Abstract
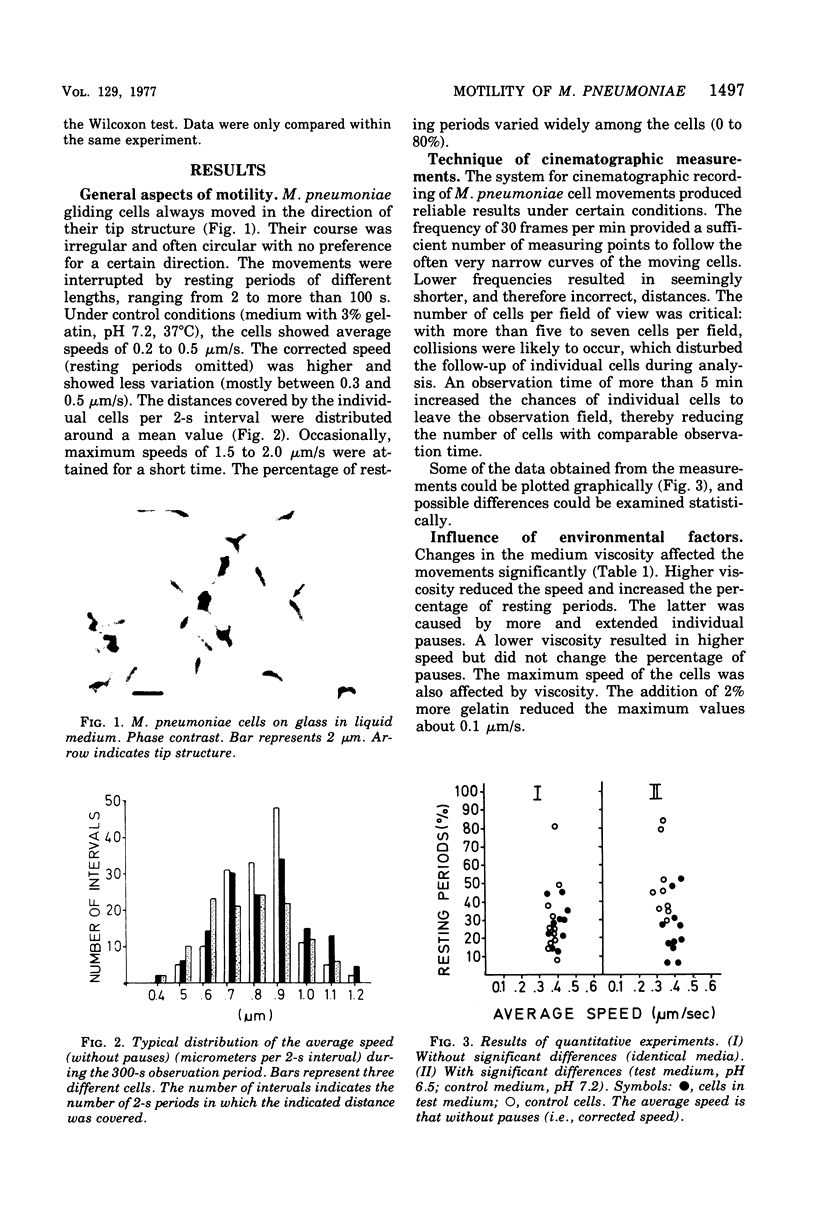
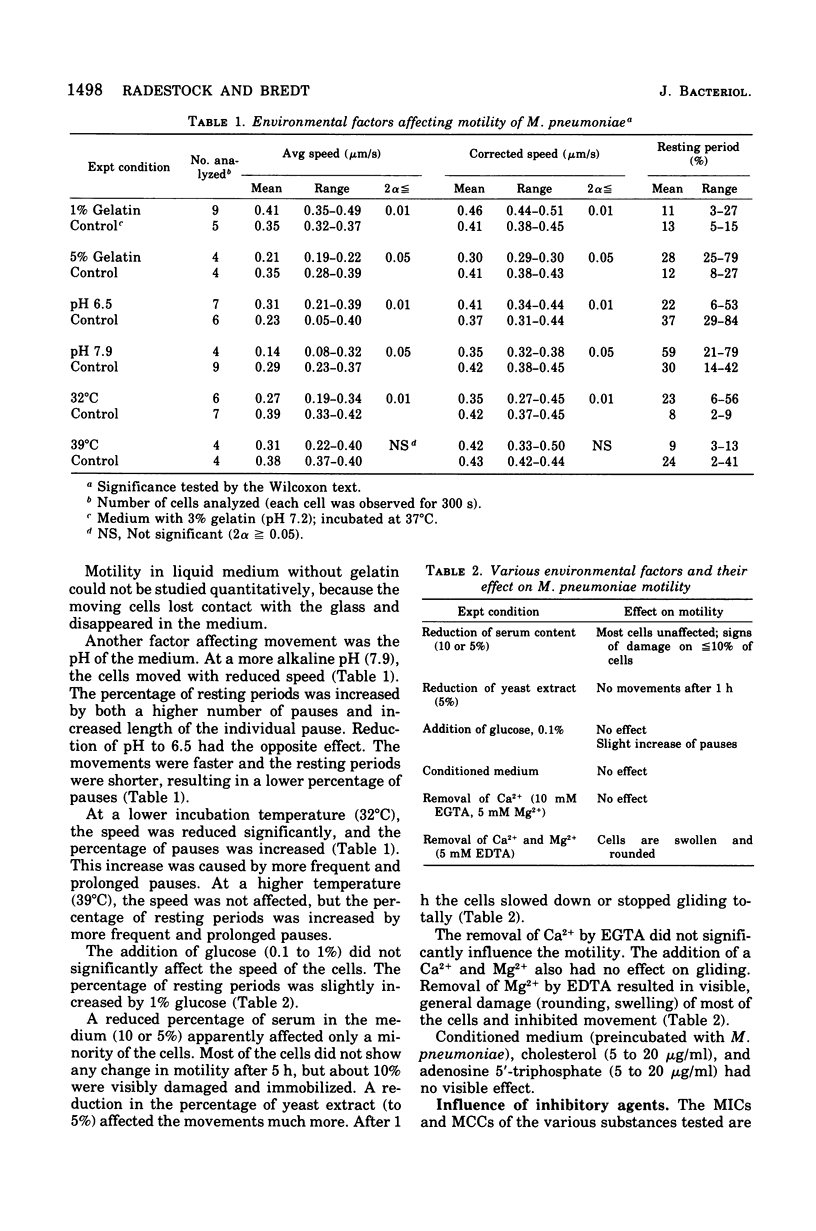
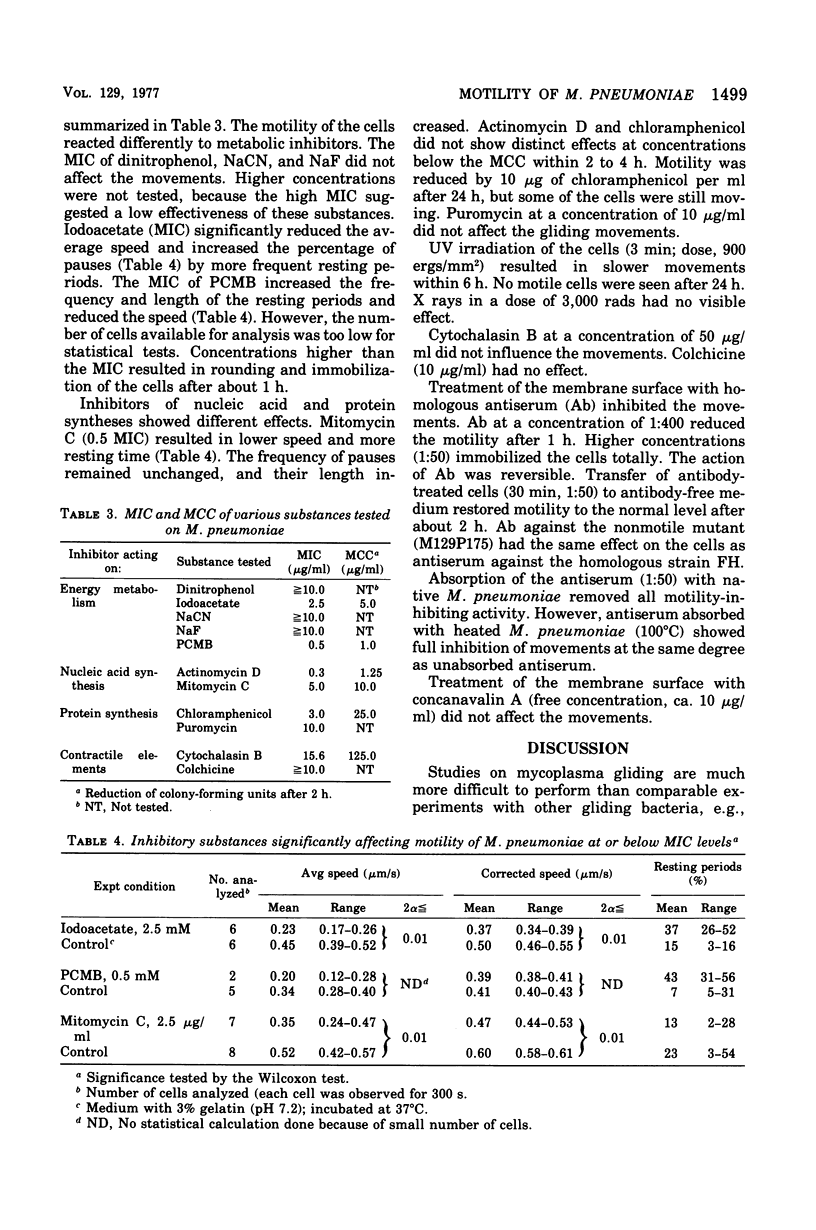
Cell of Mycoplasma pneumoniae FH gliding on a glass surface in liquid medium were examined by microscopic observation and quantitatively by microcinematography (30 frames per min). Comparisons were made only within the individual experiments. The cells moved in an irregular pattern with numerous narrow bends and circles. They never changed their leading end. The average speed (without pauses) was relatively constant between o.2 and 0.5 mum/s. The maximum speed was about 1.5 to 2.0 mum/s. The movements were interrupted by resting periods of different lengths and frequency. Temperature, viscosity, pH, and the presence of yeast extract in the medium influenced the motility significantly; changes in glucose, calcium ions, and serum content were less effective. The movements were affected by iodoacetate, p-mercuribenzoate, and mitomycin C at inhibitory or subinhibitory concentrations. Sodium fluoride, sodium cyanide, dinitrophenol, chloramphenicol, puromycin, cholchicin, and cytochalasin B at minimal inhibitory concentrations did not affect motility. The movements were effectively inhibited by anti-M. pneumoniae antiserum. Studies with absorbed antiserum suggested that the surface components involved in motility are heat labile. The gliding of M. pneumoniae cells required an intact energy metabolism and the proteins involved seemed to have a low turnover.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Black F. T. DNA base composition of human T strain mycoplasmas. Nature. 1968 Sep 7;219(5158):1044–1045. doi: 10.1038/2191044a0. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt W., Bitter-Suermann D. Interactions between Mycoplasma pneumoniae and guinea pig complement. Infect Immun. 1975 Mar;11(3):497–504. doi: 10.1128/iai.11.3.497-504.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt W. Growth morphology of Mycoplasma pneumoniae strain FH on glass surface. Proc Soc Exp Biol Med. 1968 Jun;128(2):338–340. doi: 10.3181/00379727-128-33009. [DOI] [PubMed] [Google Scholar]
- Bredt W., Höfling K. H., Heunert H. H., Milthaler B. Messungen an beweglichen Zellen von Mycoplasma pneumoniae. Z Med Mikrobiol Immunol. 1970;156(1):39–43. [PubMed] [Google Scholar]
- Bredt W. Motility and multiplication of Mycoplasma pneumoniae. A phase contrast study. Pathol Microbiol (Basel) 1968;32(6):321–326. doi: 10.1159/000162074. [DOI] [PubMed] [Google Scholar]
- Burchard R. P. Studies on gliding motility in Myxococcus xanthus. Arch Microbiol. 1974;99(3):271–280. doi: 10.1007/BF00696242. [DOI] [PubMed] [Google Scholar]
- Freundt E. A. Present status of the medical importance of mycoplasmas. Pathol Microbiol (Basel) 1974;40(3):155–187. doi: 10.1159/000162521. [DOI] [PubMed] [Google Scholar]
- Furcht L. T., Scott E. Effect of vinblastine sulfate, colchicine and lumicolchicine on membrane organization of normal and transformed cells. Exp Cell Res. 1975 Dec;96(2):271–282. doi: 10.1016/0014-4827(75)90257-8. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Henrichsen J. Bacterial surface translocation: a survey and a classification. Bacteriol Rev. 1972 Dec;36(4):478–503. doi: 10.1128/br.36.4.478-503.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrichsen J. Gliding and twitching motility of bacteria unaffected by cytochalasin B. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):623–624. doi: 10.1111/j.1699-0463.1972.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Jahn T. L., Bovee E. C. Movement and locomotion of microorganisms. Annu Rev Microbiol. 1965;19:21–58. doi: 10.1146/annurev.mi.19.100165.000321. [DOI] [PubMed] [Google Scholar]
- Morton H. E., Roberts R. J. Production of anti-Mycoplasma (PPLO) antibodies in rabbits. Proc Soc Exp Biol Med. 1967 Jun;125(2):538–543. doi: 10.3181/00379727-125-32140. [DOI] [PubMed] [Google Scholar]
- NELSON J. B. The behavior of murine PPLO in HeLa cell cultures. Ann N Y Acad Sci. 1960 Jan 15;79:450–457. doi: 10.1111/j.1749-6632.1960.tb42710.x. [DOI] [PubMed] [Google Scholar]
- Nelson J. B., Lyons M. J. Phase-contrast and electron microscopy of murine strains of Mycoplasma. J Bacteriol. 1965 Dec;90(6):1750–1763. doi: 10.1128/jb.90.6.1750-1763.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senterfit L. B., Jensen K. E. Antimetabolic antibodies to Mycoplasma pneumoniae measured by tetrazolium reduction inhibition. Proc Soc Exp Biol Med. 1966 Jul;122(3):786–790. doi: 10.3181/00379727-122-31252. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]