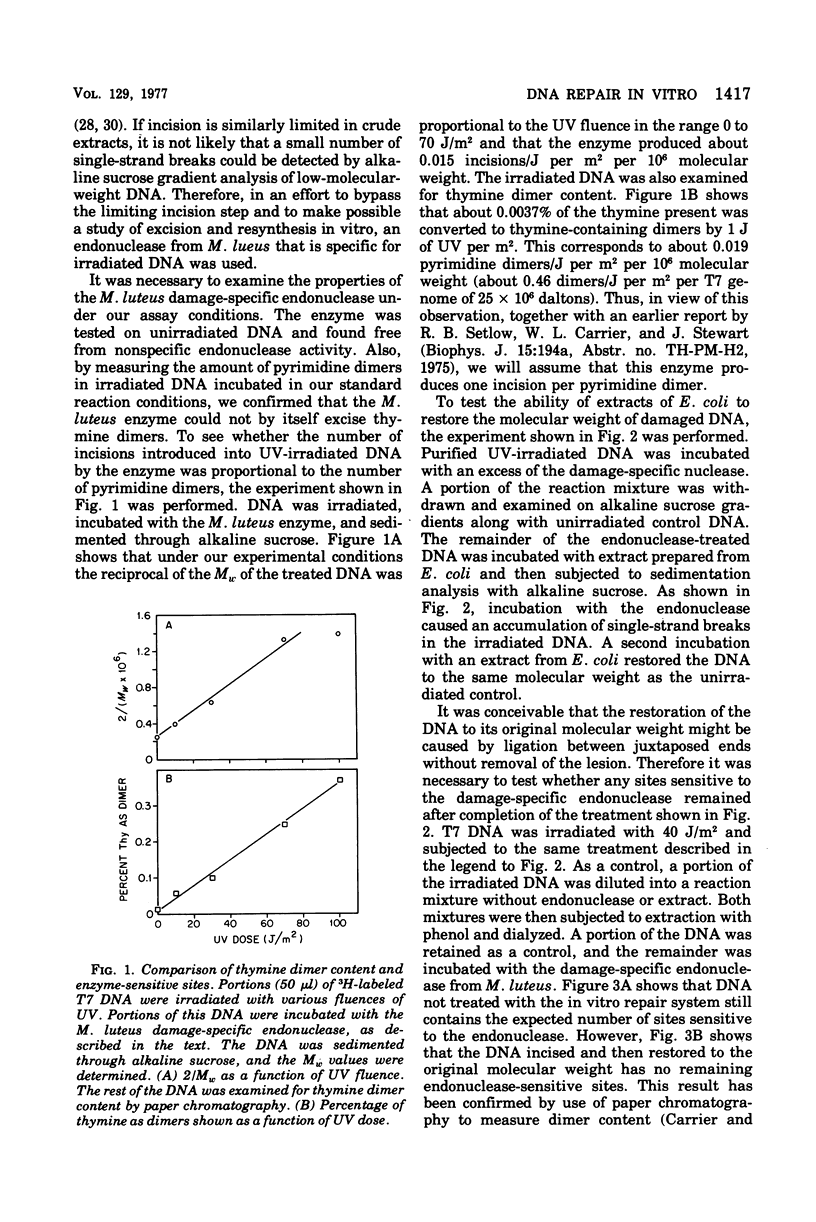
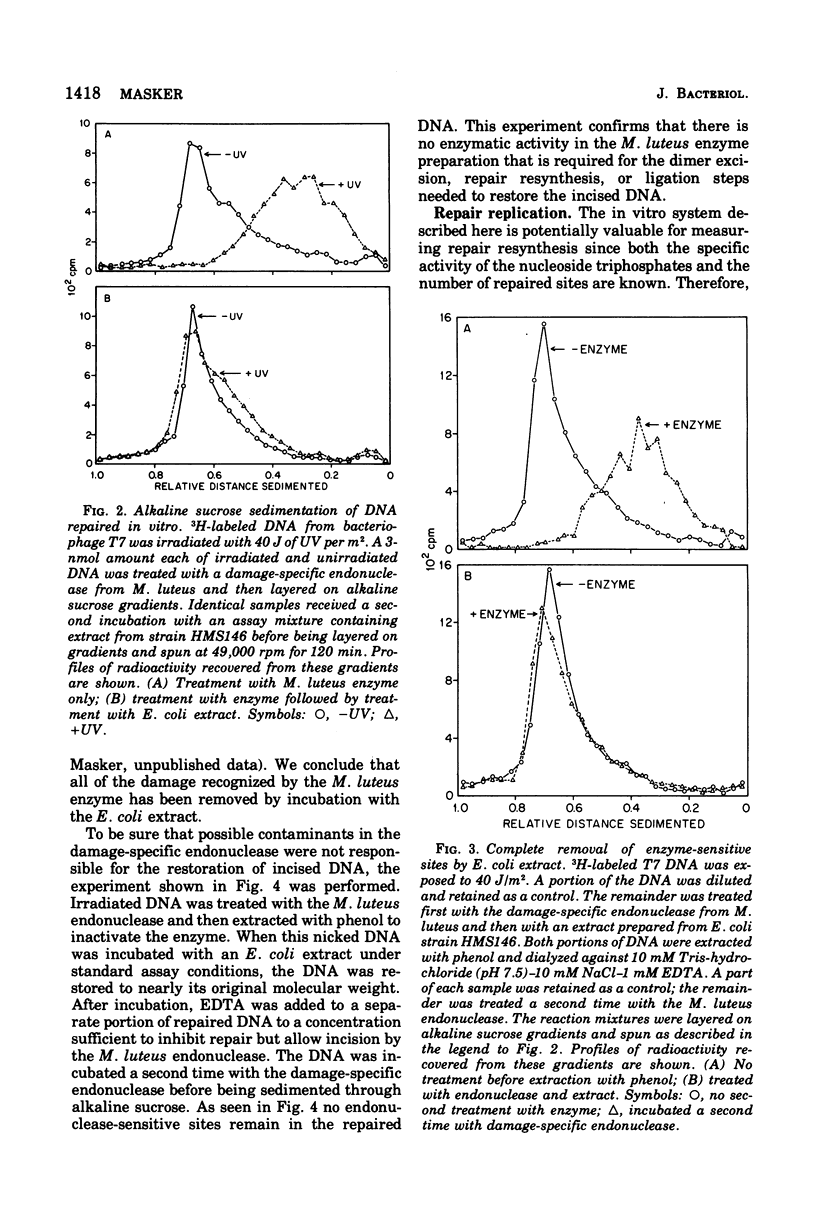
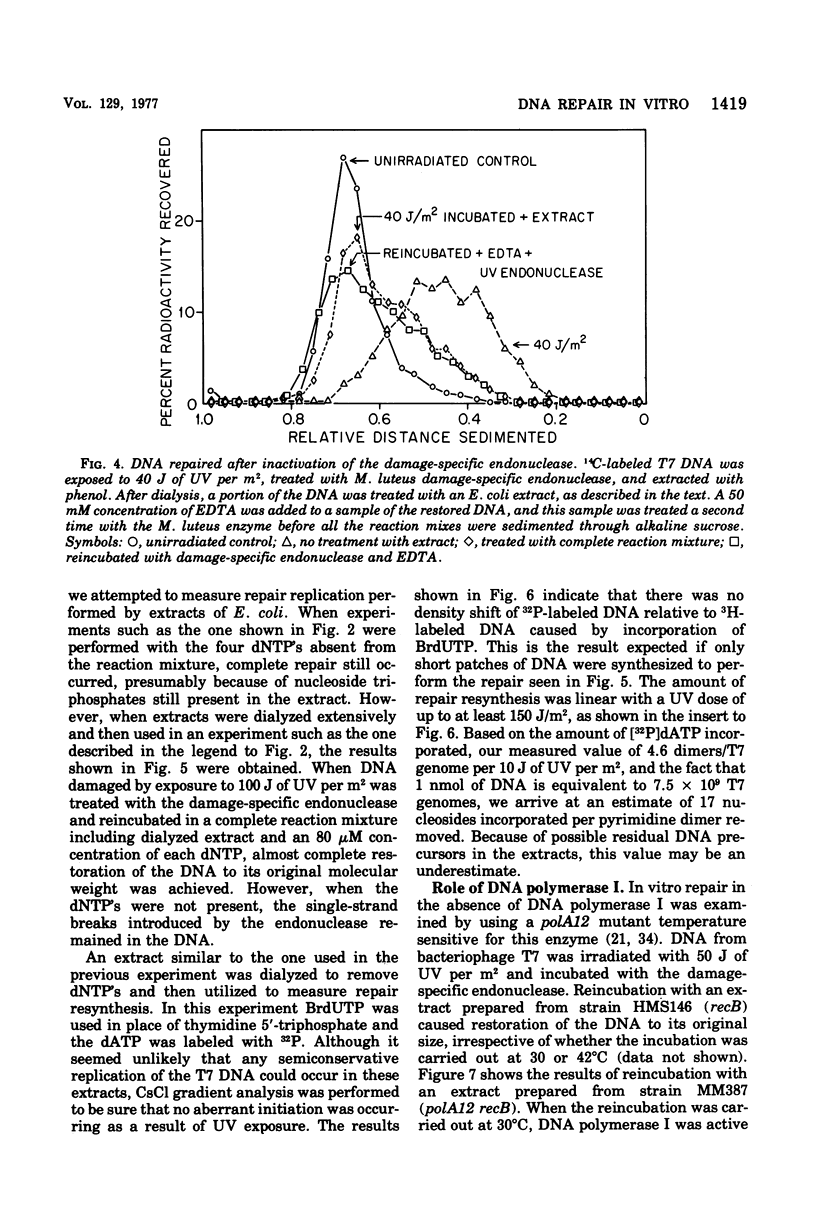
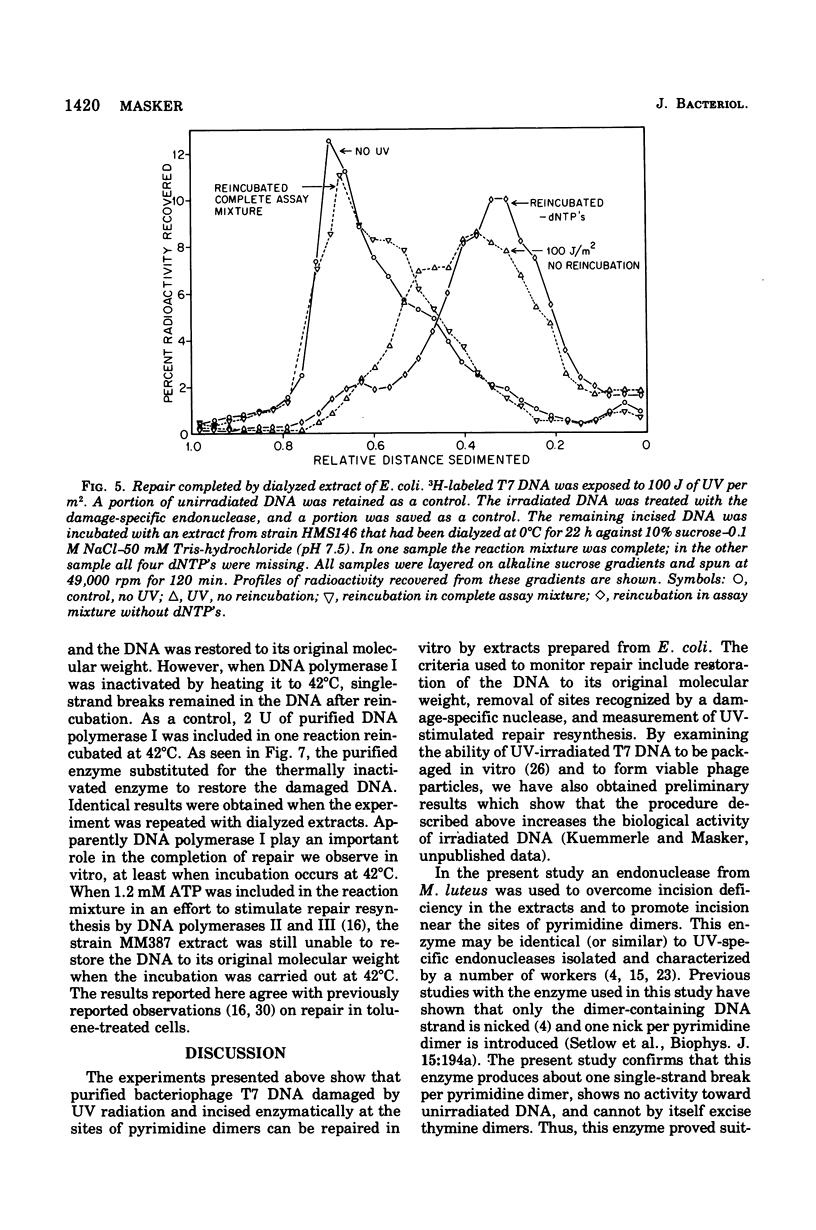
Abstract
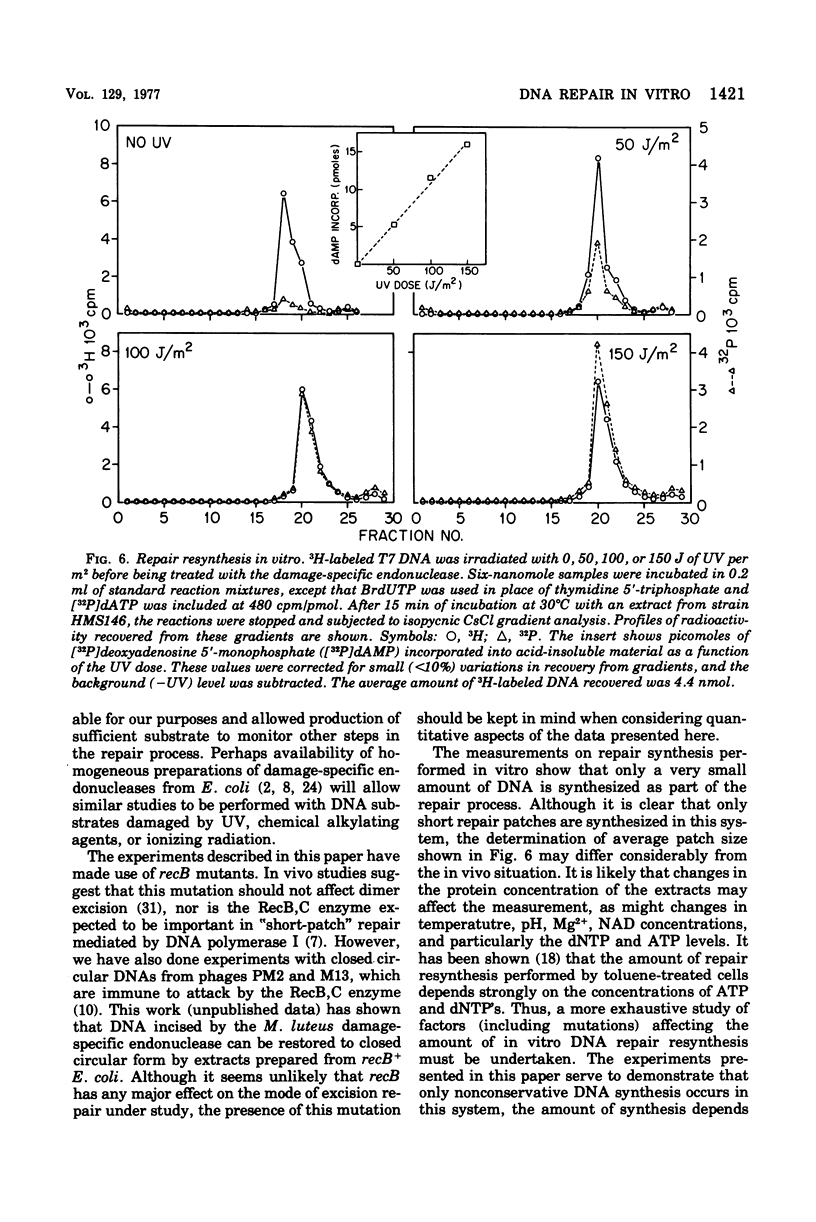
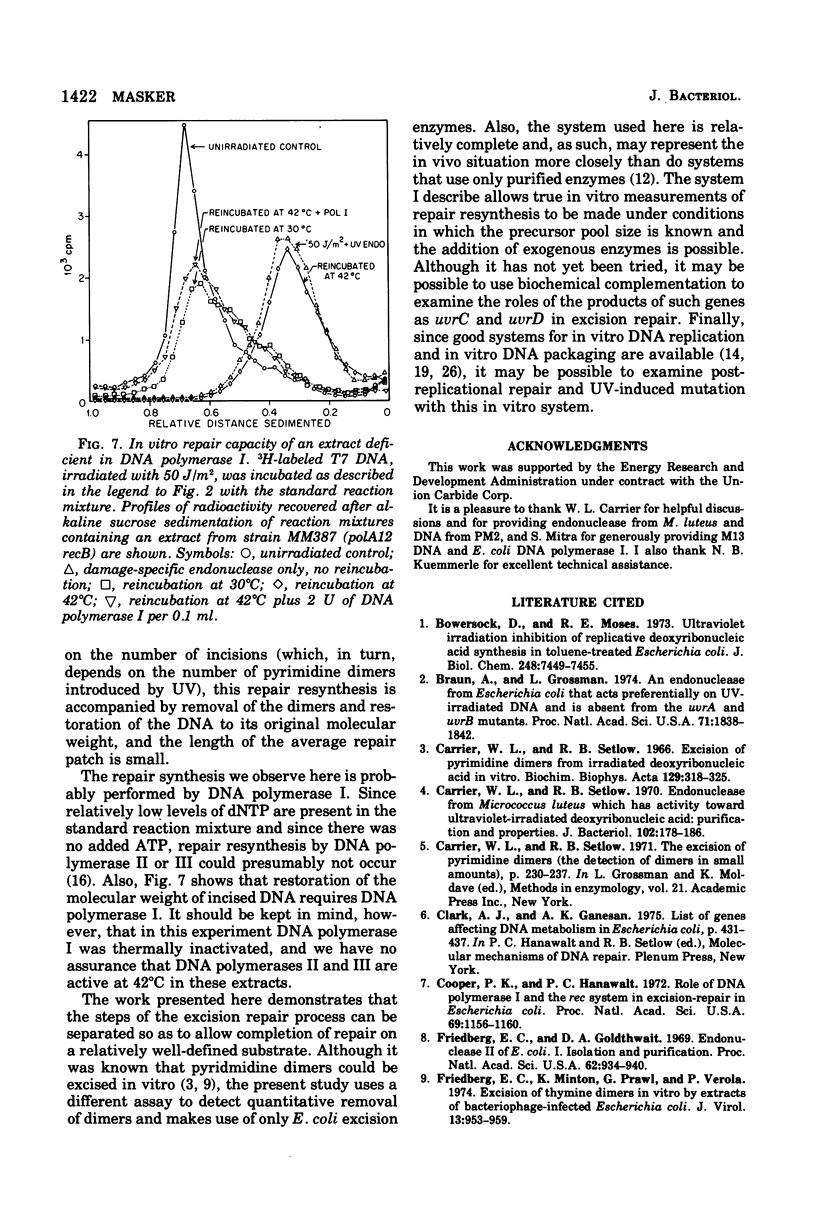
Deoxyribonucleic acid (DNA) from bacteriophage T7 has been used to monitor the capacity of gently lysed extracts of Escherichia coli to perform repair resynthesis after ultraviolet (UV) irradiation. Purified DNA damaged by up to 100 J of UV radiation per m2 was treated with an endonuclease from Micrococcus luteus that introduces single-strand breaks in irradiated DNA. This DNA was then used as a substrate to study repair resynthesis by extracts of E. coli. It was found that incubation with the extract and exogenous nucleoside triphosphates under suitable assay conditions resulted in removal of all pyrimidine dimers and restoration of the substrate DNA to its original molecular weight. Repair resynthesis, detected as nonconservative, UV-stimulated DNA synthesis, was directly proportional tothe number of pyrimidine dimers introduced by radiation. The repair mode described here appears to require DNA polymerase I since it does no occur at the restrictive temperature in polA12 mutants, which contain a thermolabile polymerase. The addition of purified DNA polymerase I to extracts made from a polA mutant restores the ability to complete repair at the restrictive temperature.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowersock D., Moses R. E. Ultraviolet irradiation inhibition of replicative deoxyribonucleic acid synthesis in toluene-treated Escherichia coli. J Biol Chem. 1973 Nov 10;248(21):7449–7455. [PubMed] [Google Scholar]
- Braun A., Grossman L. An endonuclease from Escherichia coli that acts preferentially on UV-irradiated DNA and is absent from the uvrA and uvrB mutants. Proc Natl Acad Sci U S A. 1974 May;71(5):1838–1842. doi: 10.1073/pnas.71.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Ganesan A. Lists of genes affecting DNA metabolism in Escherichia coli. Basic Life Sci. 1975;5B:431–437. doi: 10.1007/978-1-4684-2898-8_2. [DOI] [PubMed] [Google Scholar]
- Cooper P. K., Hanawalt P. C. Role of DNA polymerase I and the rec system in excision-repair in Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1156–1160. doi: 10.1073/pnas.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Goldthwait D. A. Endonuclease II of E. coli. I. Isolation and purification. Proc Natl Acad Sci U S A. 1969 Mar;62(3):934–940. doi: 10.1073/pnas.62.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Minton K., Pawl G., Verzola P. Excision of thymine dimers in vitro by extracts of bacteriophage-infected Escherichia coli. J Virol. 1974 May;13(5):953–959. doi: 10.1128/jvi.13.5.953-959.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmark P. J., Linn S. Purification and properties of the recBC DNase of Escherichia coli K-12. J Biol Chem. 1972 Mar 25;247(6):1849–1860. [PubMed] [Google Scholar]
- Grossman L., Braun A., Feldberg R., Mahler I. Enzymatic repair of DNA. Annu Rev Biochem. 1975;44:19–43. doi: 10.1146/annurev.bi.44.070175.000315. [DOI] [PubMed] [Google Scholar]
- Hamilton L., Mahler I., Grossman L. Enzymatic repair of deoxyribonucleic acid; the biochemical and biological repair properties of a deoxyribonucleic acid polymerase from micrococcus luteus. Biochemistry. 1974 Apr 23;13(9):1886–1896. doi: 10.1021/bi00706a017. [DOI] [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Excision of damaged thymine residues from gamma-irradiated poly(dA-dT) by crude extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3532–3536. doi: 10.1073/pnas.71.9.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkle D. C., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. Requirements for deoxyribonucleic acid synthesis and characterization of the product. J Biol Chem. 1974 May 10;249(9):2974–2980. [PubMed] [Google Scholar]
- Kaplan J. C., Kushner S. R., Grossman L. Enzymatic repair of DNA, 1. Purification of two enzymes involved in the excision of thymine dimers from ultraviolet-irradiated DNA. Proc Natl Acad Sci U S A. 1969 May;63(1):144–151. doi: 10.1073/pnas.63.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Nucleoside triphosphate dependence of repair replication in toluenized Escherichia coli. J Mol Biol. 1974 Sep 5;88(1):13–23. doi: 10.1016/0022-2836(74)90292-7. [DOI] [PubMed] [Google Scholar]
- Masker W. E., Hanawalt P. C. Ultraviolet-stimulated DNA synthesis in toluenzied Escherichia coli deficient in DNA polymerase I. Proc Natl Acad Sci U S A. 1973 Jan;70(1):129–133. doi: 10.1073/pnas.70.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Richardson C. C. Bacteriophage T7 deoxyribonucleic acid replication in vitro. V. Synthesis of intact chromosomes of bacteriophage T7. J Mol Biol. 1976 Feb 5;100(4):543–556. doi: 10.1016/s0022-2836(76)80044-7. [DOI] [PubMed] [Google Scholar]
- Masker W. E. The ATP dependence of the incision and resynthesis steps of excision repair. Biochim Biophys Acta. 1976 Aug 18;442(2):162–173. doi: 10.1016/0005-2787(76)90487-1. [DOI] [PubMed] [Google Scholar]
- Monk M., Kinross J. Conditional lethality of recA and recB derivatives of a strain of Escherichia coli K-12 with a temperature-sensitive deoxyribonucleic acid polymerase I. J Bacteriol. 1972 Mar;109(3):971–978. doi: 10.1128/jb.109.3.971-978.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H., Okubo S., Takagi Y. Repair of ultraviolet-damaged DNA in Micrococcus lysodeikticus. I. An endonuclease specific for ultraviolet-irradiated DNA. Biochim Biophys Acta. 1971 Jan 1;228(1):67–82. doi: 10.1016/0005-2787(71)90547-8. [DOI] [PubMed] [Google Scholar]
- Radman M. An endonuclease from Escherichia coli that introduces single polynucleotide chain scissions in ultraviolet-irradiated DNA. J Biol Chem. 1976 Mar 10;251(5):1438–1445. [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- Sadowski P. D., Vetter D. Genetic recombination of bacteriophage T7 DNA in vitro. Proc Natl Acad Sci U S A. 1976 Mar;73(3):692–696. doi: 10.1073/pnas.73.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E., Strike P. Excision repair of ultraviolet-irradiated deoxyribonucleic acid in plasmolyzed cells of Escherichia coli. J Bacteriol. 1976 Mar;125(3):787–795. doi: 10.1128/jb.125.3.787-795.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segev N., Miller C., Sharon R., Ben-Ishai R. Excision repair of ultraviolet radiation damage in toluene treated Escherichia coli. Biochem Biophys Res Commun. 1973 Jun 19;52(4):1241–1245. doi: 10.1016/0006-291x(73)90633-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Sharon R., Miller C., Ben-Ishai R. Two modes of excision repair in toluene-treated Escherichia coli. J Bacteriol. 1975 Sep;123(3):1107–1114. doi: 10.1128/jb.123.3.1107-1114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Anderson J. A., Barbour S. D. Excision repair properties of isogenic rec mutants of Escherichia coli K-12. J Bacteriol. 1972 Sep;111(3):723–730. doi: 10.1128/jb.111.3.723-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Uyemura D., Lehman I. R. Biochemical characterization of mutant forms of DNA polymerase I from Escherichia coli. I. The polA12 mutation. J Biol Chem. 1976 Jul 10;251(13):4078–4084. [PubMed] [Google Scholar]
- Waldstein E. A., Sharon R., Ben-Ishai R. Role of ATP in excision repair of ultraviolet radiation damage in Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2651–2654. doi: 10.1073/pnas.71.7.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngs D. A., Van der Schueren E., Smith K. C. Separate branches of the uvr gene-dependent excision repair process in ultraviolet-irradiated Escherichia coli K-12 cells; their dependence upon growth medium and the polA, recA, recB, and exrA genes. J Bacteriol. 1974 Feb;117(2):717–725. doi: 10.1128/jb.117.2.717-725.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


