Abstract
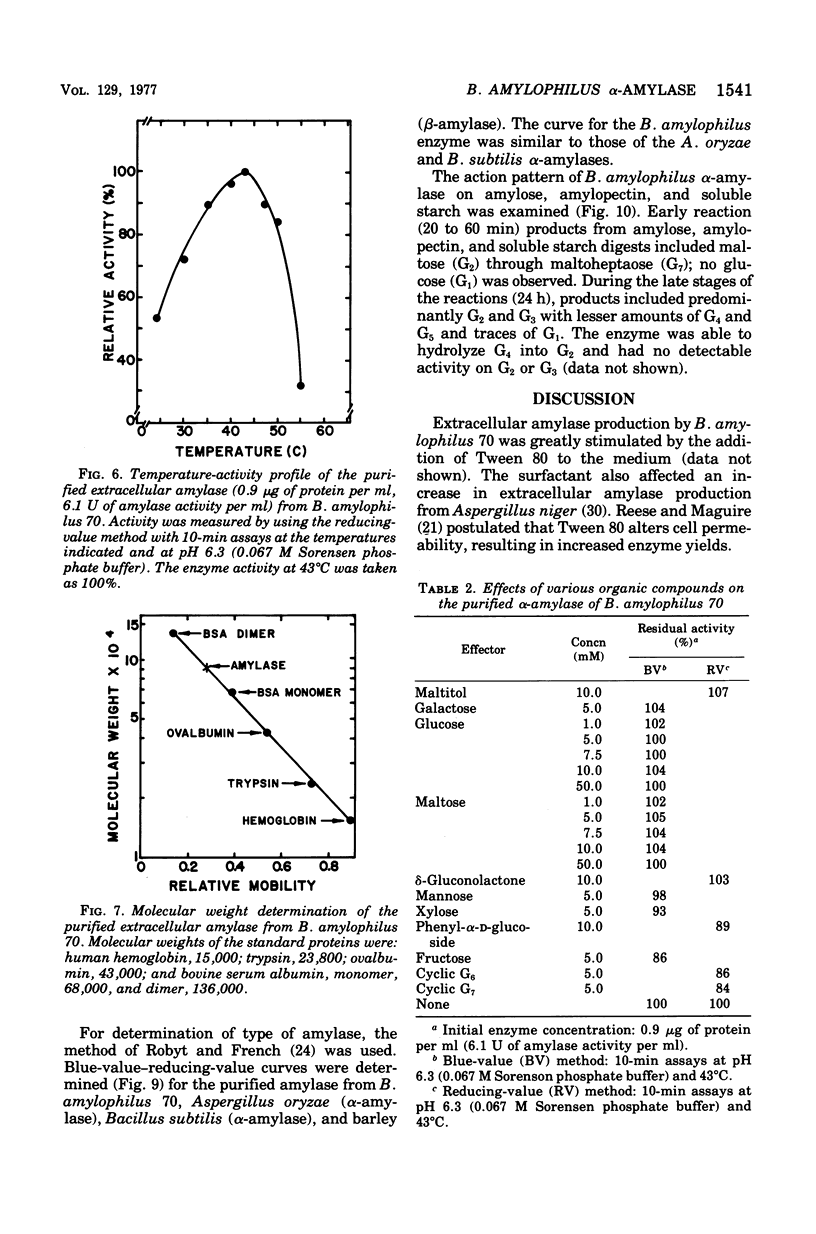
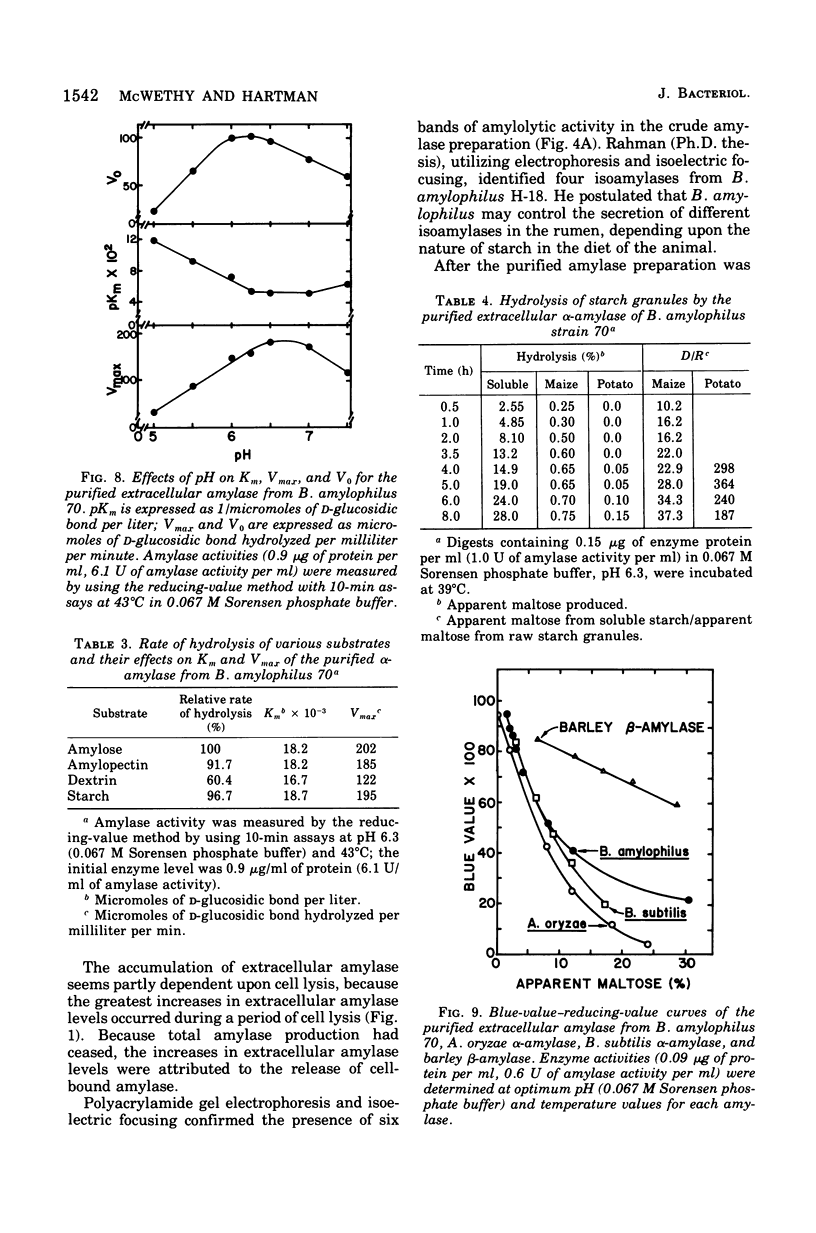
A medium was developed to obtain maximum yields of extracellular amylase from Bacteroides amylophilus 70. Crude enzyme preparation, obtained by ammonium sulfate precipitation of cell-free broth, contained six amylolytic isoenzymes that were detected by isoelectric focusing and polyacrylamide gel electrophoresis. One of these amylases was purified by diethylaminoethyl-Sephadex A-50 ion-exchange chromatography and Sephadex G-200 gel filtration techniques. Some properties of the purified extracellular alpha-amylase were: optimum pH, 6.3; optimum temperature, 43 degrees C: PH stability range, 5.8 to 7.5; isoelectric point, pH 4.6; molecular weight, 92,000 (by sodium dodecyl sulfatedisc gel electrophoresis); and sugars causing inhibition, cyclomaltoheptaose, cyclomaltohexaose, and alpha-d-phenylglucoside. In addition, Ca2+ and Co2+ were strong activators,and Hg2+ was a strong inhibitior; all other cations were slightly stimulatory. Dialysis against 0.01 M ethylenediaminetetraacetic acid caused a 58% loss of activity that was restored to 92% of the original by the addition of 0.04 M Ca2+. The enzyme affected a blue-value-reducing-value curve characteristic of alpha-type amylases. The relative rates of hydrolysis of amylose, soluble starch, amylopectin, and dextrin were 100, 97, 92, and 60%, respectively; Michaelis constants for these substrates were 18.2, 18.7, 18.2, and 16.7 mumol of d-glucosidic bond/liter, respectively. The enzyme degraded maize (corn) starch granules to some extent and had relatively little activity on potato starch granules.
Full text
PDF
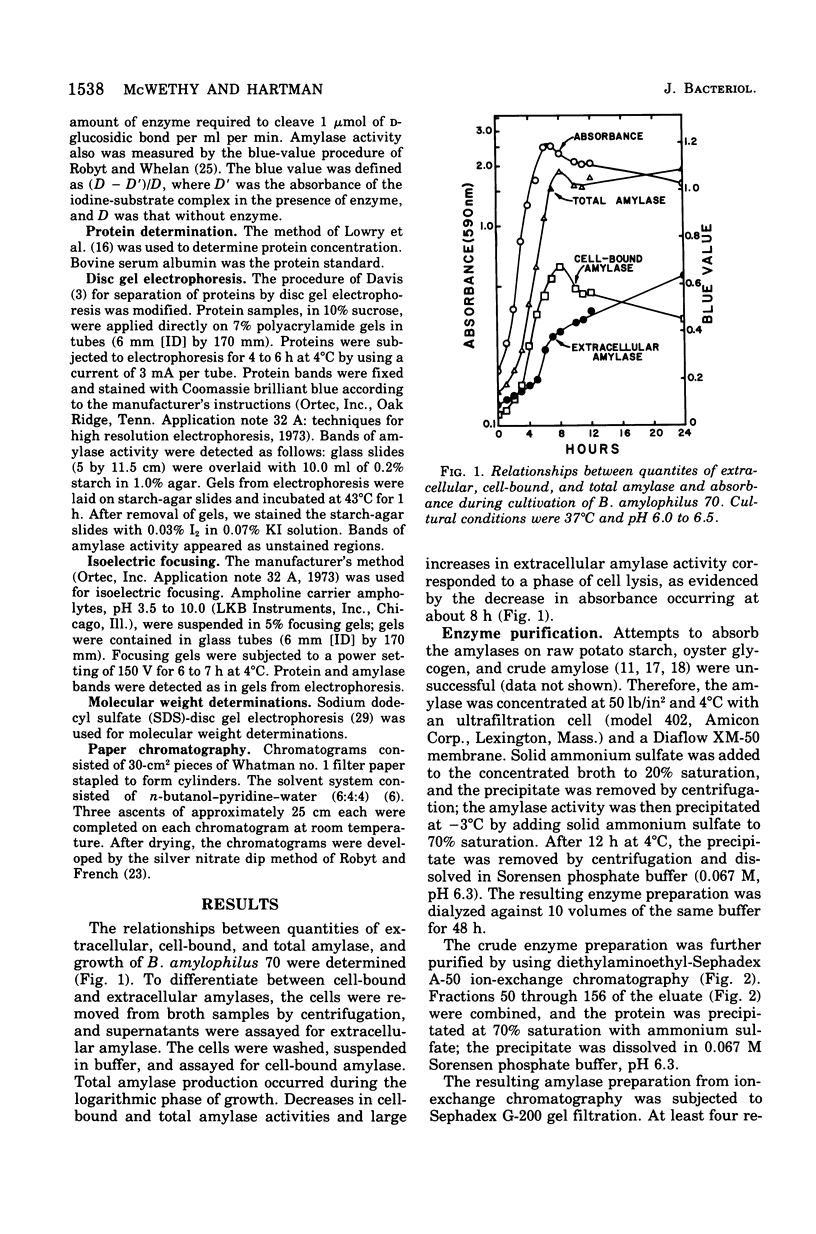
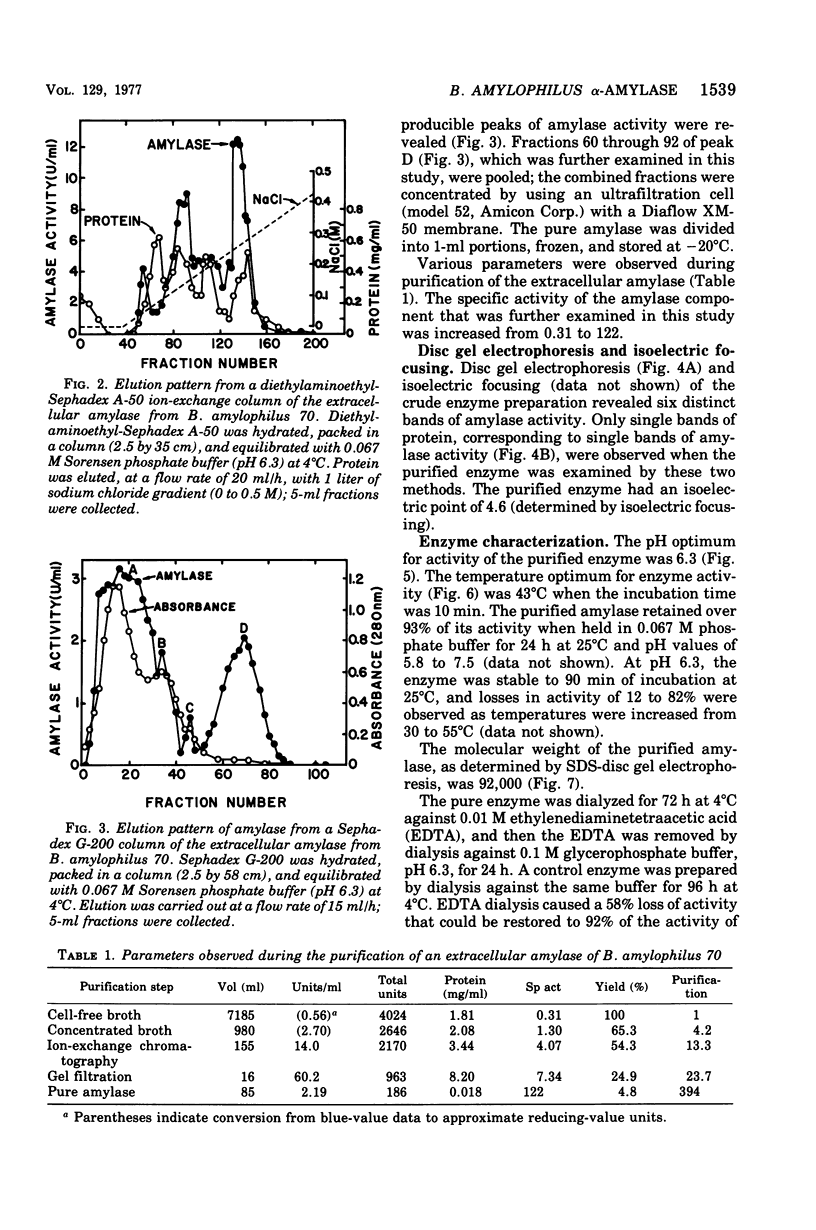
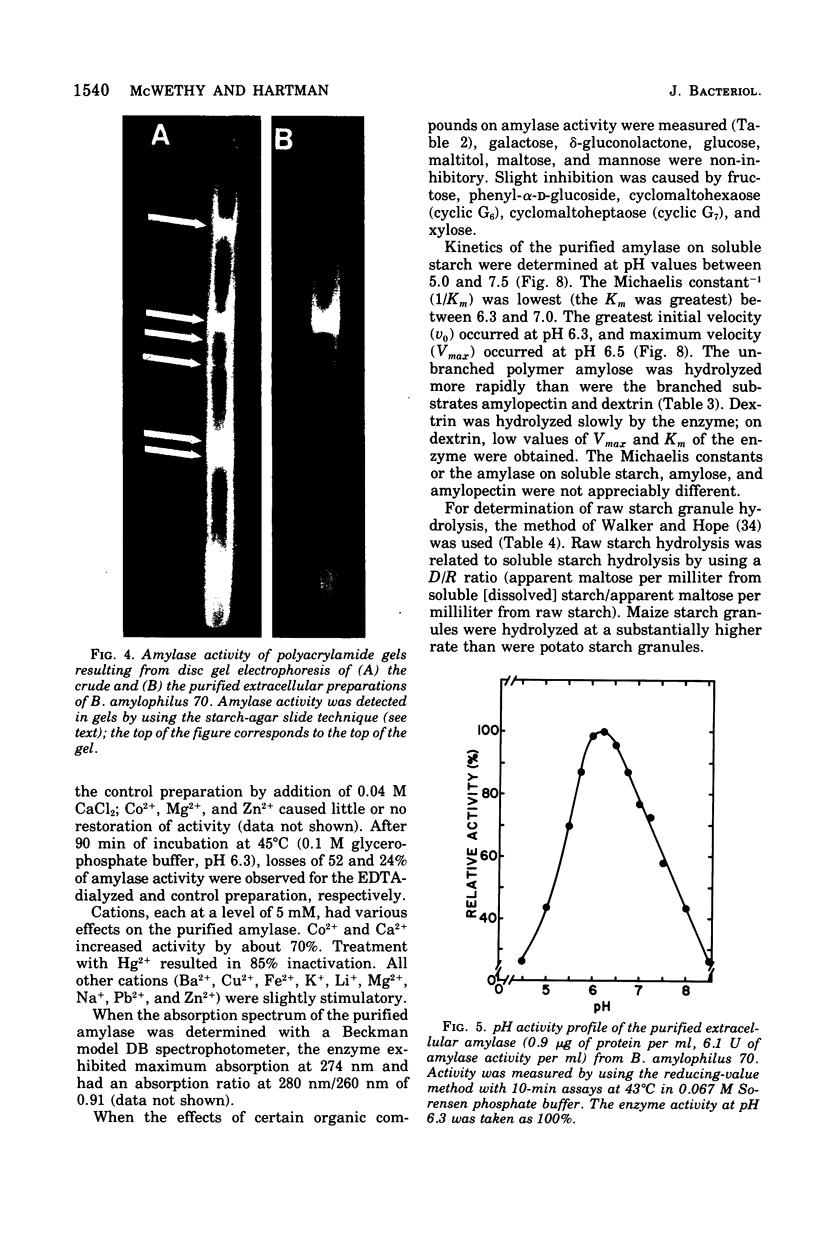


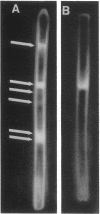
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- French D., Mancusi J. L., Abdullah M., Brammer G. L. Separation of starch oligosaccharides by high temperature paper chromatography. J Chromatogr. 1965 Aug;19(2):445–447. doi: 10.1016/s0021-9673(01)99480-4. [DOI] [PubMed] [Google Scholar]
- Greenwood C. T., MacGregor A. W., Milne E. A. Action pattern of broad bean alpha-amylase. Arch Biochem Biophys. 1965 Dec;112(3):466–470. doi: 10.1016/0003-9861(65)90081-0. [DOI] [PubMed] [Google Scholar]
- HAMLIN L. J., HUNGATE R. E. Culture and physiology of a starch-digesting bacterium (Bacteroides amylophilus n. sp.) from the bovine rumen. J Bacteriol. 1956 Oct;72(4):548–554. doi: 10.1128/jb.72.4.548-554.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOBSON P. N., MACPHERSON M. Amylases of Clostridium butyricum and a Streptococcus isolated from the rumen of the sheep. Biochem J. 1952 Dec;52(4):671–679. doi: 10.1042/bj0520671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenhull D. J., Herbert D. The amylase and maltase of Clostridium acetobutylicum. Biochem J. 1945;39(1):102–106. doi: 10.1042/bj0390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- Pitt-Rivers R., Impiombato F. S. The binding of sodium dodecyl sulphate to various proteins. Biochem J. 1968 Oct;109(5):825–830. doi: 10.1042/bj1090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBYT J., FRENCH D. Action pattern and specificity of an amylase from Bacillus subtilis. Arch Biochem Biophys. 1963 Mar;100:451–467. doi: 10.1016/0003-9861(63)90112-7. [DOI] [PubMed] [Google Scholar]
- Reese E. T., Maguire A. Surfactants as stimulants of enzyme production by microorganisms. Appl Microbiol. 1969 Feb;17(2):242–245. doi: 10.1128/am.17.2.242-245.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J. Structure and function of amylases. II. Multiple forms of bacillus subtilis -amylase. Arch Biochem Biophys. 1973 Apr;155(2):445–451. doi: 10.1016/0003-9861(73)90135-5. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., French D. Multiple attach hypothesis of alpha-amylase action: action of porcine pancreatic, human salivary, and Aspergillus oryzae alpha-amylases. Arch Biochem Biophys. 1967 Oct;122(1):8–16. doi: 10.1016/0003-9861(67)90118-x. [DOI] [PubMed] [Google Scholar]
- STEIN E. A., FISCHER E. H. The resistance of alpha-amylases towards proteolytic attack. J Biol Chem. 1958 Jun;232(2):867–879. [PubMed] [Google Scholar]
- Stoklosa J. T., Latz H. W. Molecular weight determinations of proteins by polyacrylamide gel electrophoresis with sodium dodecyl sulfate in just the sample solution. Biochem Biophys Res Commun. 1974 May 7;58(1):74–79. doi: 10.1016/0006-291x(74)90892-4. [DOI] [PubMed] [Google Scholar]
- VALLEE B. L., STEIN E. A., SUMERWELL W. N., FISCHER E. H. Metal content of alpha-amylases of various origins. J Biol Chem. 1959 Nov;234:2901–2905. [PubMed] [Google Scholar]
- WALKER G. J., HOPE P. M. The action of some alpha-amylases on starch granules. Biochem J. 1963 Mar;86:452–462. doi: 10.1042/bj0860452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G. J. THE CELL-BOUND ALPHA-AMYLASES OF STREPTOCOCCUS BOVIS. Biochem J. 1965 Feb;94:289–298. doi: 10.1042/bj0940289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Hope P. M. Degradation of starch granules by some amylolytic bacteria from the rumen of sheep. Biochem J. 1964 Feb;90(2):398–408. doi: 10.1042/bj0900398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Crystallization and properties of alpha-amylase from five strains of Bacillus amyloliquefaciens. Biochemistry. 1967 Dec;6(12):3681–3689. doi: 10.1021/bi00864a010. [DOI] [PubMed] [Google Scholar]