Abstract
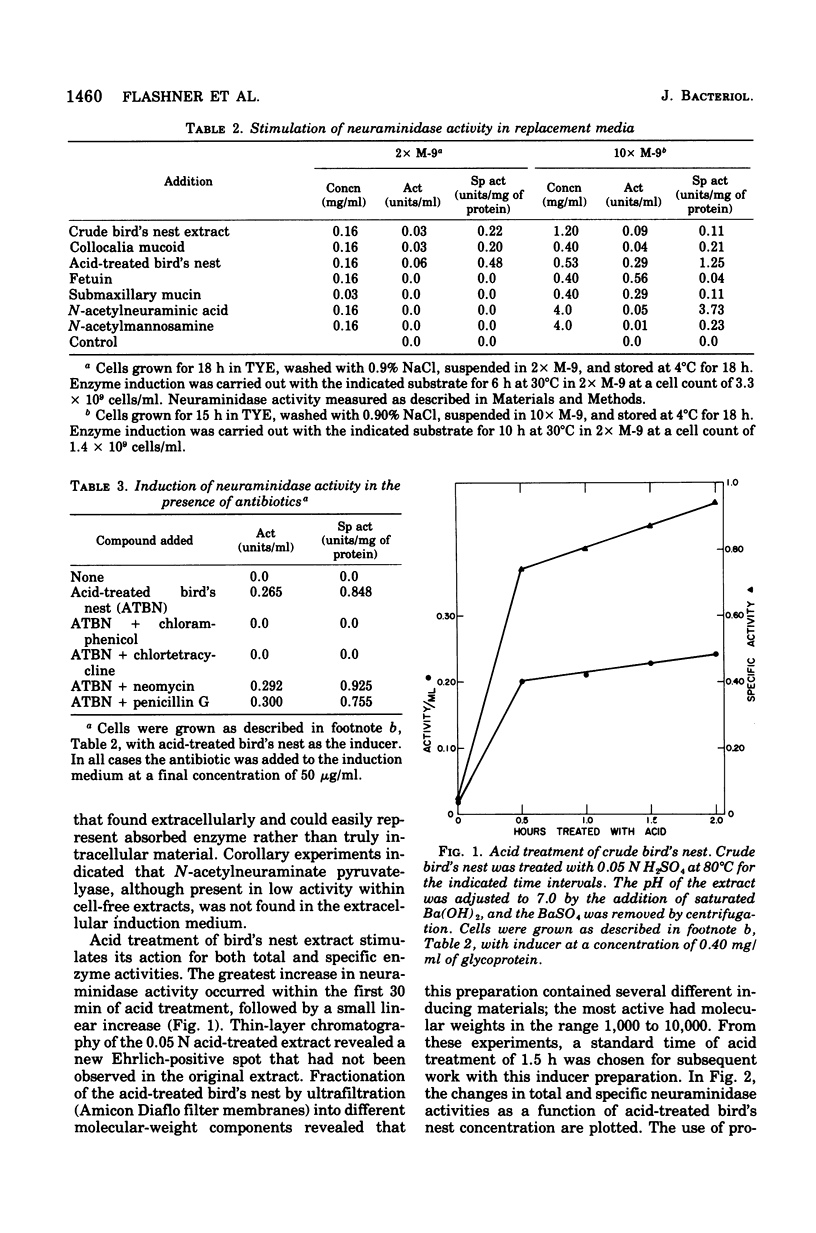
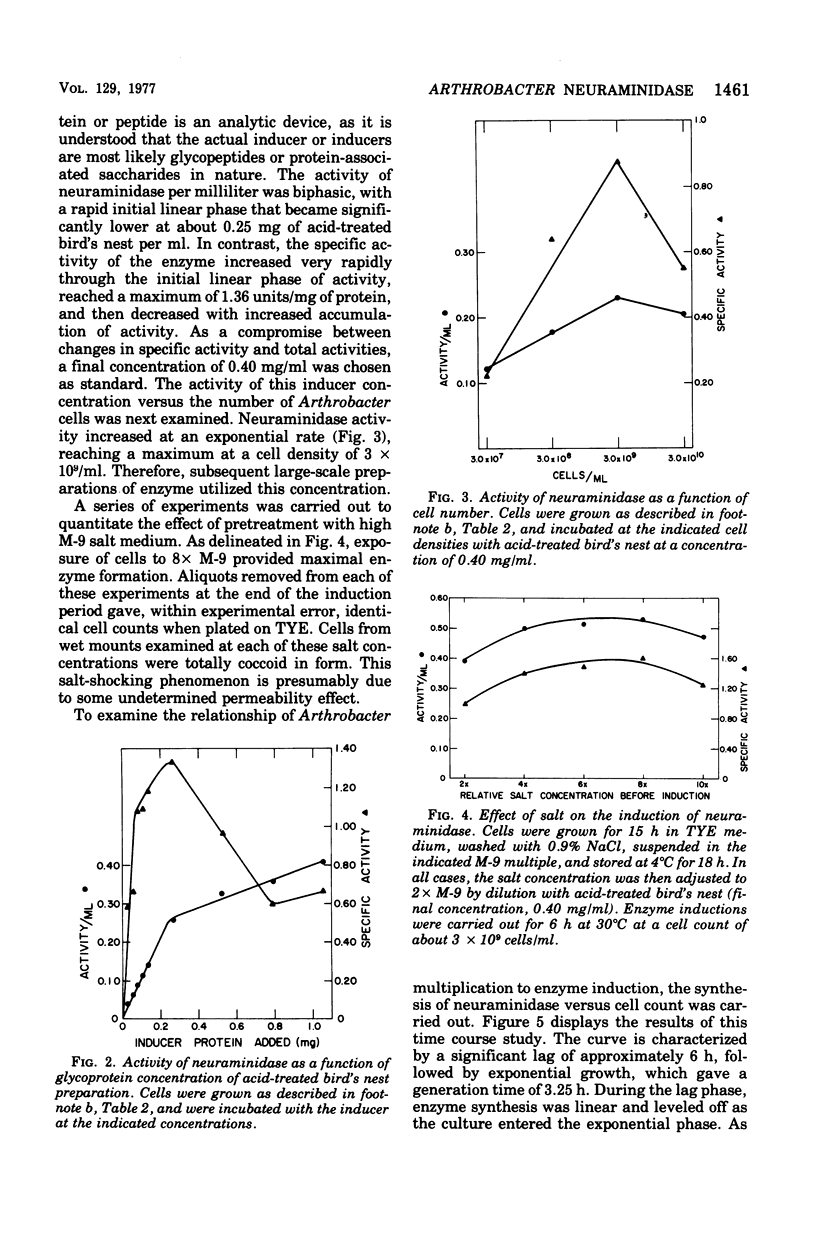
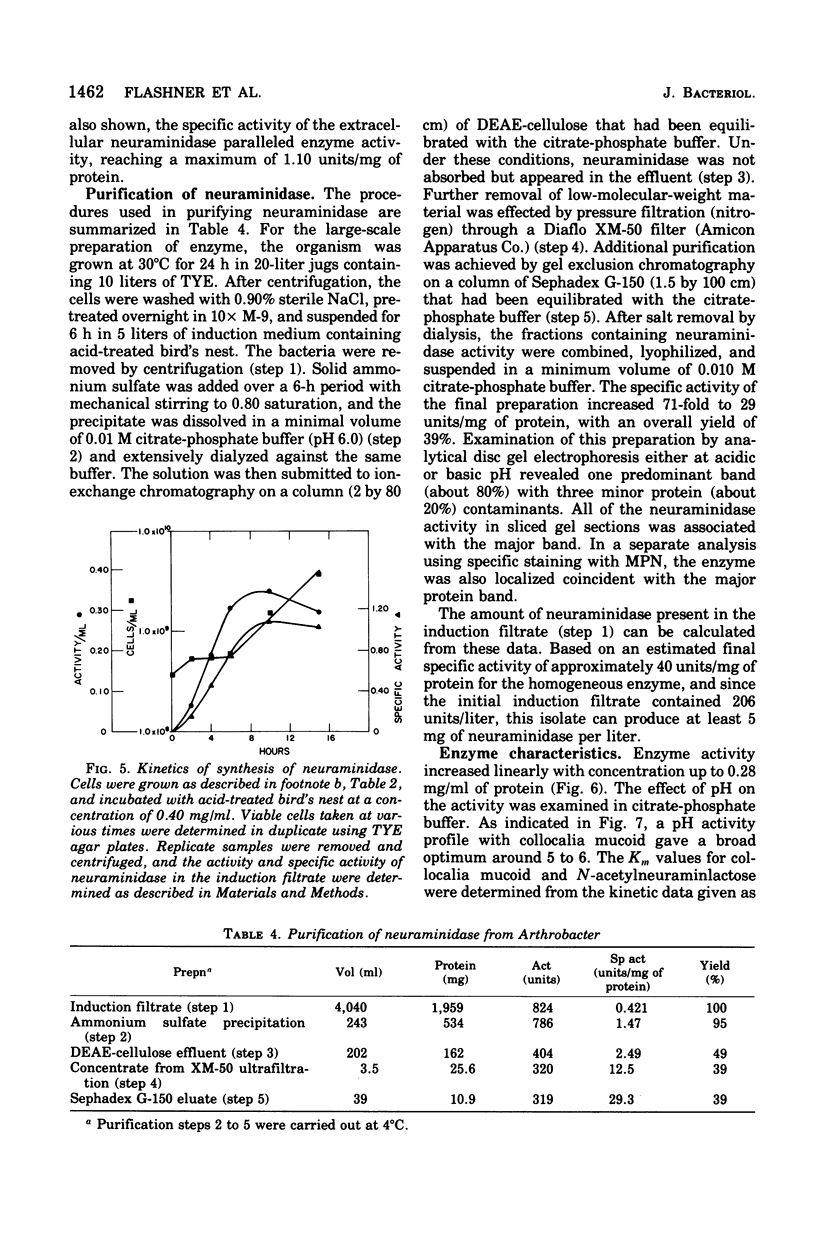
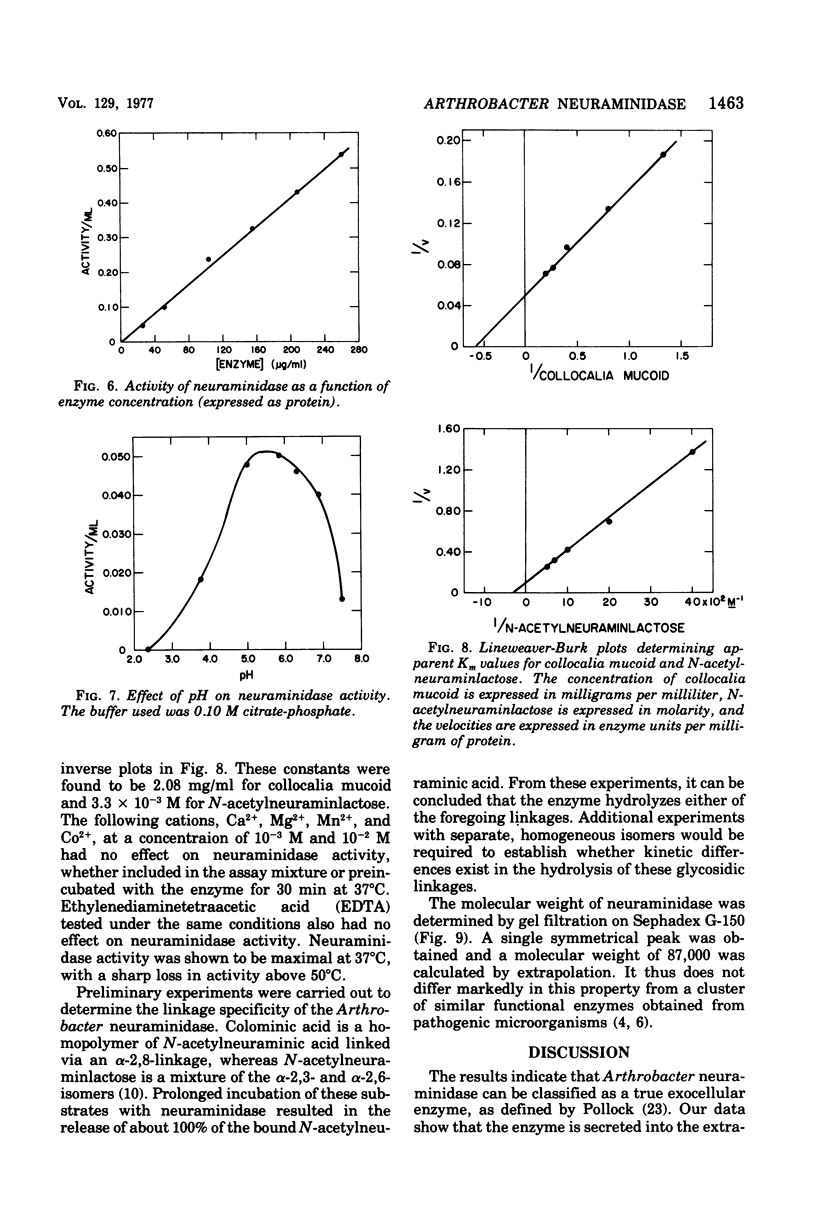
The elective isolation of a soil microorganism, tentatively assigned to the genus Arthrobacter, which produced an extracellular neuraminidase is described. The secretion of neuraminidase from washed cells in minimal medium required the presence of sialo-containing glycoproteins, whereas free N-acetyl-neuraminic asid of N-acetylmannosamine were poor inducers. No enzyme could be dected in the induction fitrated of cells, in the absence of inducer or in the culture filtrate of cells grown in a complete medium. The routine enzyme inducer was a hot-water extract of "edible bird's nest." Mild acid treatment (0.05 N H2SO4) of this extract increased enzyme activity two--to threefold and the specific activity about eightfold. Neuraminidase induction with acid-treated bird's nest was manifested at a linear rate for 6 h without increase in cell number. No other anticipated glycohydrolase or protease activities were foud. The amount of enzyme located within the cells was barely detectable as compared to that found in the induction filtrate. Experiments with chloramphenicol or chlortetracycline indicate that de novo protein synthesis was required for neuraminidase production and that this exoenzyme was not released from a preformed pool. Neuraminidase from this source has an apparent molecular weight of 87,000, a pH optimum of 5 to 6, and an apparent Km of 2.08 mg/ml for collocalia mucoid and 3.3 X 10(-3) M for N-acetylneuraminlactose and is insensitive both to Ca2+ ions and ethylenediaminetetraacetic acid. Preliminary studies indicate that the enzyme can hydrolyze alpha-2,3-, alpha-2,6-, or alph-2-8-N-acetylneuraminylglycosidic linkages. From total activity data and purification criteria, it would appear that this isolate can produce about 5 mg of enzyme per liter of induction medium.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden S. B., Chang W. H., Barksdale L. Distribution of neuraminidase and n-acetylneuraminate lyase activities among corynebacteria, mycobacteria, and nocardias. J Bacteriol. 1972 Dec;112(3):1206–1212. doi: 10.1128/jb.112.3.1206-1212.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke E., Scharmann W., Drzeniek R. Die Bestimmung des Molekulargewichtes bakterieller Neuraminidasen mit Hilfe der Gel-Filtration. Zentralbl Bakteriol Orig A. 1974;229(1):55–67. [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Geisow M. J. An improved method for purifying sialidase. Biochem J. 1975 Oct;151(1):181–183. doi: 10.1042/bj1510181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWE C., LEE L. T., ROSE H. M. Collocalia mucoid: a substrate for myxovirus neuraminidase. Arch Biochem Biophys. 1961 Dec;95:512–520. doi: 10.1016/0003-9861(61)90184-9. [DOI] [PubMed] [Google Scholar]
- Hatton M. W., Regoeczi E. A simple method for the purification of commercial neuraminidase preparations free from proteases. Biochim Biophys Acta. 1973 Nov 15;327(1):114–120. doi: 10.1016/0005-2744(73)90108-3. [DOI] [PubMed] [Google Scholar]
- Kathan R. H., Weeks D. I. Structure studies of collocalia mucoid. I. Carbohydrate and amino acid composition. Arch Biochem Biophys. 1969 Nov;134(2):572–576. doi: 10.1016/0003-9861(69)90319-1. [DOI] [PubMed] [Google Scholar]
- Kelly R. T., Greiff D., Farmer S. Neuraminidase activity in Diplococcus pneumoniae. J Bacteriol. 1966 Feb;91(2):601–603. doi: 10.1128/jb.91.2.601-603.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Greiff D. Toxicity of pneumococcal neuraminidase. Infect Immun. 1970 Jul;2(1):115–117. doi: 10.1128/iai.2.1.115-117.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto S., Aoyagi T., Takeuchi T., Umezawa H. Purification and characterization of Streptomyces sialidases. J Bacteriol. 1974 Aug;119(2):394–400. doi: 10.1128/jb.119.2.394-400.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litchfield C. D., Prescott J. M. Regulation of proteolytic enzyme production by Aeromonas proteolytica. I. Extracellular endopeptidase. Can J Microbiol. 1970 Jan;16(1):17–22. doi: 10.1139/m70-003. [DOI] [PubMed] [Google Scholar]
- Myhill M. K., Cook T. M. Extracellular neuraminidase of Streptomyces albus. Can J Microbiol. 1972 Jul;18(7):1007–1014. doi: 10.1139/m72-157. [DOI] [PubMed] [Google Scholar]
- Nees S., Veh R. W., Schauer R. Purification and characterization of neuraminidase from Clostridium perfringens. Hoppe Seylers Z Physiol Chem. 1975 Jun;356(6):1027–1042. doi: 10.1515/bchm2.1975.356.s1.1027. [DOI] [PubMed] [Google Scholar]
- Schauer R., Wember M., Wirtz-Peitz F., Ferreira do Amaral C. Studies on the substrate specificity of acylneuraminate pyruvate-lyase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1073–1080. doi: 10.1515/bchm2.1971.352.2.1073. [DOI] [PubMed] [Google Scholar]
- Tanenbaum S. W., Sun S. C. Some molecular properties of pneumoccal neuraminidase isoenzymes. Biochim Biophys Acta. 1971 Mar 23;229(3):824–828. doi: 10.1016/0005-2795(71)90301-1. [DOI] [PubMed] [Google Scholar]
- Tuppy H., Palese P. A chromogenic substrate for the investigation of neuraminidases. FEBS Lett. 1969 Apr;3(1):72–75. doi: 10.1016/0014-5793(69)80100-6. [DOI] [PubMed] [Google Scholar]
- Uchida Y., Tsukada Y., Sugimori T. Production of microbial neuraminidases induced by colominic acid. Biochim Biophys Acta. 1974 Jun 18;350(2):425–431. doi: 10.1016/0005-2744(74)90517-8. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


