Abstract
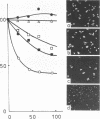
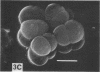
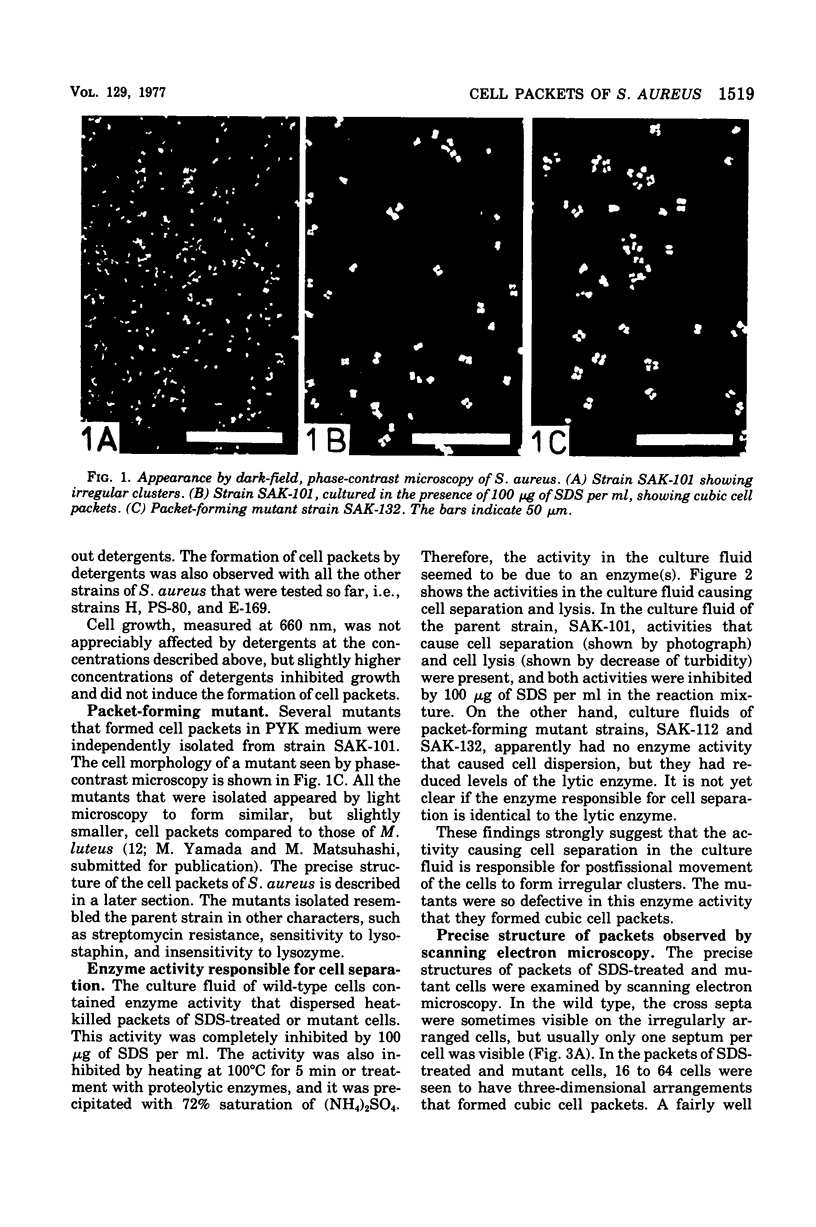
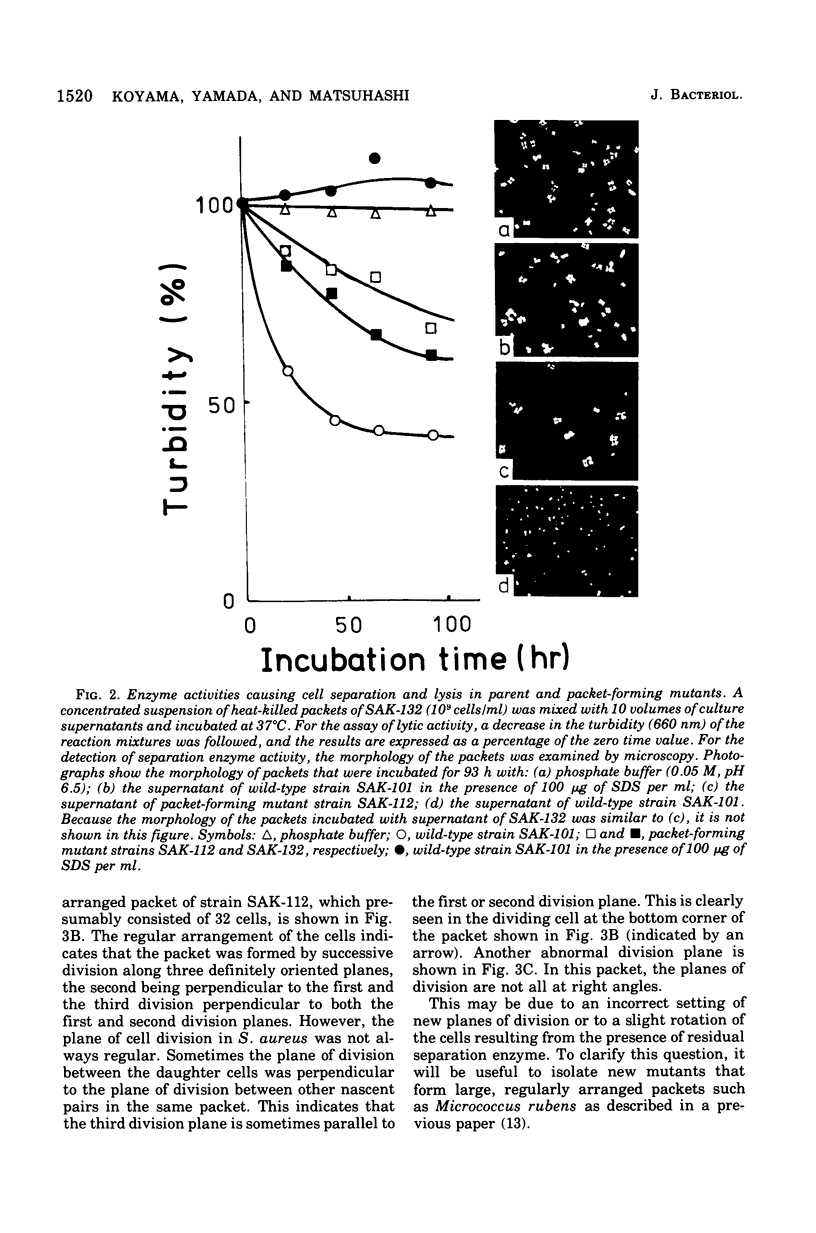
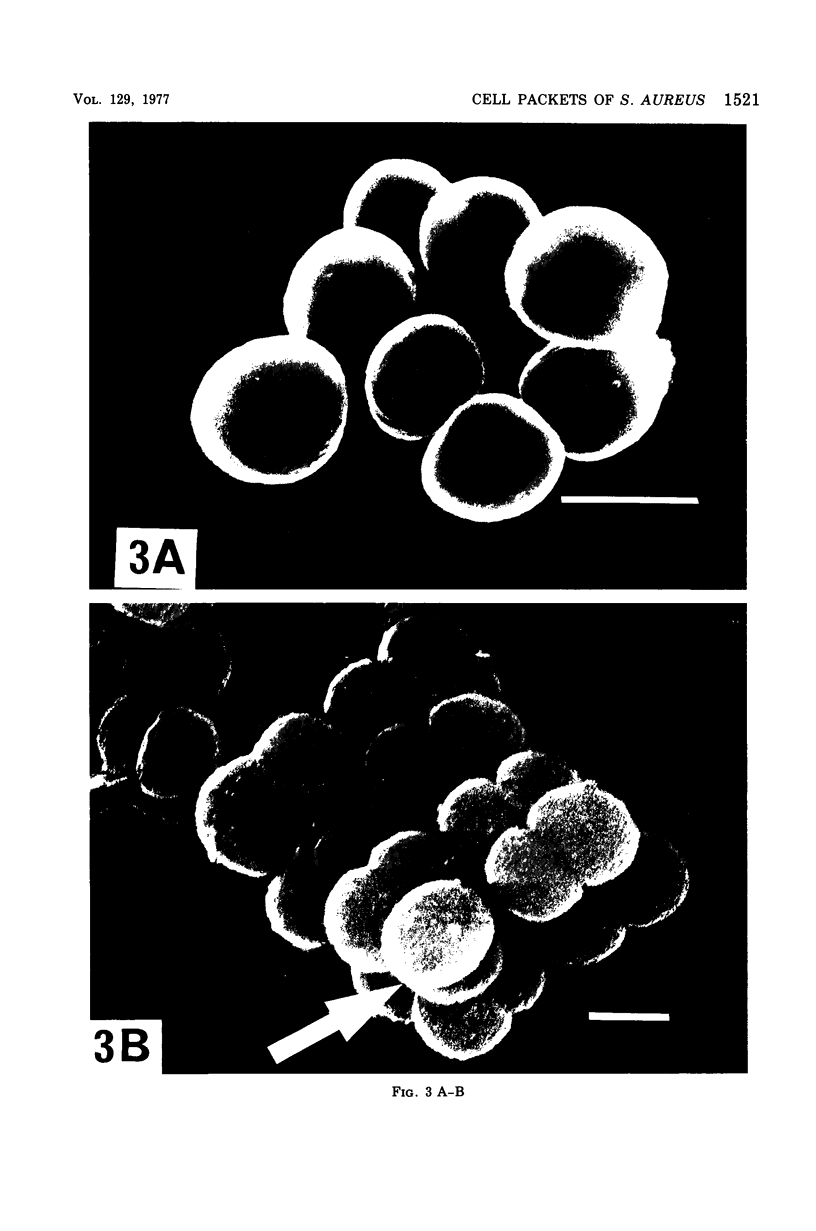
Staphylococcus aureus, which usually forms grape-like clusters, has the ability to form regularly arranged cell packets. These regular cell packets are formed when the activity of its separation enzyme(s) is lost either by treatment with detergents, such as sodium dodecyl sulfate or Trition X-100, or by mutation of the cells. These cell packets consisted of 8 to 64 spherical cells that have a three-dimensional arrangement. Some irregularity in the arragement of cells in packets, however, can be observed by scanning electron microscopy. It is concluded that S. aureus fundametally divides along three definitely oriented planes that are located at right angles to each other. After cell division, the cells usually become translocated due to the action of a separation enzyme(s) to form grape-like clusters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatterjee A. N., Mirelman D., Singer H. J., Park J. T. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J Bacteriol. 1969 Nov;100(2):846–853. doi: 10.1128/jb.100.2.846-853.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Wong W., Young F. E., Gilpin R. W. Isolation and characterization of a mutant of Staphylococcus aureus deficient in autolytic activity. J Bacteriol. 1976 Mar;125(3):961–967. doi: 10.1128/jb.125.3.961-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Scanning electron microscopy of Staphyloccus aureus exposed to some common anti-staphylococcal agents. J Gen Microbiol. 1972 Apr;70(2):263–270. doi: 10.1099/00221287-70-2-263. [DOI] [PubMed] [Google Scholar]
- Huff E., Silverman C. S., Adams N. J., Awkard W. S. Extracellular cell wall lytic enzyme from Staphylococcus aureus: purification and partial characterization. J Bacteriol. 1970 Sep;103(3):761–769. doi: 10.1128/jb.103.3.761-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMINSKI I., CAMERON J., WYLLIE G. Chaining and unchaining Streptococcus faecalis; a hypothesis of the mechanism of bacterial cell separation. Nature. 1958 May 24;181(4621):1477–1477. doi: 10.1038/1811477a0. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D., Higgins M. L., Porres-Juan J. Some properties of two autolytic-defective mutants of Streptococcus faecalis ATCC 9790. J Bacteriol. 1972 Jan;109(1):423–431. doi: 10.1128/jb.109.1.423-431.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Hirose A., Matsuhashi M. Association of lack of cell wall teichuronic acid with formation of cell packets of Micrococcus lysodeikticus (luteus) mutants. J Bacteriol. 1975 Aug;123(2):678–686. doi: 10.1128/jb.123.2.678-686.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Koyama T., Matsuhashi M. Interconversion of large packets and small groups of cells of Micrococcus rubens: dependence upon magnesium and phosphate. J Bacteriol. 1977 Mar;129(3):1513–1517. doi: 10.1128/jb.129.3.1513-1517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]