Abstract
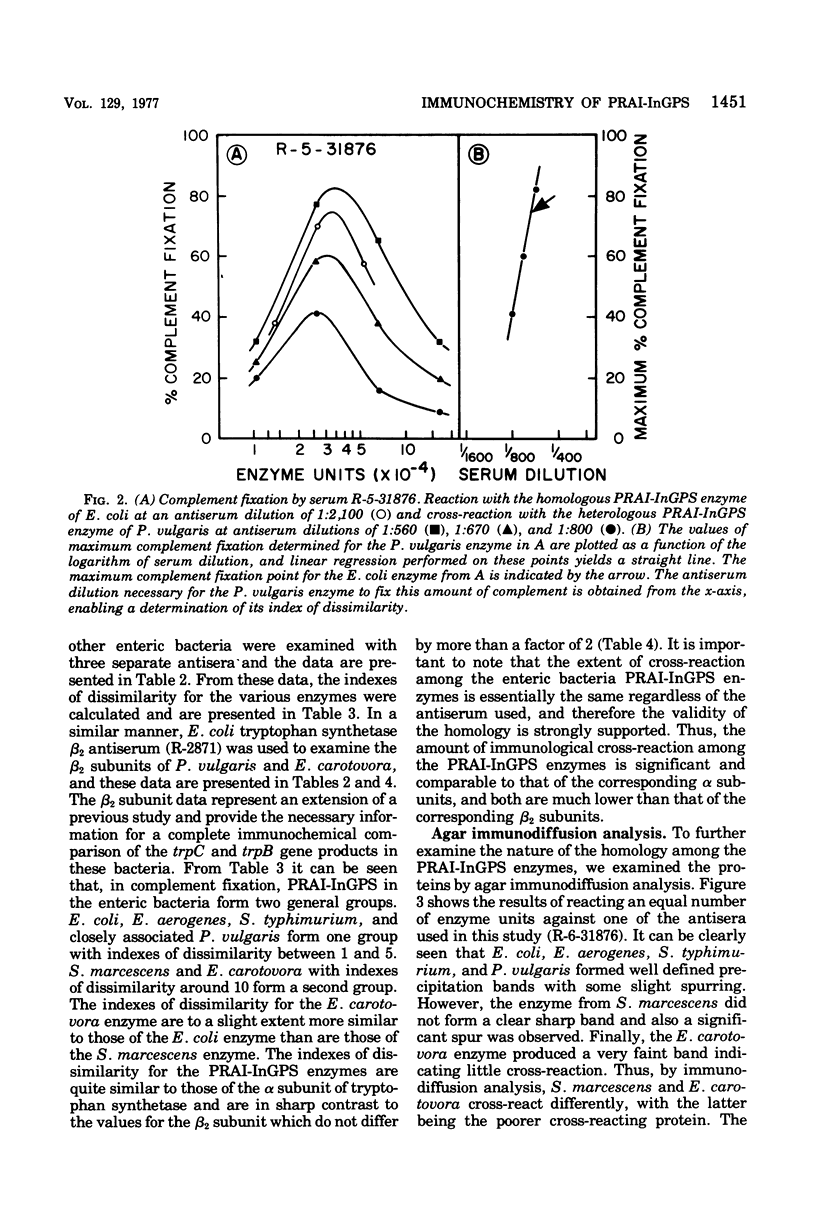
The bifunctional enzyme of the tryptophan operon, phosphoribosylanthranilate isomerase-indoleglycerol phosphate synthetase (PRAI-InGPS;EC 4.1.1.48), was characterized by an immunochemical study of six representative members of the Enterobacteriaceae: Escherichia coli, Salmonella typhimurium, Enterobacter aerogenes, Serratia marcescens, Erwinia carotovora, and Proteus vulgaris. PRAI-InGPS was purified from E. coli, and antisera were prepared in rabbits. These antisera were utilized in quantitative microcomplement fixation allowing for a comparison of the overall antigenic surface structure of the various homologous enzymes. These data showed E. coli PRAI-InGPS and S. marcescens and E. carotovora PRAI-InGPS (taken as a group) to have an index of dissimilarity of approximately 10, whereas the other organisms had values intermediate. In addition, antiserum to E. coli tryptophan synthetase beta2 subunit was used in microcomplement fixation to extend the previous comparison of this subunit (Rocha, Crawford, and Mills, 1972) to E. carotovora and P. vulgaris. Indexes of dissimilarity for E. coli compared to P. vulgaris of E. carotovora were 1.0 and 1.7, respectively. Agar immunodiffusion using PRAI-Ingps antisera showed significant cross-reaction among E. coli, E. aerogenes, S. typhimurium, and P. vulgaris whereas the enzymes from S. marcescens and E. carotovora cross-reacted to a lesser extent, with the latter reaction being quite weak. Comparative enzyme neutralization using E. coli PRAI-InGPS antisera showed significant cross-reactions among the enzymes in that all were neutralized at least 25%. The data taken together indicate that the trpC gene products in the Enterobacteriaceae are a homologous group of proteins, that the genetic divergene of the trpC gene is basically the same as the trpA gene, and that both are less conserved than the trpB gene. Furthermore, the PRAI-InGPS, enzyme active site appears to represent a more evolutionarily conserved region of the protein. These findings indicate that, with respect to PRAI-InGPS, similarity to E. coli among the organisms examined is in the following order: (E. aerogenes, S. typhimurium, P. vulgaris) greater than (S. marcescens, E. carotovora).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Helinski D. R., Somerville R. L., Yanofsky C. Comparison of the tryptophan synthetase alpha-subunits of several species of Enterobacteriaceae. J Bacteriol. 1966 May;91(5):1819–1826. doi: 10.1128/jb.91.5.1819-1826.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E., Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616–4624. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Denney R. M., Yanofsky C. Detection of tryptophan messenger RNA in several bacterial species and examination of the properties of heterologous DNA-RNA hybrids. J Mol Biol. 1972 Mar 14;64(2):319–339. doi: 10.1016/0022-2836(72)90501-3. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Margoliash E. Construction of phylogenetic trees. Science. 1967 Jan 20;155(3760):279–284. doi: 10.1126/science.155.3760.279. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Drapeau G. R., Carlton B. C., Yanofsky C. The amino acid sequence of the A protein (alpha subunit) of the tryptophan synthetase of Escherichia coli. J Biol Chem. 1967 Nov 25;242(22):5442–5446. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li S. L., Yanofsky C. Amino acid sequences of fifty residues from the amino termini of the tryptophan synthetase chains of several enterobacteria. J Biol Chem. 1972 Feb 25;247(4):1031–1037. [PubMed] [Google Scholar]
- McQuade J. F., 3rd, Creighton T. E. Purification and comparison of the N-(5'-phosphoribosyl)anthranilic acid isomerase-indole-3-glycerol phosphate synthetase of tryptophan biosynthesis from three species of Enterobacteriaceae. Eur J Biochem. 1970 Oct;16(2):199–207. doi: 10.1111/j.1432-1033.1970.tb01072.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager E. M., Wilson A. C. The dependence of immunological cross-reactivity upon sequence resemblance among lysozymes. I. Micro-complement fixation studies. J Biol Chem. 1971 Oct 10;246(19):5978–5989. [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Generation time and genomic evolution in primates. Science. 1973 Mar 16;179(4078):1144–1147. doi: 10.1126/science.179.4078.1144. [DOI] [PubMed] [Google Scholar]
- Smith O. H. Structure of the trpC cistron specifying indoleglycerol phosphate synthetase, and its localization in the tryptophan operon of Escherichia coli. Genetics. 1967 Sep;57(1):95–105. doi: 10.1093/genetics/57.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wegman J., Crawford I. P. Tryptophan synthetic pathway and its regulation in Chromobacterium violaceum. J Bacteriol. 1968 Jun;95(6):2325–2335. doi: 10.1128/jb.95.6.2325-2335.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]




