Abstract
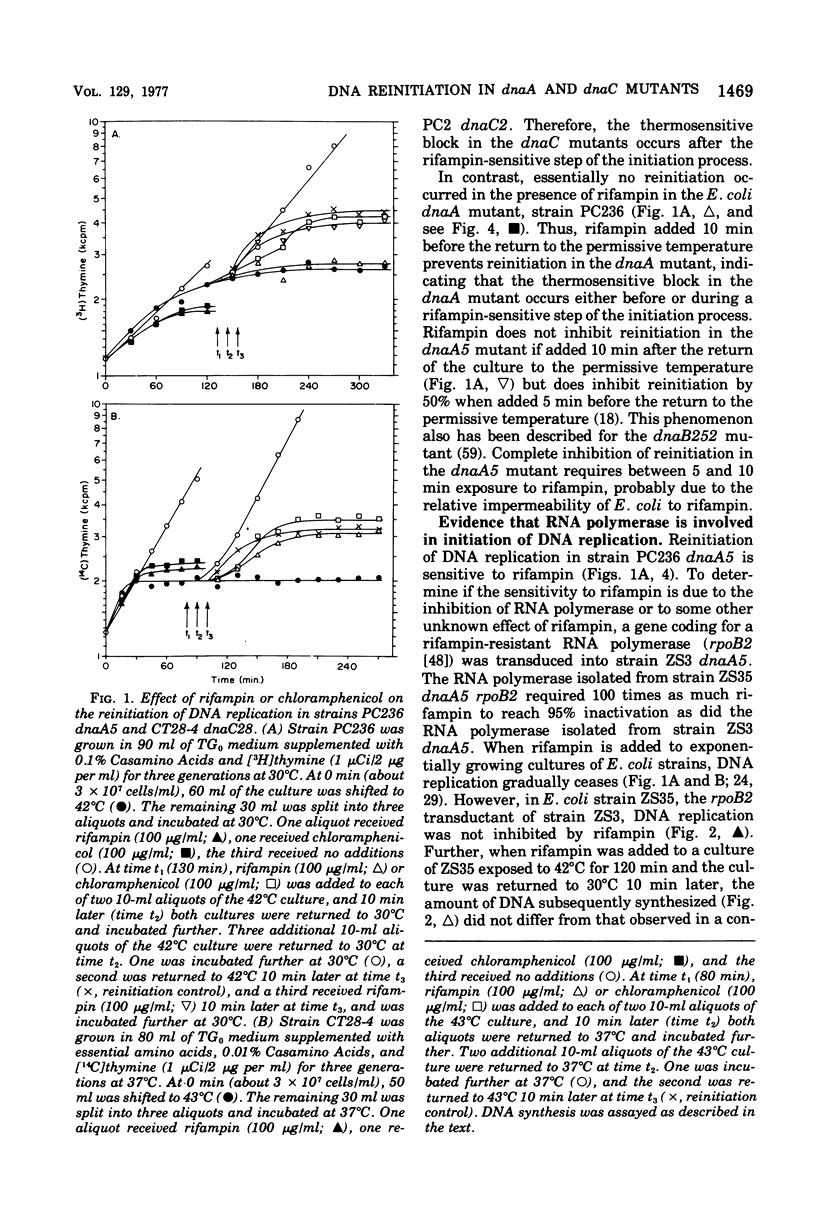
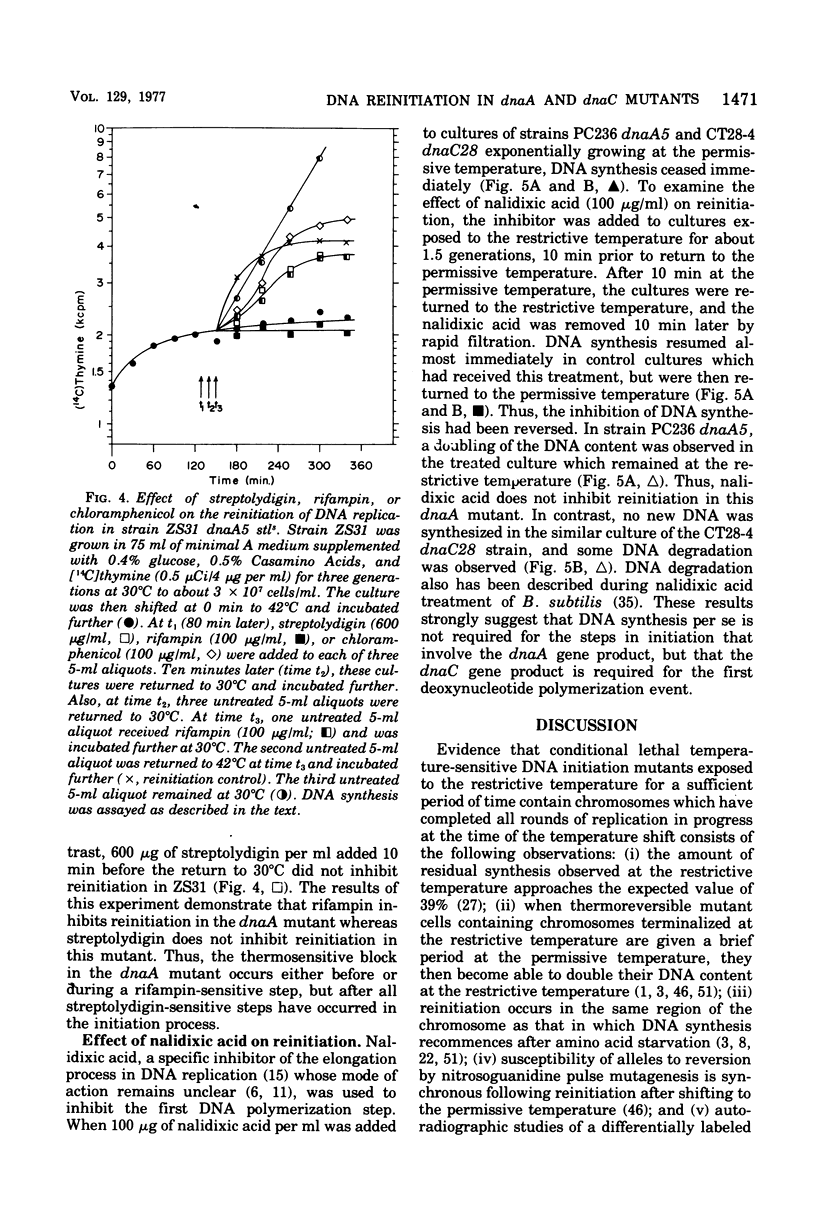
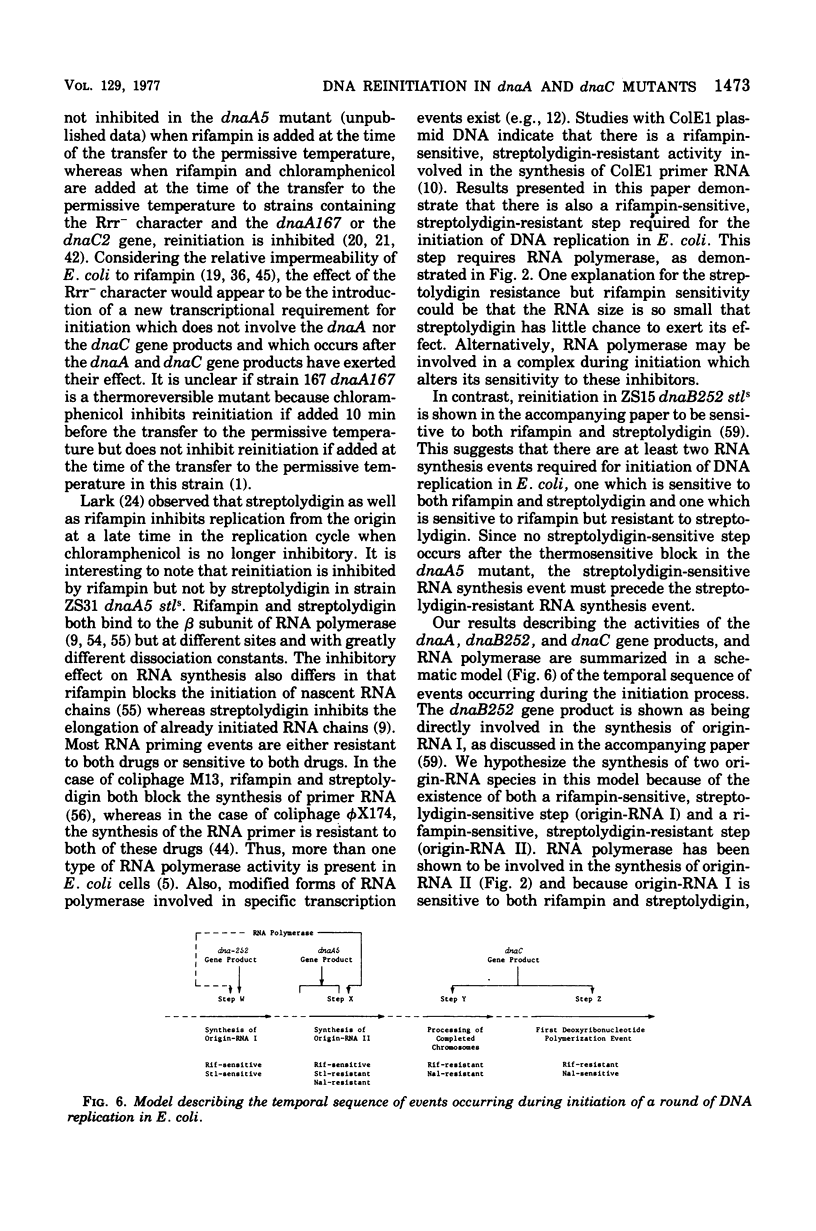
Three thermosensitive deoxyribonucleic acid (DNA) initiation mutants of Escherichia coli exposed to the restrictive temperature for one to two generations were examined for the ability to reinitiate DNA replication after returning to the permissive temperature in the presence of rifampin, chloramphenicol, or nalidixic acid. Reinitiation in the dnaA mutant was inhibited by rifampin but not by chloramphenicol, whereas renitiation was not inhibited by rifampin but not by chloramphenicol, whereas reinitiation was not inhibited in two dnaC mutants by either rifampin or chloramphenicol. To observe the rifampin inhibition, the antibiotic must be added at least 10 min before return to the permissive temperature. The rifampin inhibition of reinitiation was not observed when a rifampin-resistant ribonucleic acid ((RNA) polymerase gene was introduced into the dnaA mutant, demonstrating that RNA polymerase synthesizes one or more RNA species required for the initation of DNA replication (origin-RNA). Reinitiation at 30 degrees C was not inhibited by streptolydigin in a stretolydigin-sensitive dnaA muntant. Incubation in the presence of nalidixic acid prevented subsequent reinitiation in the dnaC28 mutant but did not inhibit reinitiation in the dnaA5 muntant. These results demonstrate that the dnaA gene product acts before or during the synthesis of an origin-RNA, RNA polymerase synthesizes this origin RNA, and the dnaC gene product is involved in a step after this RNA synthesis event. Furthermore, these results suggest that the dnaC gene product is involved in the first deoxyribounucleotide polymerization event wheareas the dnaA gene product acts prior to this event. A model is presented describing the temporal sequence of events that occur during initiation of a round of DNA replication, based on results in this and the accompanying paper.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Tomizawa J. Chromosome replication in Escherichia coli K12 mutant affected in the process of DNA initiation. Genetics. 1971 Sep;69(1):1–15. doi: 10.1093/genetics/69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D., Messer W., Schlicht M. Mutants of Escherichia coli B-r defective in deoxyribonucleic acid initiation: dnaI, a new gene for replication. J Bacteriol. 1974 Jun;118(3):783–789. doi: 10.1128/jb.118.3.783-789.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D., Schlicht M., Schuster H. Temperature-sensitive initiation of DNA replication in a mutant of Escherichia coli K12. Mol Gen Genet. 1971;111(2):145–158. doi: 10.1007/BF00267789. [DOI] [PubMed] [Google Scholar]
- Bird R. E., Louarn J., Martuscelli J., Caro L. Origin and sequence of chromosome replication in Escherichia coli. J Mol Biol. 1972 Oct 14;70(3):549–566. doi: 10.1016/0022-2836(72)90559-1. [DOI] [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carl P. L. Escherichia coli mutants with temperature-sensitive synthesis of DNA. Mol Gen Genet. 1970;109(2):107–122. doi: 10.1007/BF00269647. [DOI] [PubMed] [Google Scholar]
- Cassani G., Burgess R. R., Goodman H. M., Gold L. Inhibition of RNA polymerase by streptolydigin. Nat New Biol. 1971 Apr 14;230(15):197–200. doi: 10.1038/newbio230197a0. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Evenchik B. G. Effects of rifampicin, streptolydigin and actinomycin D on the replication of Col E1 plasmid DNA in Escherichia coli. J Mol Biol. 1973 Apr 15;75(3):503–513. doi: 10.1016/0022-2836(73)90457-9. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Smith J. T. Nalidixic acid and bacterial chromosome replication. Nature. 1976 Apr 15;260(5552):643–645. doi: 10.1038/260643a0. [DOI] [PubMed] [Google Scholar]
- Duffy J. J., Petrusek R. L., Geiduschek E. P. Conversion of Bacillus subtilis RNA polymerase activity in vitro by a protein induced by phage SP01. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2366–2370. doi: 10.1073/pnas.72.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans I. M., Eberle H. Accumulation of the capacity of initiation of deoxyribonucleic acid replication in Escherichia coli. J Bacteriol. 1975 Mar;121(3):883–891. doi: 10.1128/jb.121.3.883-891.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Eisenstark A. Bi-directional chromosomal replication in Salmonella typhimurium. J Bacteriol. 1973 Jul;115(1):168–176. doi: 10.1128/jb.115.1.168-176.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C., Engbaek F., Flammang T., Burgess R. Rapid micromethod for the purification of Escherichia coli ribonucleic acid polymerase and the preparation of bacterial extracts active in ribonucleic acid synthesis. J Bacteriol. 1976 Oct;128(1):382–389. doi: 10.1128/jb.128.1.382-389.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J. D. DNA replication in bacteria. Curr Top Microbiol Immunol. 1972;57:39–74. doi: 10.1007/978-3-642-65297-4_2. [DOI] [PubMed] [Google Scholar]
- Hanna M. H., Carl P. L. Reinitiation of deoxyribonucleic acid synthesis by deoxyribonucleic acid initiation mutants of Escherichia coli: role of ribonucleic acid synthesis, protein synthesis, and cell division. J Bacteriol. 1975 Jan;121(1):219–226. doi: 10.1128/jb.121.1.219-226.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn R., Vogel M., Brown R. D. Conservation of the rifamycin sensitivity of transcription during T4 development. Nature. 1969 Mar 1;221(5183):836–838. doi: 10.1038/221836a0. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Saito T. Initiation of DNA replication in Escherichia coli. II. Effect of rifampicin on the resumption of replication of F episome and chromosome upon the returning of dna mutants from a non-permissive to a permissive temperature. Mol Gen Genet. 1975;137(3):239–248. doi: 10.1007/BF00333019. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Saitoh T. Initation of DNA replication in Escherichia coli. I. Characteristics of the initation process in dna mutants. Mol Gen Genet. 1974;132(1):49–62. doi: 10.1007/BF00268230. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Mordoh J., Jacob F. On the process of cellular division in Escherichia coli. 3. Thermosensitive mutants of Escherichia coli altered in the process of DNA initiation. J Mol Biol. 1970 Nov 14;53(3):369–387. doi: 10.1016/0022-2836(70)90072-0. [DOI] [PubMed] [Google Scholar]
- Jonasson J. Evidence for bidirectional chromosome replication in Escherichia coli C based on marker-frequency analysis by DNA-DNA hybridization with P2 and lambda prophages. Mol Gen Genet. 1973 Jan 18;120(1):68–90. doi: 10.1007/BF00332985. [DOI] [PubMed] [Google Scholar]
- LARK K. G., REPKO T., HOFFMAN E. J. THE EFFECT OF AMINO ACID DEPRIVATION ON SUBSEQUENT DEOXYRIBONUCLEIC ACID REPLICATION. Biochim Biophys Acta. 1963 Sep 17;76:9–24. [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Laurent S. J. Initiation of deoxyribonucleic acid replication in a temperature-sensitive mutant of B. subtilis: evidence for a transcriptional step. J Bacteriol. 1973 Oct;116(1):141–145. doi: 10.1128/jb.116.1.141-145.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- Masters M., Broda P. Evidence for the bidirectional replications of the Escherichia coli chromosome. Nat New Biol. 1971 Aug 4;232(31):137–140. doi: 10.1038/newbio232137a0. [DOI] [PubMed] [Google Scholar]
- Messer W. Initiation of deoxyribonucleic acid replication in Escherichia coli B-r: chronology of events and transcriptional control of initiation. J Bacteriol. 1972 Oct;112(1):7–12. doi: 10.1128/jb.112.1.7-12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Meselson M. Periodic replication of DNA in steadily growing Escherichia coli: the localized origin of replication. Cold Spring Harb Symp Quant Biol. 1968;33:553–557. doi: 10.1101/sqb.1968.033.01.062. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Eisenstark A. Sequence of genes replicated in Salmonella typhimurium as examined by transduction techniques. J Bacteriol. 1970 May;102(2):320–333. doi: 10.1128/jb.102.2.320-333.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. M., Kuempel P. L. Bidirectional replication of the chromosome in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2842–2845. doi: 10.1073/pnas.69.10.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramareddy G., Reiter H. Specific loss of newly replicated deoxyribonucleic acid in nalidixic acid-treated Bacillus subtilis 168. J Bacteriol. 1969 Nov;100(2):724–729. doi: 10.1128/jb.100.2.724-729.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P., Speyer J. Rifampicin inhibition of ribonucleic acid and protein synthesis in normal and ethylenediaminetetraacetic acid-treated Escherichia coli. J Bacteriol. 1970 Oct;104(1):376–389. doi: 10.1128/jb.104.1.376-389.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R. L., Dalbey M. S., Davern C. I. Autoradiographic evidence for bidirectional DNA replication in Escherichia coli. J Mol Biol. 1973 Mar 15;74(4):599–604. doi: 10.1016/0022-2836(73)90050-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. L., Davern C. I. Direction of deoxyribonucleic acid replication in Escherichia coli under various conditions of cell growth. J Bacteriol. 1976 Jan;125(1):346–352. doi: 10.1128/jb.125.1.346-352.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder O. A., Smith D. W. Isolation of membrane-associated folded chromosomes from Escherichia coli: effect of protein synthesis inhibition. J Bacteriol. 1974 Dec;120(3):1356–1363. doi: 10.1128/jb.120.3.1356-1363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder O. A., Smith D. W. Properties of membrane-associated folded chromosomes of E. coli related to initiation and termination of DNA replication. Cell. 1975 Apr;4(4):337–345. doi: 10.1016/0092-8674(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Saito T., Hiraga S. Initiation of DNA replication in Escherichia coli. III. Genetic analysis of the dna mutant exhibiting rifampicin-sensitive resumption of replication. Mol Gen Genet. 1975;137(3):249–261. doi: 10.1007/BF00333020. [DOI] [PubMed] [Google Scholar]
- Sakai H., Hashimoto S., Komano T. Replication of deoxyribonucleic acid in Escherichia coli C mutants temperature sensitive in the initiation of chromosome replication. J Bacteriol. 1974 Sep;119(3):811–820. doi: 10.1128/jb.119.3.811-820.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Wickner W., Westergaard O., Brutlag D., Geider K., Bertsch L. L., Kornberg A. Initiation of DNA synthesis: synthesis of phiX174 replicative form requires RNA synthesis resistant to rifampicin. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2691–2695. doi: 10.1073/pnas.69.9.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R. Isolation and characterization of streptolydigin resistant RNA polymerase. Nature. 1969 Sep 6;223(5210):1068–1069. doi: 10.1038/2231068a0. [DOI] [PubMed] [Google Scholar]
- Schubach W. H., Whitmer J. D., Davern C. I. Genetic control of DNA initiation in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):205–221. doi: 10.1016/0022-2836(73)90107-1. [DOI] [PubMed] [Google Scholar]
- Snyder L. R. An RNA polymerase mutant of Escherichia coli defective in the T4 viral transcription program. Virology. 1972 Nov;50(2):396–403. doi: 10.1016/0042-6822(72)90391-1. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. 3. A temperature-sensitive mutation(dnaP) affecting DNA replication. Genetics. 1974 Jun;77(2):199–220. doi: 10.1093/genetics/77.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake R. G. Visualization of reinitiated chromosomes in Bacillus subtilis. J Mol Biol. 1972 Jul 28;68(3):501–509. doi: 10.1016/0022-2836(72)90102-7. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wehrli W., Knüsel F., Schmid K., Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. Actions of the rifamycins. Bacteriol Rev. 1971 Sep;35(3):290–309. doi: 10.1128/br.35.3.290-309.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyskind J. W., Smith D. W. NOVEL Escherichia coli dnaB mutant: direct involvement of the dnaB252 gene product in the synthesis of an origin-ribonucleic acid species during initiaion of a round of deoxyribonucleic acid replication. J Bacteriol. 1977 Mar;129(3):1476–1486. doi: 10.1128/jb.129.3.1476-1486.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


