Abstract
Angiostatin, a potent naturally occurring inhibitor of angiogenesis and growth of tumor metastases, is generated by cancer-mediated proteolysis of plasminogen. Human prostate carcinoma cells (PC-3) release enzymatic activity that converts plasminogen to angiostatin. We have now identified two components released by PC-3 cells, urokinase (uPA) and free sulfhydryl donors (FSDs), that are sufficient for angiostatin generation. Furthermore, in a defined cell-free system, plasminogen activators [uPA, tissue-type plasminogen activator (tPA), or streptokinase], in combination with one of a series of FSDs (N-acetyl-l-cysteine, d-penicillamine, captopril, l-cysteine, or reduced glutathione] generate angiostatin from plasminogen. An essential role of plasmin catalytic activity for angiostatin generation was identified by using recombinant mutant plasminogens as substrates. The wild-type recombinant plasminogen was converted to angiostatin in the setting of uPA/FSD; however, a plasminogen activation site mutant and a catalytically inactive mutant failed to generate angiostatin. Cell-free derived angiostatin inhibited angiogenesis in vitro and in vivo and suppressed the growth of Lewis lung carcinoma metastases. These findings define a direct mechanism for cancer-cell-mediated angiostatin generation and permit large-scale production of bioactive angiostatin for investigation and potential therapeutic application.
Because tumor growth and metastases are dependent upon angiogenesis (1–3), the identification of agents that inhibit angiogenesis now represents a potential therapeutic approach for the control of cancer (4–7). Angiostatin, consisting of the first four of five kringle domains of plasminogen (8), is one of a number of angiogenesis inhibitors that are internal fragments of larger nonangiogenic precursor proteins (8–14); however, the mechanisms by which these fragments are generated in vivo remains unknown. Although the activity sufficient to cleave plasminogen to angiostatin is present in tumor-bearing animals and serum-free conditioned medium (SFCM) of human prostate carcinoma cells (9), the cancer-dependent mechanism of angiostatin generation has remained unknown. Recently, macrophage-derived metalloelastase was shown to produce angiostatin from plasminogen and may contribute to angiostatin generation in the murine Lewis lung carcinoma model (15). We now describe the enzymatic mechanism for the direct generation of human angiostatin from plasminogen by human prostate cancer cells and demonstrate the generation of bioactive angiostatin from human plasminogen in a defined cell-free system.
MATERIALS AND METHODS
Angiostatin Generation.
Angiostatin was generated from PC-3 cell SFCM as described (9). To generate angiostatin in a cell-free system, human plasminogen (0.2 μM) was incubated with 0.2 nM recombinant human urokinase (uPA; Abbott), 1.0 nM recombinant human two-chain tissue-type plasminogen activator (tPA; a gift from Henry Berger, Glaxo–Wellcome), or 8.0 nM streptokinase (Sigma) and with 100 μM N-acetyl-l-cysteine (NAC), d-penicillamine, captopril, l-cysteine, or reduced glutathione (Sigma) at 37°C overnight. To confirm the requirement for plasmin catalytic activity, recombinant plasminogens (16) (0.2 μM) were added to 100-μl aliquots of 50 mM Tris, pH 9.0/20 mM NaCl/0.2 nM human recombinant uPA (Abbott)/100 μM NAC (Sigma) and incubated at 37°C overnight. The angiostatin product was examined by Western blot as described (9).
Protein Purification.
SFCM was applied to Reactive Red 120-agarose (Sigma) equilibrated with 50 mM Tris⋅HCl, pH 7.5/140 mM NaCl (TBS), and proteins were eluted with 1.0 M KCl. The eluate was dialyzed against TBS by using a 6- to 8-kDa cutoff membrane. Human plasminogen (0.2 μM) was incubated in 100-μl aliquots of SFCM, Reactive Red 120-agarose flow-through, dialyzed eluate, or combined flow-through and eluate at 37°C for 18 h. For anion-exchange chromatography, SFCM was diluted 1:5 in 50 mM Tris (pH 10.0) and applied to a High Q anion-exchange resin (Bio-Rad), and a linear gradient (50–300 mM NaCl/50 mM Tris, pH 10.0) was used for elution. The protein content of each fraction was estimated by measuring the absorbance at 280 nm and fractions were analyzed for angiostatin-generating activity. For isoelectric focusing, the SFCM was concentrated 10-fold by ultrafiltration (Amicon) using a molecular mass cutoff of 10 kDa and diluted 1:5 with sterile water to lower the NaCl concentration to 20 mM, and ampholyte carriers (pH 3.5–9.5, Bio-Rad) were added. The sample was then fractionated in a Rotofor Cell (Bio-Rad) that stabilized the proteins into 20 focused zones from pH 3.0 to 10.0. Each fraction was analyzed for pH and angiostatin-generating activity.
Cofactor Detection.
Human plasminogen (0.2 μM) was added to 100-μl aliquots of the dialyzed Reactive Red 120-agarose eluate supplemented with components in RPMI 1640 medium: salts, phenol red, a vitamin mixture, or an amino acid mixture (GIBCO/BRL). To define the cofactor necessary for angiostatin generation, the dialyzed eluate was incubated with individual components of RPMI 1640 medium, and samples were tested for angiostatin-generating activity by Western blot.
Plasminogen Activator Detection.
Fractions from the anion-exchange and Reactive Red 120-agarose eluates were examined with a coupled assay that measures plasminogen activation by monitoring the amidolytic activity of generated plasmin. Briefly, the eluates were dialyzed against TBS and incubated with plasminogen (0.3 μM) and the plasmin substrate d-Val-Leu-Lys-p-nitroanilide (0.3 mM; Sigma) at 37°C. Substrate cleavage was determined by monitoring the absorbance at 405 nm using a kinetic plate reader (Molecular Devices).
Plasmin Generation.
Human plasminogen (0.2 μM) in 100-μl aliquots of 50 mM Tris, pH 9.5/20 mM NaCl was incubated with 10 μl of uPA-Sepharose (Calbiochem) for 2 h at 37°C. After incubation, the sample was centrifuged to sediment the uPA-Sepharose, and the supernatant containing plasmin was collected. The complete conversion of plasminogen to plasmin was confirmed by analysis of the supernatant on reduced Coomassie-stained polyacrylamide gels. Plasmin was then incubated for 18 h with 100 μM NAC, and samples were analyzed for the presence of angiostatin.
Bioactivity of Angiostatin.
The angiostatin, generated in a cell-free system, was purified by affinity chromatography on lysine-Sepharose (Pharmacia Biotech) and examined on Western blots as described in Gately et al. (9). Endothelial cell migration assays were performed in a modified Boyden chamber with bovine adrenal capillary endothelial cells (a gift from J. Folkman) as described (17). The mouse corneal angiogenesis assays were performed as described (18).
The Lewis lung carcinoma metastasis model was performed as described by O’Reilly et al. (8). In brief, 1 × 106 low-metastatic Lewis lung carcinoma cells were inoculated subcutaneously into C57BL6/J mice (The Jackson Laboratory). When tumors reached approximately 1200–1800 mg in size (12–14 days after implantation), animals were randomly divided into one of three treatment conditions: For the positive control group, mice were left with tumors intact (n = 10); for the remaining animals, tumors were surgically resected. Tumor-resected mice received either cell-free-derived angiostatin, (0.15 mg, twice daily, subcutaneously) beginning on day 2 after surgery (n = 6) or, for negative control, received twice daily subcutaneous injections of phosphate-buffered saline (n = 6). Mice were sacrificed on days 25–27, and lung mass was measured to quantitate the growth of metastatic lung tumors.
RESULTS
Purification of the Factors Responsible for the Production of Angiostatin.
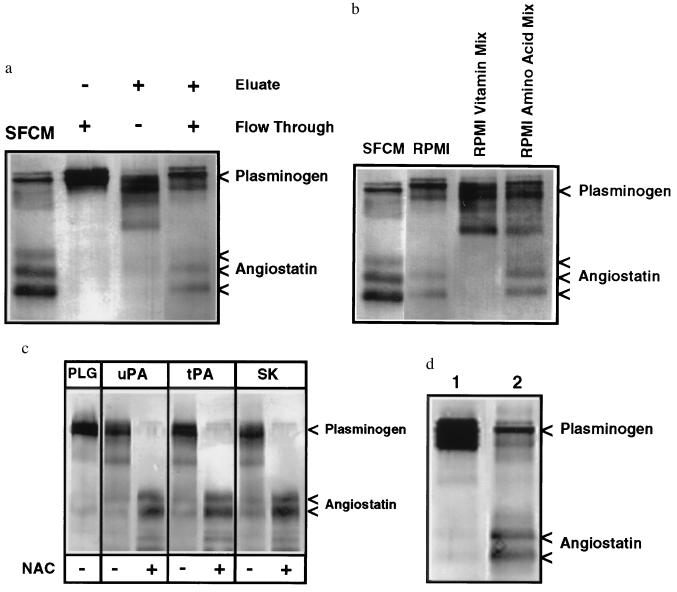
A significant loss of angiostatin-generating activity from human PC-3 prostate carcinoma cell SFCM (9) was observed after dialysis using 6- to 8-kDa molecular mass cutoff membranes, suggesting that a low molecular weight cofactor was required. Fractionation of SFCM on Reactive Red 120-agarose indicated that complementary components were required for angiostatin generation. The flow-through and the dialyzed eluate alone failed to generate angiostatin; however, combination of the flow-through and the eluate fraction restored angiostatin-generating activity (Fig. 1a). The flow-through component was stable to boiling, suggesting that this factor was not likely to be a protein. In contrast, the eluate component was thermolabile and was retained after dialysis consistent with the eluate containing a protein(s).
Figure 1.
Western blot analysis of angiostatin generation. (a) The angiostatin-generating activity of PC-3 SFCM required two distinct fractions from Reactive Red 120-agarose chromatography. Angiostatin was generated when plasminogen was incubated with SFCM. No angiostatin generation was detected by incubation of plasminogen with the Reactive Red 120-agarose flow-through or 1.0 M KCl eluate dialyzed against TBS. When the flow-through and dialyzed eluate were combined, however, angiostatin generation was restored. (b) A cofactor for angiostatin generation was present in unconditioned RPMI 1640 medium and its amino acid mixture. Plasminogen incubated with PC-3 SFCM generates angiostatin. Plasminogen incubated with the dialyzed Reactive Red 120-agarose eluate supplemented with unconditioned RPMI 1640 medium also generates angiostatin. The cofactor activity of RPMI 1640 medium, necessary for angiostatin generation, was not present in the RPMI 1640 vitamin mixture but was present in the RPMI 1640 amino acid mixture. (c) Angiostatin was generated in a cell-free system consisting of human 0.2 μM plasminogen (PLG), uPA, tPA, or streptokinase and the sulfhydryl donor N-acetyl-l-cysteine (NAC). Human plasminogen incubated with uPA (0.2 nM), tPA (1.0 nM), or streptokinase (8.0 nM) generated angiostatin only in the presence of NAC (100 μM). (d) Human PC-3 prostate carcinoma cells, cultured for 24 h in the sulfhydryl-depleted RPMI 1640 medium, secreted sufficient FSDs and uPA to generate angiostatin from plasminogen. Human plasminogen was incubated with sulfhydryl-depleted RPMI 1640 medium and uPA (lane 1) or with identical sulfhydryl-depleted RPMI 1640 medium conditioned by human PC-3 prostate carcinoma cells (lane 2).
The Flow-Through Component Necessary for Angiostatin Generation Was Identified as a Free Sulfhydryl Donor (FSD).
Addition of nonconditioned RPMI 1640 medium to the Reactive Red 120-agarose eluate resulted in the generation of angiostatin (Fig. 1b), indicating a component of RPMI 1640 medium could serve as a cofactor. Individual constituents of nonconditioned RPMI 1640 medium were then incubated with the dialyzed eluate. The amino acid mixture could complement the eluate for angiostatin generation (Fig. 1b), and testing of individual amino acids at concentrations present in RPMI 1640 medium indicated that l-cysteine is the only amino acid capable of complementing the eluate. The RPMI vitamin mixture (Fig. 1b) and other RPMI constituents could not serve as a cofactor. Because l-cysteine is a FSD, reduced glutathione (100 μM) and NAC (100 μM) were evaluated and also found to effectively complement the eluate for angiostatin generation.
The Protein in the Elution Necessary for Angiostatin Generation Is a Plasminogen Activator.
Fractionation of SFCM using isoelectric focusing indicated that angiostatin generation was associated with an isoelectric point of approximately 9.2, similar to uPA (19). Furthermore, anion-exchange chromatography of SFCM resulted in the copurification of the angiostatin-generating activity with uPA, and the Reactive Red 120-agarose eluate contained plasminogen activator activity. The inability to separate uPA from angiostatin-generating activity suggested a role for uPA in angiostatin generation. The observation that plasmin was also converted to angiostatin by PC-3 SFCM (9) suggested that prior conversion of plasminogen to plasmin would not be inhibitory for angiostatin generation. Human plasminogen was therefore incubated with catalytic amounts of uPA, tPA, and streptokinase with and without NAC (100 μM; Fig. 1c). These data demonstrate that a plasminogen activator and NAC were sufficient for the complete conversion of plasminogen to angiostatin. Additional experiments indicated that other FSDs (100 μM l-cysteine, 100 μM reduced glutathione, 100 μM d-penicillamine, or 100 μM captopril) could substitute for NAC for the production of angiostatin. Incubation of plasminogen with uPA and a nonsulfhydryl reducing agent, dexrazoxane (Zinecard, Pharmacia), did not generate angiostatin, demonstrating the specific requirement for a FSD. These data indicate that incubation of human plasminogen with a plasminogen activator and a FSD is sufficient for conversion to angiostatin.
Prostate Carcinoma Cells Release FSDs in Vitro.
Because RPMI 1640 medium contains FSDs in the form of l-cysteine and glutathione, to determine whether PC-3 cells release sufficient FSD to convert plasminogen/plasmin to angiostatin, PC-3 cells were cultured for 24 h in defined RPMI 1640 medium lacking reduced glutathione, l-cysteine, and l-methionine. This PC-3 SFCM was found to efficiently catalyze the conversion of plasminogen to angiostatin, indicating the cells release sufficient FSD and uPA for angiostatin generation (Fig. 1d).
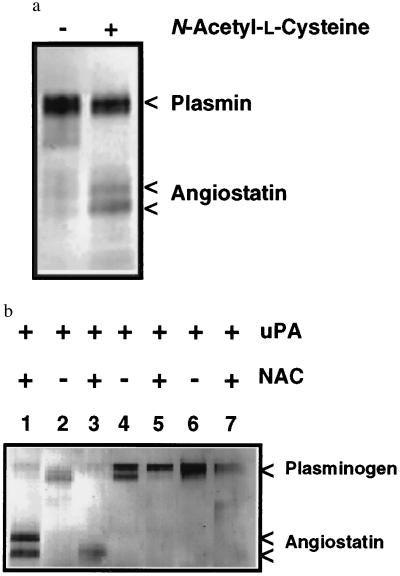
Plasmin as a Substrate for Angiostatin Generation.
Purified human plasmin, in the absence of uPA or other plasminogen activators, is converted to angiostatin in the presence of a FSD, suggesting a direct effect of the sulfhydryl donor on plasmin enzymatic activity or substrate specificity (Fig. 2a). To confirm a role for plasminogen conversion to plasmin and the catalytic role of plasmin in angiostatin generation, recombinant plasminogens (16) were evaluated as substrates for angiostatin generation. The R561A plasminogen activation site mutant is not susceptible to cleavage by plasminogen activators, whereas the D646E mutant yields a catalytically inactive plasmin due to a substitution of an essential amino acid in the serine proteinase catalytic domain. Both plasma-derived plasminogen and the wild-type recombinant plasminogen were converted to angiostatin when incubated with uPA and 100 μM NAC (Fig. 2b). However, the R561A mutant was not cleaved to plasmin or angiostatin under these conditions, providing further evidence that plasmin is an essential intermediate in angiostatin generation. The D646E mutant was converted to two-chain plasmin but angiostatin was not generated, demonstrating that plasmin catalytic activity is necessary for angiostatin generation (Fig. 2b).
Figure 2.
(a) Plasmin is converted to angiostatin in the presence of a FSD. Human plasminogen was converted to plasmin by incubation with uPA-Sepharose. Plasmin was only converted to angiostatin in the presence of 100 μM NAC. (b) Plasmin generation and catalytic activity is essential for angiostatin generation. Plasma-derived human plasminogen (0.2 μM), incubated with uPA (0.2 nM) and NAC (NAC) generates angiostatin (lane 1). The recombinant wild-type plasminogen (lanes 2 and 3) is also converted to angiostatin by the addition of uPA and NAC. The R561A activation site mutant (lanes 4 and 5), not susceptible to activation by plasminogen activators, failed to generate angiostatin when incubated with uPA and NAC. The D646E catalytically inactive mutant (lanes 6 and 7) also failed to generate angiostatin, demonstrating the requirement for plasmin catalytic activity.
Bioactivity of Affinity-Purified Cell-Free-Derived Angiostatin.
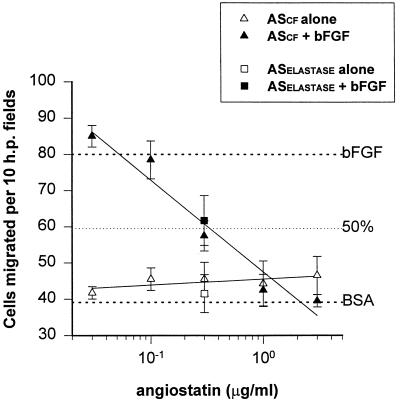
The affinity-purified angiostatin produced in the cell-free system (ASCF) was biologically active, suppressing basic fibroblast growth factor (bFGF)-induced endothelial cell proliferation with an ED50 of approximately 15 μg/ml, similar to the PC-3-derived angiostatin (9). Inhibition of bFGF-induced endothelial cell migration in vitro by the cell-free angiostatin was comparable to the PC-3-derived (9) and elastase-generated angiostatin (generously provided by Michael O’Reilly, Harvard Medical School), with an observed ED50 of 0.33 μg/ml (Fig. 3). As shown in Table 1, the cell-free angiostatin inhibited bFGF-induced angiogenesis in the mouse cornea as was shown for the PC-3-derived angiostatin (9).
Figure 3.
Inhibition of bovine capillary endothelial cell migration by angiostatin produced in a cell-free system (ASCF). Endothelial cell migration in a Boyden chamber toward a range of concentrations of angiostatin was measured in the presence (solid symbol) or absence (open symbol) of stimulatory bFGF. As a control, single points using elastase-generated angiostatin (ASElastase) are shown (squares). These data demonstrate that ASCF inhibits bFGF-induced endothelial cell migration in a dose-dependent manner, with an ED50 of 0.33 μg/ml.
Table 1.
In vivo inhibitory activity of cell-free produced angiostatin
| Compound tested | No. positive corneas/ total no. implanted |
|---|---|
| bFGF (50 ng per pellet) | 4/4 |
| Angiostatin (200 ng per pellet) | 0/4 |
| bFGF + angiostatin | 0/4 |
Pellets were formulated with the indicated compounds and implanted into the corneas of mice, and neovascularization was assessed by slit-lamp microscopy 5 days later. Vigorous growth of vessels into the normally avascular cornea was scored as a positive response.
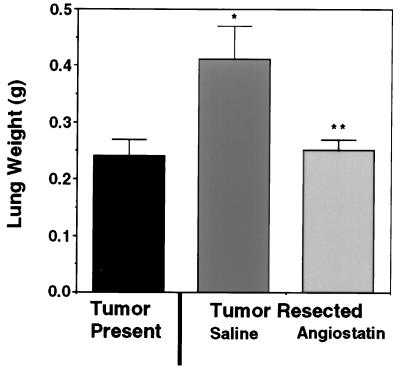
Administration of the cell-free-produced angiostatin to mice significantly inhibited the growth of Lewis lung carcinoma metastases (Fig. 4). Surgical resection of primary subcutaneous Lewis lung tumors in mice resulted in numerous macroscopic metastases and a 71% increase in lung mass compared with animals in which the primary tumors were not resected (Fig. 4). By contrast, administration of angiostatin produced in a cell-free system suppressed the increase in lung weight to a comparable level as observed in animals with the primary tumor intact, and only microscopic metastases were observed. These data not only support the model that primary tumors can suppress the growth of metastases by the generation of an inhibitor of angiogenesis, angiostatin, but also confirm the biological activity of the cell-free-produced angiostatin.
Figure 4.
Angiostatin produced in a cell-free system suppresses the growth Lewis lung carcinoma lung metastases after resection of the primary tumor. The presence of the primary subcutaneous Lewis lung tumor suppressed the expansion of lung metastases (tumor present control). By contrast resection of the Lewis lung tumor and administration of saline resulted in a significant increase in the mean lung mass compared with the tumor present control, confirming primary tumor-mediated suppression of metastatic tumor growth (∗, P < 0.01). Subcutaneous administration of angiostatin after removal of the primary tumor, significantly suppressed the expansion of lung metastases to levels comparable to the tumor control group (angiostatin compared with saline; ∗∗, P < 0.01).
DISCUSSION
The results presented demonstrate the mechanism by which human prostate carcinoma cells convert plasminogen to the angiogenesis inhibitor angiostatin. Plasminogen is first converted to the two-chain serine proteinase plasmin, by uPA, and in the presence of a FSD, plasmin serves as both the substrate and enzyme for the generation of angiostatin (Fig. 5). This pathway was confirmed by the ability to convert plasminogen to angiostatin in a cell-free system using one of three available plasminogen activators and one of a series of physiological or pharmacological FSDs. Furthermore, the angiostatin generated in the cell-free system was shown to be bioactive, demonstrating antiangiogenic activity in vitro and in vivo and suppressing the growth of lung metastases in the mouse Lewis lung carcinoma model.
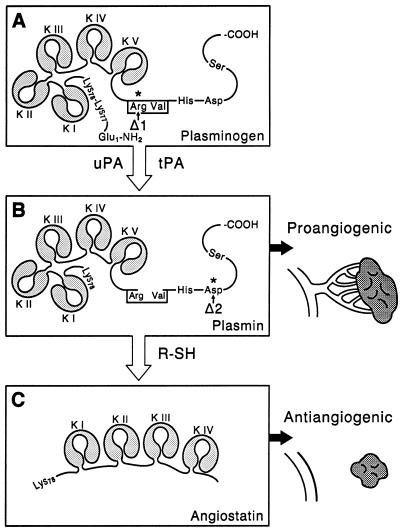
Figure 5.
Conversion of the proangiogenic proteinase plasmin to the angiogenesis inhibitor angiostatin. (A) The zymogen plasminogen is converted to the active proteinase plasmin by cleavage of the Arg560-Val561 peptide bond by plasminogen activators such as uPA and tPA. (B) Plasmin is a proangiogenic proteinase capable of degrading a variety of extracellular matrix proteins, facilitating endothelial cell migration and angiogenesis. (C) Plasmin in the presence of a FSD is converted to the angiogenesis inhibitor angiostatin. The plasminogen activation site mutant R561A, indicated by Δ1, is not cleaved by plasminogen activators, preventing conversion of plasminogen to the plasmin intermediate required for angiostatin generation. The plasminogen mutant D646E, indicated by Δ2, is cleaved by plasminogen activators, but the resulting two-chain plasmin is inactive due to the substitution of a catalytically essential aspartic acid residue in the serine proteinase catalytic triad. In the presence of a FSD, the inactive D646E mutant plasmin is not converted to angiostatin, demonstrating the requirement for plasmin catalytic activity.
The local or systemic availability of FSDs may be an important regulatory point in the angiogenic cascade in physiologic and pathologic settings. The role of the FSD is not yet known but could be involved in modification of the conformation of plasmin, altering enzymatic activity or allowing plasmin to be cleaved at previously unrecognized sites. The observation that a FSD is required for angiostatin generation suggests that the reported antiangiogenic properties of pharmacologic sulfhydryl donors such as d-penicillamine and captopril (20–24) may be due to their ability to promote the conversion plasmin, a normally proangiogenic proteinase (25), to the angiogenic inhibitor angiostatin. The potential loss of plasmin catalytic activity that would result from plasmin conversion to angiostatin (Fig. 5) may contribute to reduced fibrinolysis and the hypercoagulable state often observed in patients with cancer (26).
The angiostatin-generating activity released by human prostate carcinoma cells was not blocked by inhibitors of elastase or metal-dependent proteinases (9). These data suggest a direct mechanism of angiostatin generation by human prostate cancer cells, in contrast to the indirect mechanism of angiostatin generation, dependent upon expression of metalloelastase by tumor-infiltrating macrophages (15). Thus, these data demonstrate alternative models of angiostatin generation, suggesting there may be multiple pathways for the generation of angiostatin.
The identification of a direct mechanism of human prostate cancer-mediated angiostatin generation and the recapitulation of this process in a cell-free system allow for the efficient large-scale production of angiostatin that is antiangiogenic and capable of suppressing the growth of Lewis lung carcinoma metastases. The ability to produce angiostatin in a cell-free system will allow for large-scale production of this protein for in vivo testing as an novel anticancer agent. In addition, the elucidation of the components required for plasminogen conversion to angiostatin could permit the direct in vivo generation of angiostatin in the patient by administration of a plasminogen activator with a pharmacologic FSD.
Acknowledgments
We thank M.S. O’Reilly for providing the elastase-generated angiostatin and R. Bacallao for assistance with isoelectric focusing. This work was supported in part by The Feinberg Cardiovascular Research Institute; a grant-in-aid from the American Cancer Society, Illinois Division; National Institutes of Health Grants CA71875 (to G.A.S.), CA58900 (to M.S.S.), and HL13423 (to F.J.C.); and Veterans Administration Merit Review Research Grant (to H.C.K.).
ABBREVIATIONS
- uPA
urokinase-type plasminogen activator
- tPA
tissue-type plasminogen activator
- NAC
N-acetyl-l-cysteine
- bFGF
basic fibroblast growth factor
- FSD
free sulfhydryl donor
- SFCM
serum-free conditioned medium
- ASCF
angiostatin produced in the cell-free system
References
- 1.Folkman J. J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J, Watson K, Ingber D, Hanahan D. Nature (London) 1989;339:58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- 3.Weidner N, Semple J P, Welch W R, Folkman J. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 4.Fotsis T, Zhang Y, Pepper M S, Adlercreutz H, Montesano R, Nawroth P P, Schweigerer L. Nature (London) 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 5.Kim K J, Li B, Winer J, Armanini M, Gillett N, Phillips H S, Ferrara N. Nature (London) 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 6.O’Reilly M S, Holmgren L, Chen C, Folkman J. Nat Med. 1996;2:689–692. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren L, O’Reilly M S, Folkman J. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 8.O’Reilly M S, Holmgren L, Shing Y, Chen C, Rosenthal R A, Moses M, Lane W S, Cao Y, Sage E H, Folkman J. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 9.Gately S, Twardowski P, Stack M S, Patrick M, Boggio L, Cundiff D L, Schnaper H W, Madison L, Volpert O, Bouck N, Enghild J, Kwaan H C, Soff G A. Cancer Res. 1996;56:4887–4890. [PubMed] [Google Scholar]
- 10.O’Reilly M S, Boehm T, Shing Y, Fukai N, Vasios G, Lane W S, Flynn E, Birkhead J R, Olsen B R, Folkman J. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S K, Hassel T, Singh J P. Proc Natl Acad Sci USA. 1995;92:7799–7803. doi: 10.1073/pnas.92.17.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sage E H, Bassuk J A, Yost J C, Folkman M J, Lane T F. J Cell Biochem. 1995;57:127–140. doi: 10.1002/jcb.240570113. [DOI] [PubMed] [Google Scholar]
- 13.Clapp C, Martial J A, Guzman R C, Rentier-Delure F, Weiner R I. Endocrinology. 1993;133:1292–1299. doi: 10.1210/endo.133.3.7689950. [DOI] [PubMed] [Google Scholar]
- 14.Good D J, Polverini P J, Rastinejad F, Le Beau M M, Lemons R S, Frazier W A, Bouck N P. Proc Natl Acad Sci USA. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong Z, Kumar R, Yang X, Fidler I J. Cell. 1997;88:801–810. doi: 10.1016/s0092-8674(00)81926-1. [DOI] [PubMed] [Google Scholar]
- 16.Grella D, Castellino F J. Blood. 1997;89:1585–1589. [PubMed] [Google Scholar]
- 17.Rastinejad F, Polverini P J, Bouck N P. Cell. 1989;56:345–355. doi: 10.1016/0092-8674(89)90238-9. [DOI] [PubMed] [Google Scholar]
- 18.Kenyon B M, Voest E E, Chen C C, Flynn E, Folkman J, D’Amato R J. Invest Ophthalmol Visual Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 19.Colombi M, Rebessi L, Boiocchi M, Barlati S. Cancer Res. 1986;46:5748–5753. [PubMed] [Google Scholar]
- 20.Volpert O V, Ward W F, Lingen M W, Chesler L, Solt D B, Johnson M D, Molteni A, Polverini P J, Bouck N P. J Clin Invest. 1996;98:671–679. doi: 10.1172/JCI118838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brem S S, Zagzag D, Tsanaclis A M, Gately S, Elkouby M P, Brien S E. Am J Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
- 22.Matsubara T, Saura R, Hirohata K, Ziff M. J Clin Invest. 1989;83:158–167. doi: 10.1172/JCI113853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsubara T, Ziff M. J Clin Invest. 1987;79:1440–1446. doi: 10.1172/JCI112972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch A E, Burrows J C, Polverini P J, Cho M, Leibovich S J. Agents Actions. 1991;34:350–357. doi: 10.1007/BF01988728. [DOI] [PubMed] [Google Scholar]
- 25.Mignatti P, Rifkin D B. Curr Top Microbiol Immunol. 1996;213:33–50. doi: 10.1007/978-3-642-61107-0_3. [DOI] [PubMed] [Google Scholar]
- 26.Green K B, Silverstein R L. Hematol Oncol Clin N Am. 1996;10:499–530. doi: 10.1016/s0889-8588(05)70349-x. [DOI] [PubMed] [Google Scholar]